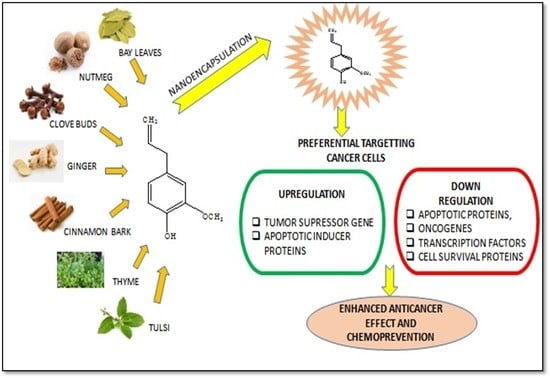
Molecular Mechanisms of Action of Eugenol in Cancer: Recent Trends and Advancement
Abstract
:1. Introduction
2. Methodology for Literature Search and Selection of Anticancer Studies
3. Eugenol Characteristics
4. Anticancer Potential of Eugenol
4.1. Effect of Eugenol on Breast Cancer
4.2. Effect of Eugenol on Cervical Cancer
4.3. Effect of Eugenol on Colorectal Cancers
4.4. Effect of Eugenol on Gastric Cancers
4.5. Effect of Eugenol on Lung Cancer
4.6. Effect of Eugenol on Leukemias
4.7. Effect of Eugenol on Liver Cancer
4.8. Effect of Eugenol on Gliomas
4.9. Effect of Eugenol on Melanomas
4.10. Effect of Eugenol on Osteosarcoma Cells
4.11. Effect on Prostate Cancer
4.12. Effect of Eugenol on Skin Tumors
4.13. Effect of Eugenol on Oral Cancers
4.14. Effect of Eugenol on Ovarian Cancer
| Cancer Type | Tested Compound | * Cell Line Used | Effect and Mechanism | IC50/EC50 Value (µM)/mM | References |
|---|---|---|---|---|---|
| Breast cancer | Eugenol (EUG) | MCF7 cells, T47-D cells, MDA-MB-231 cells, and non-tumorigenic MCF 10A cell | ↑ Apoptosis, ↓ E2F1/surviving ┴ NF-κB and cyclin D1 oncogenes | 2.4 µM | [41] |
| EUG | MCF-7 cells | ↑ DNA fragmentation, ↓ intracellular glutathione level, ↑ intracellular H2O2 and lipid peroxidation, ↑ apoptosis | 1–4 mM | [43] | |
| EUG and cisplatin | MDA-MB-231, MDA-MB-468, and BT-20 cells | ┴ NF-κB and ALDH | EUG (1.0 μM) and cisplatin (30 μM) | [30] | |
| EUG | MCF-7 cells | ↑ Expression of Bcl-2, ↓ intracellular ATP, ↓ membrane potential, ┴ mitochondrial function, ↓ Cyt-c release and LDH | 0.9 mM | [32] | |
| Benzoxazine and aminomethyl derivatives of EUG (6-allyl-3-(furan-2-yl-methyl)-8-methoxy-3,4-dihydro-2H-benzo[e][1,3]oxazine) | MCF-7 cells | ↑ Cytotoxicity | 21.7 µM | [33] | |
| EUG | MDA-MB-231 and MCF-7 cells | ↓ Mitochondria membrane potential (ΔΨm), ↓ proliferation Cell Nuclear Antigen (PCNA) level, ↑ Bax overexpression, ↑ DNA damage | 15.09 µM in MDA-MB 231 cells 22.75 µM in MCF7 cells | [31] | |
| EUG | MCF-7 cells | ↓ MMP-9 expression, ↓ paxilin gene expression, Suppress metastasis | 1 and 1.5 µg/mL after 24 and 48h, respectively | [34] | |
| EUG | MCF-7, BT-474, SKBR-3, and H-ras oncogene transfected MCF10A (MCF10A-ras) human breast epithelial cells | Dose-dependent selective cytotoxicity in MCF10A-ras cells but not in MCF10A cells, ┴ OXPHOS and FAO, ↓ c-Myc/PGC-1β/ERR-α pathways, ┴ ROS generation | 160–200 µM | [35] | |
| EUG | MDA-MB-231 cells | ↓ mRNA expressions of MMP-1, -3, -7, -9, -11, ┴ cancer metastasis, ↑ antiproliferative action | 2.89 mM | [42] | |
| Doxorubicin (DOX) + EUG/astaxanthin (AST) | MCF-7 cells | EUG and AST augmented DOX cytotoxicity, ↑ caspase 3 ↓ CK7 and LC3BI/II ratio | DOX −0.5 μM DOX + EUG 0.088 μM DOX + AST 0.06 μM | [36] | |
| EUG | SK-BR-3 and MDA-MB-231 | ↑ Caspase-3, -7, and -9 expressions, ↓ MMP2 and MMP9 gene expression, ↓ triple-negative and HER2-positive breast cancer metastasis, ↑ anti-metastatic effect | - | [37] | |
| Hesperidin (HES) and EUG in hybrid nanoformulation | MCF-7 cells, L929 fibroblast cells | FA conjugated carrier targets FA receptor-positive breast cancer cells with higher efficacy; ↑ anti-cancer efficacy of HES and EUG by more than 30-folds. | EUG- 36.27 μg/mL; Hesperidin- 39.72 μg/mL; hybrid nanoformulation- 8.75 μg/mL | [29] | |
| Syzygium aromaticum essential oil (SAEO) or EUG-loaded chitosan nanoparticles | MDA-MB-468 and A-375 cells | ↑ antioxidant activity of SAEO and EUG (IC50: 204 and 109 μg mL−1, respectively). ↑ anticancer potential when formulated chitosan nanoparticles | 79 μg mL−1 in (A-375 cells; 51 μg mL−1 in MDA-MB-468 cells | [45] | |
| Molecular hybrids of new sulfonamides with EUG or dihydroEUG (4b) | HepG2, A549, HT-144 MCF-7 cell | ↓ Cyclin D1 and cyclin E expression, ┴ cell cycle at G1/S transition, ↑ apoptosis in MCF-7 cells | - | [40] | |
| EUG | HER2 positive (SK-BR-3) and triple-negative (MDA-MB-231) cells | ↑ Autophagy by microtubule-associated protein 1 light chain 3, ↑ AKT serine/threonine kinase 1 (AKT), ↓ Nucleoporin 62, ↑ forkhead box O3 (FOXO3a), ↑ cyclin-dependent kinase inhibitor (p27), and Caspase-3 and -9, ↑ cyclin-dependent kinase inhibitor 1A (p21), ↑ apoptosis, ┴ PI3K/AKT/FOXO3a pathway | 5, 10 µM | [44] | |
| 1, 2, 3-triazole-isoxazoline derivatives of EUG | MCF-7, MDA-MB-231 | ┴ Proliferation, ↑ cytotoxicity | 17.32–25.94 µM | [65] | |
| Cervical cancer | DCM-EUG extract of Syzygium aromaticum | HeLa cells | ↑ Cytotoxicity, ↑ apoptosis | 200 mg/mL | [47] |
| Myricetin, methyl EUG, and cisplatin | HeLa cells | ↑ Anti-cancer activity, ↑ apoptosis, ┴ cell cycle, ↓ mitochondrial membrane potential, ↑ caspase-3, ↑ lactate dehydrogenase release | (60 μM methyl eugenol + 1 μM cisplatin) or (60 μM Myricetin + 1 μM cisplatin) | [46] | |
| EUG, cisplatin, radiation | HeLa cells | ↓ Proliferation rate, ↑ LDH release, ↑ caspase-3 and -9 activity, ↑ expression of Bax, ↓ expression of B-cell lymphoma (Bcl)-2, ↑ cytochrome c (Cyt-c), ↓ interleukin-1 beta (IL-1β), ↓ cyclooxygenase-2 (Cox-2) | 350 µM (EUG), 0.5–2.5 µM (cisplatin) and 4–6 Gy X-rays | [48] | |
| EUG | SIHA cells | ΔΨm didn’t decrease possibly due to resistance, ↓ PCNA levels, ↑ caspase-3 activation, ↑ Bax overexpression, ↑ DNA damage | 18.31 µM | [31] | |
| Gemcitabine + EUG | HeLa cells | ↑ Apoptosis and inflammation, ↓ Bcl-2, COX-2, and IL-1β | 15–25 mM (Gemcitabine) 150 µM (EUG) | [24] | |
| Sulforaphane + EUG | HeLa cells | ↓ Bcl-2, IL-β, and COX-2 expressions, ↑ caspase-3 | 2.5–8 μM (Sulforaphane) EUG (100–350 μM) | [50] | |
| 5-fluorouracil + EUG | HeLa cells | ↑ cytotoxic, ↓ G2/M phase, ┴ cell cycle in the S phase and G1/G0 phase, | 316 µM (EUG), 21 µM (5-fluorouracil) or combination (153 µM EUG and 10.5 µM 5FU) | [49] | |
| Colon cancer | EUG | Caco-2, SW-620, and NCM-460 cells | ↑ Late-apoptosis and necrosis in Caco-2, ↓ reduce cell proliferation, ↓ G2 phase or G1 phase of cell cycle | 218, 166, and 92 µM, respectively, for the NCM-460, Caco-2, and SW-620 cells, respectively at 24 h | [52] |
| EUG | HCT-15 and HT29 cells | ↑ Apoptosis, ↓ MMP dissipation, ↑ caspase-3 and polyadenosine diphosphate-ribose polymerase (PARP), ↑ p53 tumor suppressor gene, ↑ ROS generation | 300 µM and 500 µM for HCT-15 and HT-29 cell, respectively | [55] | |
| EUG | Lipopolysaccharide-activated mouse macrophage RAW264.7 cells, HT-29 cells | ↓ COX-2 expression in lipopolysaccharide-stimulated macrophage RAW264.7 cells, ┴ mRNA expression of COX-2 in HT-29 cells | 0.37 µM | [53] | |
| EUG (32%) and oleanonic Acid (26%) present in the active fraction of clove | HCT-116 cells | ↑ Dose- and time-dependent apoptosis via autophagy mediated by PI3K/Akt/mTOR | 113.5 µM | [54] | |
| 4-trifluoromethyl benzoic acid (TFBA) + EUG | HCT116, WiDr cells | ↑ Cytotoxicity and anticancer activity | 20.7 and 20.1 µM for HCT116 and WiDr cells, respectively. | [57] | |
| EUG-canola oil or EUG-medium chain triglyceride nanoemulsions | HTB37 cells | Apoptotic cell death via ROS generation, ┴ cell cycle at sub G1/S phase | 750 µM | [56] | |
| Gastric Cancer | EUG and Capsaicin | AGS cells | ↑ Apoptotic activity of capsaicin is p53-dependent, ↑ expression of proapoptotic proteins (Bax, caspase-3 and -8). EUG ┴ cell proliferation and ↑ apoptotic activity which was independent of p53, ↑ caspase-8, and -3 expression | 250 μM and 1 mM, respectively, for capsaicin and EUG | [60] |
| Fibrosarcoma | 1, 2, 3-triazole-isoxazoline derivatives of EUG | HT-1080 | ┴ Proliferation | 15.31–18.81 µM | [65] |
| Gliomas | EUG | DBTRG-05MG human glioblastoma cells | ↑ Mitochondrial pathway of apoptosis in a Ca2+dissociated manner, ↓ Mitochondrial membrane potential, ↑ ROS production, ↑ caspase-9 and -3, ↑ cytochrome c | 100–300 µM | [69] |
| EUG-loaded chitosan nanosystem | Rat C6 glioma cells | ↓ Expression of NF-κB and epithelial to mesenchymal transition (EMT) protein, ↑ apoptosis | 7.5 µM | [68] | |
| Leukemia | EUG | HL60 | ↑ ROS generated apoptosis, ↑ Mitochondrial permeability transition, ↓ Bcl-2, ↑ cytochrome c release | 23.7 µM | [66] |
| EUG and 3,3′-dimethoxy-5,5′-di-2-propenyl-1,1′-biphenyl-2,2′-diol (bis-EUG) | HL-60 | ↑ Cytotoxicity, ↑ apoptosis, ┴ COX-2 gene expression | 0.18 mM (bis EUG) and 0.38 mM (EUG) | [67] | |
| Liver cancer | EUG-canola oil or EUG-medium chain triglyceride nanoemulsions | HB8065 cells | ↑ Apoptotic cell death via ROS generation, ┴ cell cycle at sub G1/S phase | 500 µM | [56] |
| Lung carcinoma | 1, 2, 3-triazole-isoxazoline derivatives of EUG | A549 cells | ┴ Proliferation | 17.32–25.4 µM | [65] |
| EUG | Normal mouse fibroblast cells and A549 cells | ↑ Cytotoxicity against cancer cells but it is non-toxic against normal cells, ↓ expression of β-catenin, ↓ CD44, EpCAM, Oct4, and Notch1 expression, ↑ β-catenin and GSK-3β, ↑ N-terminal phosphorylated Ser37 | 5 μM | [64] | |
| EUG | MRC-5 and A549 cells | ↑ Antiproliferative and antimetastatic effects, ↓ phosphate-Akt, ↓ MMP2 | 800 µM and 400 µM in MRC-5 and A549, respectively | [62] | |
| Melanoma | EUG-related biphenyl (S)-6,6′-dibromo-dehydrodi eugenol | WM266-4, SK-Mel28, LCM-Mel, LCP-Mel, PNP-Mel, A-13443, CN-Mel, GR-Mel cells and SbCl2, NB, GI-LI-N, LAN-5 cells | ↑ Cytotoxic, ↑ apoptosis, ↑ caspase activation | 16–27 µM | [71] |
| Hyaluronic acid-coated dacarbazine-EUG liposomes | SK-MEL-28 and B16F10 cells | ↓ E2F1/survivin pathway, ┴ cell cycle at S phase, ↑ apoptosis and cytotoxicity, ↓ migration and proliferation | - | [73] | |
| EUG | SK-Mel-28 and A2058 cells | ↑ caspase-3 activation A2058 cells, ↑ Bax overexpression, ↑ DNA damage | 7.201 μM (SK-Mel-28) and 12.17 μM (A2058) | [31] | |
| G361 cells | ↑ Apoptosis, ┴ cell cycle at S phase, ↓ expressions of cyclin A, cyclin D3, cyclin E, cdc2, cdk2, and cdk4, ↑ cleavage of DFF45 and PARP, ↑ caspase-3, and -9 | 1 mM | [74] | ||
| Oral squamous cell carcinoma | EUG | HSC-2 cells | ↑ Apoptotic cell death | 0.5 mM–2 mM | [83] |
| Silver nanoconjugates of EUG | KB cells | ↑ Cytotoxicity, ┴ cell cycle at S and G2/M phase | 2.5–50 µM | [85] | |
| Hydroalcoholic extract of Cinnamonium verum containing EUG | SCC25 cells | ↑ Cytotoxicity, ↑ apoptosis, ┴ cell cycle at the S phase | 24.71 µM | [81] | |
| Osteosarcoma | EUG | HOS cell | ↑ Apoptosis by caspase-3 activation, ↑ expression of the p53 tumor suppressor gene, ↑ cleavage of PARP and lamin ↓DFF-45 | 1.5 µM | [75] |
| Ovarian cancer | Cisplatin + EUG | SKOV3 and OV2774 cells | ┴ Ovarian cell growth, ↑ apoptosis by ↓ Notch-Hes1 signaling, ↑ Hes1 promoted stemness ↓ drug resistance ABC transporter genes | 5–10 µM (Cisplatin) + 1 µM (EUG) | [84] |
| Prostate cancer | EUG radiolabeled with I131 | PC3 cells | Cytotoxic, better uptake | 89.44 µM | [77] |
| EUG + 2-methyl estradiol | LNCaP, PC-3, DU 145 cells | ┴ Cell cycle at G2/M phase, ↑ Bcl-2-dependent apoptotic cell death, ↑ proapoptotic protein Bax | 0.5 µM (EUG) + 41 µM (2-methyl estradiol) in LNCaP cells | [78] |
5. Discussion
6. Toxicity of Eugenol
7. Challenges and Future Scope
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassanpour, S.H.; Dehghani, M. Review of cancer from perspective of molecular. J. Cancer Res. Pract. 2017, 4, 127–129. [Google Scholar] [CrossRef]
- Ghanbari-Movahed, M.; Kaceli, T.; Mondal, A.; Farzaei, M.; Bishayee, A. Recent Advances in Improved Anticancer Efficacies of Camptothecin Nano-Formulations: A Systematic Review. Biomedicines 2021, 9, 480. [Google Scholar] [CrossRef]
- Aljuffali, I.A.; Fang, C.-L.; Chen, C.-H.; Fang, J.-Y. Nanomedicine as a strategy for natural compound delivery to prevent and treat cancers. Curr. Pharm. Des. 2016, 22, 4219–4231. [Google Scholar] [CrossRef] [PubMed]
- Lagoa, R.; Silva, J.; Rodrigues, J.R.; Bishayee, A. Advances in phytochemical delivery systems for improved anticancer activity. Biotechnol. Adv. 2020, 38, 107382. [Google Scholar] [CrossRef]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharmacol. 2019, 10, 1614, Erratumin Front. Pharmacol. 2020, 11, 175. [Google Scholar] [CrossRef] [Green Version]
- Bhalla, Y.; Gupta, V.K.; Jaitak, V. Anticancer activity of essential oils: A review. J. Sci. Food Agric. 2013, 93, 3643–3653. [Google Scholar] [CrossRef]
- Barboza, J.N.; Da Silva Maia Bezerra Filho, C.; Silva, R.O.; Medeiros, J.V.R.; de Sousa, D.P. An Overview on the Anti-inflammatory Potential and Antioxidant Profile of Eugenol. Oxidative Med. Cell. Longev. 2018, 2018, 3957262. [Google Scholar] [CrossRef]
- Park, S.-H.; Sim, Y.-B.; Lee, J.-K.; Kim, S.-M.; Kang, Y.-J.; Jung, J.-S.; Suh, H.-W. The analgesic effects and mechanisms of orally administered eugenol. Arch. Pharmacal Res. 2011, 34, 501–507. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxidant Activity of Eugenol: A Structure–Activity Relationship Study. J. Med. Food 2011, 14, 975–985. [Google Scholar] [CrossRef]
- Chami, N.; Chami, F.; Bennis, S.; Trouillas, J.; Remmal, A. Antifungal treatment with carvacrol and eugenol of oral candidiasis in immunosuppressed rats. Braz. J. Infect. Dis. 2004, 8, 217–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchese, A.; Barbieri, R.; Coppo, E.; Orhan, I.E.; Daglia, M.; Nabavi, S.M.; Izadi, M.; Abdollahi, M.; Nabavi, S.M.; Ajami, M. Antimicrobial activity of eugenol and essential oils containing eugenol: A mechanistic viewpoint. Crit. Rev. Microbiol. 2017, 43, 668–689. [Google Scholar] [CrossRef] [PubMed]
- Benencia, F.; Courrèges, M.C. In vitro and in vivo activity of eugenol on human herpesvirus. Phytother. Res. 2000, 14, 495–500. [Google Scholar] [CrossRef]
- de Morais, S.M.; Vila-Nova, N.S.; Bevilaqua, C.; Rondon, F.C.; Lobo, C.H.; de Alencar Araripe Noronha Moura, A.; Sales, A.D.; Rodrigues, A.P.R.; de Figuereido, J.R.; Campello, C.C.; et al. Thymol and eugenol derivatives as potential antileishmanial agents. Bioorg. Med. Chem. 2014, 22, 6250–6255. [Google Scholar] [CrossRef] [Green Version]
- Pramod, K.; Ansari, S.H.; Ali, J. Eugenol: A Natural Compound with Versatile Pharmacological Actions. Nat. Prod. Commun. 2010, 5, 1999–2006. [Google Scholar] [CrossRef] [Green Version]
- Nejad, S.M.; Özgüneş, H.; Başaran, N. Pharmacological and Toxicological Properties of Eugenol. Turk. J. Pharm. Sci. 2017, 14, 201–206. [Google Scholar] [CrossRef]
- Khalil, A.A.; Rahman, U.U.; Khan, M.R.; Sahar, A.; Mehmood, T.; Khan, M. Essential oil eugenol: Sources, extraction techniques and nutraceutical perspectives. RSC Adv. 2017, 7, 32669–32681. [Google Scholar] [CrossRef] [Green Version]
- Bendre, R.S.; Rajput, J.D.; Bagul, S.D.; Karandikar, P. Outlooks on medicinal properties of eugenol and its synthetic derivatives. Nat. Prod. Chem. Res. 2016, 4, 1–6. [Google Scholar] [CrossRef]
- Fadilah, F.; Yanuar, A.; Arsianti, A.; Andrajati, R. Phenylpropanoids, eugenol scaffold, and its derivatives as anticancer. Asian J. Pharm. Clin. Res. 2017, 41–46. [Google Scholar] [CrossRef] [Green Version]
- Begum, S.N.; Ray, A.S.; Rahaman, C.H. A comprehensive and systematic review on potential anticancer activities of eugenol: From pre-clinical evidence to molecular mechanisms of action. Phytomedicine 2022, 107, 154456. [Google Scholar] [CrossRef]
- Zari, A.T.; Zari, T.A.; Hakeem, K.R. Anticancer Properties of Eugenol: A Review. Molecules 2021, 26, 7407. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, D.P.; Militão, G.C.G.; De Morais, M.C.; De Sousa, D.P. The Dual Antioxidant/Prooxidant Effect of Eugenol and Its Action in Cancer Development and Treatment. Nutrients 2017, 9, 1367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Healthcare Interventions: Explanation and Elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [Green Version]
- Hussain, A.; Brahmbhatt, K.; Priyani, A.; Ahmed, M.; Rizvi, T.A.; Sharma, C. Eugenol Enhances the Chemotherapeutic Potential of Gemcitabine and Induces Anticarcinogenic and Anti-inflammatory Activity in Human Cervical Cancer Cells. Cancer Biother. Radiopharm. 2011, 26, 519–527. [Google Scholar] [CrossRef]
- Pal, D.; Sur, S.; Roy, R.; Mandal, S.; Panda, C.K. Epigallocatechin gallate in combination with eugenol or amarogentin shows synergistic chemotherapeutic potential in cervical cancer cell line. J. Cell Physiol. 2018, 234, 825–836. [Google Scholar] [CrossRef] [Green Version]
- Kubatka, P.; Kello, M.; Kajo, K.; Samec, M.; Jasek, K.; Vybohova, D.; Uramova, S.; Líšková, A.; Sadlonova, V.; Koklesova, L.; et al. Chemopreventive and Therapeutic Efficacy of Cinnamomum zeylanicum L. Bark in Experimental Breast Carcinoma: Mechanistic in Vivo and in Vitro Analyses. Molecules 2020, 25, 1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahangir, M.A.; Taleuzzaman, M.; Beg, S.; Verma, S.; Gilani, S.J.; Alam, P. A Review of Eugenol-based Nanomedicine: Recent Advancements. Curr. Bioact. Compd. 2021, 17, 214–219. [Google Scholar] [CrossRef]
- Salama, L.; Pastor, E.R.; Stone, T.; Mousa, S.A. Emerging Nanopharmaceuticals and Nanonutraceuticals in Cancer Management. Biomedicines 2020, 8, 347. [Google Scholar] [CrossRef] [PubMed]
- Maheswari, P.U.; Muthappa, R.; Bindhya, K.P.; Begum, K.M.S. Evaluation of folic acid functionalized BSA-CaFe2O4 nanohybrid carrier for the controlled delivery of natural cytotoxic drugs hesperidin and eugenol. J. Drug Deliv. Sci. Technol. 2021, 61, 102105. [Google Scholar] [CrossRef]
- Islam, S.S.; Al-Sharif, I.; Sultan, A.; Al-Mazrou, A.; Remmal, A.; Aboussekhra, A. Eugenol potentiates cisplatin anti-cancer activity through inhibition of ALDH-positive breast cancer stem cells and the NF-κB signaling pathway. Mol. Carcinog. 2018, 57, 333–346. [Google Scholar] [CrossRef]
- Júnior, P.L.D.S.; Câmara, D.A.D.; Costa, A.S.; Ruiz, J.L.M.; Levy, D.; Azevedo, R.A.; Pasqualoto, K.F.M.; de Oliveira, C.F.; de Melo, T.C.; Pessoa, N.D.S.; et al. Apoptotic effect of eugenol envolves G2/M phase abrogation accompanied by mitochondrial damage and clastogenic effect on cancer cell in vitro. Phytomedicine 2016, 23, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Al Wafai, R.; El-Rabih, W.; Katerji, M.; Safi, R.; El Sabban, M.; El-Rifai, O.; Usta, J. Chemosensitivity of MCF-7 cells to eugenol: Release of cytochrome-c and lactate dehydrogenase. Sci. Rep. 2017, 7, 43730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudyanto, M.; Widiandani, T.; Syahrani, A. Some Benzoxazine and aminomethyl derivatives of Eugenol: Cytotoxicity on MCF-7 cell line. Int. J. Pharm. Pharm. Sci. 2015, 7, 229. [Google Scholar]
- Baharara, J.; Ramezani, T.; Mousavi, M.; Kouhestanian, K. Eugenol suppressed metastasis of breast carcinoma cells and migration by regulation of MMP-9 & paxilin gene expression. Sch. J. Agric. Vet. Sci. 2015, 2, 125–130. [Google Scholar]
- Yan, X.; Zhang, G.; Bie, F.; Lv, Y.; Ma, Y.; Ma, M.; Wang, Y.; Hao, X.; Yuan, N.; Jiang, X. Eugenol inhibits oxidative phosphorylation and fatty acid oxidation via downregulation of c-Myc/PGC-1β/ERRα signaling pathway in MCF10A-ras cells. Sci. Rep. 2017, 7, 12920. [Google Scholar] [CrossRef] [Green Version]
- Fouad, M.A.; Sayed-Ahmed, M.M.; Huwait, E.A.; Hafez, H.F.; Osman, A.-M.M. Epigenetic immunomodulatory effect of eugenol and astaxanthin on doxorubicin cytotoxicity in hormonal positive breast Cancer cells. BMC Pharmacol. Toxicol. 2021, 22, 8. [Google Scholar] [CrossRef]
- Abdullah, M.L.; Hafez, M.M.; Al-Hoshani, A.; Al-Shabanah, O. Anti-metastatic and anti-proliferative activity of eugenol against triple negative and HER2 positive breast cancer cells. BMC Complement. Altern. Med. 2018, 18, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, P.; Barua, A.; Roy, A.; Pattanayak, R.; Bhattacharyya, M.; Saha, P. Eugenol restricts Cancer Stem Cell population by degradation of β-catenin via N-terminal Ser37 phosphorylation-an in vivo and in vitro experimental evaluation. Chem. Interact. 2020, 316, 108938. [Google Scholar] [CrossRef]
- Ma, M.; Ma, Y.; Zhang, G.-J.; Liao, R.; Jiang, X.-F.; Yan, X.-X.; Bie, F.-J.; Li, X.-B.; Lv, Y.-H. Eugenol alleviated breast precancerous lesions through HER2/PI3K-AKT pathway-induced cell apoptosis and S-phase arrest. Oncotarget 2017, 8, 56296. [Google Scholar] [CrossRef] [Green Version]
- Azevedo-Barbosa, H.; Ferreira-Silva, G.; Silva, C.F.; de Souza, T.B.; Dias, D.F.; de Paula, A.C.C.; Ionta, M.; Carvalho, D.T. Phenylpropanoid-based sulfonamide promotes cyclin D1 and cyclin E down-regulation and induces cell cycle arrest at G1/S transition in estrogen positive MCF-7 cell line. Toxicol. Vitr. 2019, 59, 150–160. [Google Scholar] [CrossRef]
- Al-Sharif, I.; Remmal, A.; Aboussekhra, A. Eugenol triggers apoptosis in breast cancer cells through E2F1/survivin down-regulation. BMC Cancer 2013, 13, 600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajoriya, S.; Nandhakumar, P.; Karthik, K.; Kumar, A.; Saini, M.; Kataria, M. Study on effect of eugenol on anti-metastatic activity and expression of MMPS in TNBC MDA MB: 231 cell line. J. Pharmacogn. Phytochem. 2019, 8, 788–794. [Google Scholar]
- Vidhya, N.; Devaraj, S.N. Induction of apoptosis by eugenol in human breast cancer cells. Indian J. Exp. Biol. 2011, 49, 871–878. [Google Scholar] [PubMed]
- Abdullah, M.L.; Al-Shabanah, O.; Hassan, Z.K.; Hafez, M.M. Eugenol-Induced Autophagy and Apoptosis in Breast Cancer Cells via PI3K/AKT/FOXO3a Pathway Inhibition. Int. J. Mol. Sci. 2021, 22, 9243. [Google Scholar] [CrossRef] [PubMed]
- Valizadeh, A.; Khaleghi, A.A.; Alipanah, H.; Zarenezhad, E.; Osanloo, M. Anticarcinogenic Effect of Chitosan Nanoparticles Containing Syzygium aromaticum Essential Oil or Eugenol toward Breast and Skin Cancer Cell Lines. BioNanoScience 2021, 11, 678–686. [Google Scholar] [CrossRef]
- Yi, J.-L.; Shi, S.; Shen, Y.-L.; Wang, L.; Chen, H.-Y.; Zhu, J.; Ding, Y. Myricetin and methyl eugenol combination enhances the anticancer activity, cell cycle arrest and apoptosis induction of cis-platin against HeLa cervical cancer cell lines. Int. J. Clin. Exp. Pathol. 2015, 8, 1116–1127. [Google Scholar]
- Das, A.; Harshadha, K.; Dhinesh, K.S.K.; Hari, R.K.; Jayaprakash, B. Evaluation of Therapeutic Potential of Eugenol-A Natural Derivative of Syzygium aromaticum on Cervical Cancer. Asian Pac. J. Cancer Prev. 2018, 19, 1977–1985. [Google Scholar] [CrossRef]
- Fathy, M.; Fawzy, M.A.; Hintzsche, H.; Nikaido, T.; Dandekar, T.; Othman, E.M. Eugenol Exerts Apoptotic Effect and Modulates the Sensitivity of HeLa Cells to Cisplatin and Radiation. Molecules 2019, 24, 3979. [Google Scholar] [CrossRef] [Green Version]
- Hemaiswarya, S.; Doble, M. Combination of phenylpropanoids with 5-fluorouracil as anti-cancer agents against human cervical cancer (HeLa) cell line. Phytomedicine 2013, 20, 151–158. [Google Scholar] [CrossRef]
- Hussain, A.; Priyani, A.; Sadrieh, L.; Brahmbhatt, K.; Ahmed, M.; Sharma, C. Concurrent Sulforaphane and Eugenol Induces Differential Effects on Human Cervical Cancer Cells. Integr. Cancer Ther. 2012, 11, 154–165. [Google Scholar] [CrossRef] [Green Version]
- Permatasari, H.K.; Effendi, A.B.; Qhabibi, F.R.; Fawwaz, F.; Dominique, A. Eugenol isolated from Syzygium aromaticum inhibits HeLa cancer cell migration by altering epithelial-mesenchymal transition protein regulators. J. Appl. Pharm. Sci. 2021, 11, 049–053. [Google Scholar]
- Petrocelli, G.; Farabegoli, F.; Valerii, M.; Giovannini, C.; Sardo, A.; Spisni, E. Molecules Present in Plant Essential Oils for Prevention and Treatment of Colorectal Cancer (CRC). Molecules 2021, 26, 885. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Oh, O.-J.; Min, H.-Y.; Park, E.-J.; Kim, Y.; Park, H.J.; Han, Y.N.; Lee, S.K. Eugenol suppresses cyclooxygenase-2 expression in lipopolysaccharide-stimulated mouse macrophage RAW264.7 cells. Life Sci. 2003, 73, 337–348. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, G.; Zhang, D.; An, W.; Lai, H.; Li, X.; Cao, S.; Lin, X. Active fraction of clove induces apoptosis via PI3K/Akt/mTOR-mediated autophagy in human colorectal cancer HCT-116 cells. Int. J. Oncol. 2018, 53, 1363–1373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaganathan, S.K.; Mazumdar, A.; Mondhe, D.; Mandal, M. Apoptotic effect of eugenol in human colon cancer cell lines. Cell Biol. Int. 2011, 35, 607–615. [Google Scholar] [CrossRef]
- Majeed, H.; Antoniou, J.; Fang, Z. Apoptotic Effects of Eugenol-loaded Nanoemulsions in Human Colon and Liver Cancer Cell Lines. Asian Pac. J. Cancer Prev. 2014, 15, 9159–9164. [Google Scholar] [CrossRef] [Green Version]
- Fadilah, F.; Andrajati, R.; Yanuar, A.; Arsianti, A. In-vitro anticancer activity combination of eugenol and simple aromatic benzoate compounds against human colon HCT-116 cells and WiDr cells. J. Pharm. Sci. Res. 2017, 9, 637. [Google Scholar]
- Manikandan, P.; Vinothini, G.; Priyadarsini, R.V.; Prathiba, D.; Nagini, S. Eugenol inhibits cell proliferation via NF-κB suppression in a rat model of gastric carcinogenesis induced by MNNG. Investig. New Drugs 2010, 29, 110–117. [Google Scholar] [CrossRef]
- Manikandan, P.; Murugan, R.S.; Priyadarsini, R.V.; Vinothini, G.; Nagini, S. Eugenol induces apoptosis and inhibits invasion and angiogenesis in a rat model of gastric carcinogenesis induced by MNNG. Life Sci. 2010, 86, 936–941. [Google Scholar] [CrossRef]
- Sarkar, A.; Bhattacharjee, S.; Mandal, D.P. Induction of Apoptosis by Eugenol and Capsaicin in Human Gastric Cancer AGS Cells—Elucidating the Role of p53. Asian Pac. J. Cancer Prev. 2015, 16, 6753–6759. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Panda, C.K.; Das, S. Clove (Syzygium aromaticum L.), a potential chemopreventive agent for lung cancer. Carcinogenesis 2005, 27, 1645–1654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fangjun, L.; Zhijia, Y. Tumor suppressive roles of eugenol in human lung cancer cells. Thorac. Cancer 2018, 9, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Liu, Z.; Zeng, J.; Chen, L.; Wu, Q.; Mo, J.; Zhang, G.; Song, L.; Xu, W.; Zhang, S.; et al. Eugenol inhibits non-small cell lung cancer by repressing expression of NF-κB-regulated TRIM59. Phytother. Res. 2019, 33, 1562–1569. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, P.; Barua, A.; Roy, A.; Pattanayak, R.; Bhattacharyya, M.; Saha, P. Eugenol emerges as an elixir by targeting β-catenin, the central cancer stem cell regulator in lung carcinogenesis: An in vivo and in vitro rationale. Food Funct. 2021, 12, 1063–1078. [Google Scholar] [CrossRef] [PubMed]
- Taia, A.; Essaber, M.; Oubella, A.; Aatif, A.; Bodiguel, J.; Jamart-Grégoire, B.; Itto, M.Y.A.; Morjani, H. Synthesis, characterization, and biological evaluation of new heterocyclic systems 1, 2, 3-triazole-isoxazoline from eugenol by the mixed condensation reactions. Synth. Commun. 2020, 50, 2052–2065. [Google Scholar] [CrossRef]
- Yoo, C.-B.; Han, K.-T.; Cho, K.-S.; Ha, J.; Park, H.-J.; Nam, J.-H.; Kil, U.-H.; Lee, K.-T. Eugenol isolated from the essential oil of Eugenia caryophyllata induces a reactive oxygen species-mediated apoptosis in HL-60 human promyelocytic leukemia cells. Cancer Lett. 2005, 225, 41–52. [Google Scholar] [CrossRef]
- Okada, N.; Hirata, A.; Murakami, Y.; Shoji, M.; Sakagami, H.; Fujisawa, S. Induction of cytotoxicity and apoptosis and inhibition of cyclooxygenase-2 gene expression by eugenol-related compounds. Anticancer Res. 2005, 25, 3263–3269. [Google Scholar]
- Li, Z.; Veeraraghavan, V.P.; Mohan, S.K.; Bolla, S.R.; Lakshmanan, H.; Kumaran, S.; Aruni, W.; Aladresi, A.A.M.; Shair, O.H.; Alharbi, S.A.; et al. Apoptotic induction and anti-metastatic activity of eugenol encapsulated chitosan nanopolymer on rat glioma C6 cells via alleviating the MMP signaling pathway. J. Photochem. Photobiol. B Biol. 2020, 203, 111773. [Google Scholar] [CrossRef]
- Liang, W.-Z.; Chou, C.-T.; Hsu, S.-S.; Liao, W.-C.; Shieh, P.; Kuo, D.-H.; Tseng, H.-W.; Kuo, C.-C.; Jan, C.-R. The involvement of mitochondrial apoptotic pathway in eugenol-induced cell death in human glioblastoma cells. Toxicol. Lett. 2015, 232, 122–132. [Google Scholar] [CrossRef]
- Kim, G.-C.; Choi, D.-S.; Lim, J.-S.; Jeong, H.-C.; Kim, I.-R.; Lee, M.-H.; Park, B.-S. Caspases-dependent apoptosis in human melanoma cell by eugenol. Korean J. Anat. 2006, 245–253. [Google Scholar]
- Pisano, M.; Pagnan, G.; Loi, M.; Mura, M.E.; Tilocca, M.G.; Palmieri, G.; Fabbri, D.; Dettori, M.A.; Delogu, G.; Ponzoni, M.; et al. Antiproliferative and pro-apoptotic activity of eugenol-related biphenyls on malignant melanoma cells. Mol. Cancer 2007, 6, 8. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, R.; Nadiminty, N.; Fitzpatrick, J.E.; Alworth, W.L.; Slaga, T.J.; Kumar, A.P. Eugenol Causes Melanoma Growth Suppression through Inhibition of E2F1 Transcriptional Activity. J. Biol. Chem. 2005, 280, 5812–5819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, H.; Mishra, P.K.; Iqbal, Z.; Jaggi, M.; Madaan, A.; Bhuyan, K.; Gupta, N.; Gupta, N.; Vats, K.; Verma, R.; et al. Co-Delivery of Eugenol and Dacarbazine by Hyaluronic Acid-Coated Liposomes for Targeted Inhibition of Survivin in Treatment of Resistant Metastatic Melanoma. Pharmaceutics 2019, 11, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rachoi, B.-B.; Shin, S.-H.; Kim, U.-K.; Hong, J.-W.; Kim, G.-C. S phase cell cycle arrest and apoptosis is induced by eugenol in G361 human melanoma cells. Int. J. Oral Biol. 2011, 36, 129–134. [Google Scholar]
- Shin, S.-H.; Park, J.-H.; Kim, G.-C.; Park, B.-S.; Gil, Y.-G.; Kim, C.-H. The mechanism of apoptosis induced by eugenol in human osteosarcoma cells. J. Korean Assoc. Oral Maxillofac. Surg. 2007, 33, 20–27. [Google Scholar]
- Razak, M.A.I.A.; Hamid, H.A.; Othman, R.N.I.R.; Moktar, S.A.M.; Miskon, A. Improved Drug Delivery System for Cancer Treatment by D-Glucose Conjugation with Eugenol from Natural Product. Curr. Drug Deliv. 2021, 18, 312–322. [Google Scholar] [CrossRef]
- Dervis, E.; Kilcar, A.Y.; Medine, E.I.; Tekin, V.; Cetkin, B.; Uygur, E.; Muftuler, F.Z.B. In vitroIncorporation of Radioiodinated Eugenol on Adenocarcinoma Cell Lines (Caco2, MCF7, and PC3). Cancer Biother. Radiopharm. 2017, 32, 75–81. [Google Scholar] [CrossRef]
- Ghosh, R.; Ganapathy, M.; Alworth, W.L.; Chan, D.C.; Kumar, A.P. Combination of 2-methoxyestradiol (2-ME2) and eugenol for apoptosis induction synergistically in androgen independent prostate cancer cells. J. Steroid Biochem. Mol. Biol. 2009, 113, 25–35. [Google Scholar] [CrossRef]
- Pal, D.; Banerjee, S.; Mukherjee, S.; Roy, A.; Panda, C.K.; Das, S. Eugenol restricts DMBA croton oil induced skin carcinogenesis in mice: Downregulation of c-Myc and H-ras, and activation of p53 dependent apoptotic pathway. J. Dermatol. Sci. 2010, 59, 31–39. [Google Scholar] [CrossRef]
- Kaur, G.; Athar, M.; Alam, M.S. Eugenol precludes cutaneous chemical carcinogenesis in mouse by preventing oxidative stress and inflammation and by inducing apoptosis. Mol. Carcinog. 2010, 49, 290–301. [Google Scholar] [CrossRef]
- Varadarajan, S.; Narasimhan, M.; Balaji, T.M.; Chamundeeswari, D.P.; Sakthisekaran, D. In Vitro Anticancer Effects of Cinnamomum verum J. Presl, Cinnamaldehyde, 4 Hydroxycinnamic Acid and Eugenol on an Oral Squamous Cell Carcinoma Cell Line. J. Contemp. Dent. Pract. 2020, 21, 1027–1033. [Google Scholar]
- Duicu, O.M.; Pavel, I.Z.; Borcan, F.; Muntean, D.M.; Chevereșan, A.; Bratu, E.A.; Rusu, L.C.; Karancsi, O.L. Characterization of the Eugenol Effects on the Bioenergetic Profile of SCC-4 Human Squamous Cell Carcinoma Cell Line. Rev. Chim. 2018, 69, 2567–2570. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Park, B.-S. The effect of eugenol on the induction of apoptosis in HSC-2 human oral squamous cell carcinoma. J. Korean Soc. Dent. Hyg. 2015, 15, 523–529. [Google Scholar] [CrossRef] [Green Version]
- Islam, S.S.; Aboussekhra, A. Sequential combination of cisplatin with eugenol targets ovarian cancer stem cells through the Notch-Hes1 signalling pathway. J. Exp. Clin. Cancer Res. 2019, 38, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Preethi, R.; Padma, P. Anticancer activity of silver nanobioconjugates synthesized from Piper betle leaves extract and its active compound eugenol. Int. J. Pharm. Pharm. Sci. 2016, 8, 201–205. [Google Scholar] [CrossRef]
- Jaganathan, S.K.; Mondhe, D.; Wani, Z.A.; Pal, H.C.; Mandal, M. Effect of Honey and Eugenol on Ehrlich Ascites and Solid Carcinoma. J. Biomed. Biotechnol. 2010, 2010, 989163. [Google Scholar] [CrossRef] [Green Version]
- Baig, S.M.; Seevasant, I.; Mohamad, J.A.; Mukheem, A.; Huri, H.Z.; Kamarul, T. Potential of apoptotic pathway-targeted cancer therapeutic research: Where do we stand? Cell Death Dis. 2016, 7, e2058. [Google Scholar] [CrossRef] [Green Version]
- Yu, R.; Mandlekar, S.; Harvey, K.; Ucker, D.S.; Kong, A.N. Chemopreventive isothiocyanates induce apoptosis and caspase-3-like protease activity. Cancer Res. 1998, 58, 402–408. [Google Scholar]
- Aggarwal, B.B.; Shishodia, S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem. Pharmacol. 2006, 71, 1397–1421. [Google Scholar] [CrossRef]
- Lev-Ari, S.; Vexler, A.; Starr, A.; Ashkenazy-Voghera, M.; Greif, J.; Aderka, D.; Ben-Yosef, R. Curcumin Augments Gemcitabine Cytotoxic Effect on Pancreatic Adenocarcinoma Cell Lines. Cancer Investig. 2007, 25, 411–418. [Google Scholar] [CrossRef]
- Park, J.; Ayyappan, V.; Bae, E.-K.; Lee, C.; Kim, B.-S.; Kim, B.K.; Lee, Y.-Y.; Ahn, K.-S.; Yoon, S.-S. Curcumin in combination with bortezomib synergistically induced apoptosis in human multiple myeloma U266 cells. Mol. Oncol. 2008, 2, 317–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaganathan, S.K.; Supriyanto, E. Antiproliferative and Molecular Mechanism of Eugenol-Induced Apoptosis in Cancer Cells. Molecules 2012, 17, 6290–6304. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, A.; Ghadiri, A.; Dessauge, F.; Duhamel, M.; Rebollo, M.P.; Alvarez-Franco, F.; Rebollo, A. Modulating apoptosis as a target for effective therapy. Mol. Immunol. 2006, 43, 1065–1079. [Google Scholar] [CrossRef]
- Gemma, A.; Takenaka, K.; Hosoya, Y.; Matuda, K.; Seike, M.; Kurimoto, F.; Ono, Y.; Uematsu, K.; Takeda, Y.; Hibino, S.; et al. Altered expression of several genes in highly metastatic subpopulations of a human pulmonary adenocarcinoma cell line. Eur. J. Cancer 2001, 37, 1554–1561. [Google Scholar] [CrossRef]
- Sarkar, F.H.; Li, Y. Using Chemopreventive Agents to Enhance the Efficacy of Cancer Therapy. Cancer Res. 2006, 66, 3347–3350. [Google Scholar] [CrossRef] [Green Version]
- Youlden, D.R.; Cramb, S.M.; Yip, C.H.; Baade, P.D. Incidence and mortality of female breast cancer in the Asia-Pacific region. Cancer Biol. Med. 2014, 11, 101–115. [Google Scholar] [CrossRef] [Green Version]
- Anuj, G.; Sanjay, S. Eugenol: A potential phytochemical with multifaceted therapeutic activities. Pharmacologyonline 2010, 2, 108–120. [Google Scholar]
- Medeiros, M.H.G.; Dimascio, P.; Pinto, A.P.; Vargas, R.R.; Bechara, E.J.H. Horseradish Peroxidase-Catalyzed Conjugation of Eugenol with Basic Amino Acids. Free Radic. Res. 1996, 25, 5–12. [Google Scholar] [CrossRef]
- Someya, H.; Higo, Y.; Ohno, M.; Tsutsui, T.W.; Tsutsui, T. Clastogenic activity of seven endodontic medications used in dental practice in human dental pulp cells. Mutat. Res. Toxicol. Environ. Mutagen. 2008, 650, 39–47. [Google Scholar] [CrossRef]
- Sarrami, N.; Pemberton, M.N.; Thornhill, M.; Theaker, E.D. Adverse reactions associated with the use of eugenol in dentistry. Br. Dent. J. 2002, 193, 257–259. [Google Scholar] [CrossRef]
- Taleuzzaman, M.; Imam, S.S.; Gilani, S.J. Clove Oil/Eugenol as the Nanotechnological Perspective for Healthcare Applications. In Nanomedicine for Bioactives; Springer: Berlin/Heidelberg, Germany, 2020; pp. 413–430. [Google Scholar]
- Ahmad, N.; Ahmad, F.J.; Bedi, S.; Sharma, S.; Umar, S.; Ansari, M.A. A novel Nanoformulation Development of Eugenol and their treatment in inflammation and periodontitis. Saudi Pharm. J. 2019, 27, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, F.; Oliva, A.; Sabatino, M.; Imbriano, A.; Hanieh, P.N.; Garzoli, S.; Mastroianni, C.M.; De Angelis, M.; Miele, M.C.; Arnaut, M.; et al. Antimicrobial Essential Oil Formulation: Chitosan Coated Nanoemulsions for Nose to Brain Delivery. Pharmaceutics 2020, 12, 678. [Google Scholar] [CrossRef] [PubMed]
- Nastiti, C.M.R.R.; Ponto, T.; Mohammed, Y.; Roberts, M.S.; Benson, H.A.E. Novel Nanocarriers for Targeted Topical Skin Delivery of the Antioxidant Resveratrol. Pharmaceutics 2020, 12, 108. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Alam, A.; Ahmad, F.J.; Sarafroz, M.; Ansari, K.; Sharma, S.; Amir, M. Ultrasonication techniques used for the preparation of novel Eugenol-Nanoemulsion in the treatment of wounds healings and anti-inflammatory. J. Drug Deliv. Sci. Technol. 2018, 46, 461–473. [Google Scholar] [CrossRef]
- Rodenak-Kladniew, B.; Montoto, S.S.; Sbaraglini, M.; Di Ianni, M.; Ruiz, M.; Talevi, A.; Alvarez, V.; Durán, N.; Castro, G.; Islan, G. Hybrid Ofloxacin/eugenol co-loaded solid lipid nanoparticles with enhanced and targetable antimicrobial properties. Int. J. Pharm. 2019, 569, 118575. [Google Scholar] [CrossRef]
- de Araújo Lopes, A.; Da Fonseca, F.N.; Rocha, T.M.; De Freitas, L.B.; Araújo, E.V.O.; Wong, D.V.T.; Júnior, R.C.P.L.; Leal, L.K.A.M. Eugenol as a Promising Molecule for the Treatment of Dermatitis: Antioxidant and Anti-inflammatory Activities and Its Nanoformulation. Oxidative Med. Cell Longev. 2018, 2018, 8194849. [Google Scholar] [CrossRef] [Green Version]
- Shahabadi, N.; Akbari, A.; Karampour, F.; Falsafi, M. Cytotoxicity and antibacterial activities of new chemically synthesized magnetic nanoparticles containing eugenol. J. Drug Deliv. Sci. Technol. 2019, 49, 113–122. [Google Scholar] [CrossRef]
- Sun, X.; Veeraraghavan, V.P.; Surapaneni, K.M.; Hussain, S.; Mathanmohun, M.; Alharbi, S.A.; Aladresi, A.A.M.; Chinnathambi, A. Eugenol–piperine loaded polyhydroxy butyrate/polyethylene glycol nanocomposite-induced apoptosis and cell death in nasopharyngeal cancer (C666-1) cells through the inhibition of the PI3K/AKT/mTOR signaling pathway. J. Biochem. Mol. Toxicol. 2021, 35, e22700. [Google Scholar] [CrossRef]



| Tested Compound (s) & Cancer Type | Animal Model | Dose of EUG (mg/kg) | Therapeutic Effect | References |
|---|---|---|---|---|
| Eugenol (EUG) and honey & adenocarcinoma | Ehrlich ascites and solid carcinoma BALB/c mice model | 100 mg/kg intraperitoneal (i.p.) | ┴ Tumor growth (24.35%) in solid carcinoma, ┴ Growth of Ehrlich ascites by 28.88% | [86] |
| EUG & Breast adenocarcinoma | MDA-MB-231 induced nude mice xenografted human breast tumors | 100 mg per kg i.p. for 4 weeks. | ↓ Tumor growth (66%); ↓ survivin and E2F1 in tumor xenografts | [41] |
| Clove infusion (EUG) & lung cancer | Benzo[a]pyrene mediated lung carcinoma in strain A mice | aqueous clove infusion of 100 mL per mouse per day orally | ↑ Apoptosis, ┴ proliferation, ↑ caspase 3 activation, ↑ Bcl-2/Bax ratio, ↓ COX-2, and some oncogene (cMyc, Hras) | [61] |
| EUG & gastric cancer | N-methyl-N′-nitro-N-nitrosoguanidine (MNNG) mediated male Wistar rat carcinogenesis model | 100 mg/kg body weight three times per week by intragastric route | ┴ Cell proliferation, ↓ NF-κB, ↑ cyclin B, cyclin D1, and PCNA expression, ┴ expression of Gadd45, p21, and p53 | [58] |
| EUG & gastric cancer | MNNG mediated gastric carcinomas in male Wistar rats | Starting with MNNG therapy, intragastric eugenol 100 mg/kg body weight, three times per week | ↑ Apoptosis, ┴ invasion, ┴ angiogenesis by ↑ expression of TIMP-2 and RECK, ↓ MMPs, VEGF and VEGFR1 expression, ↓ pro-apoptotic Bax and caspase-3 expression, ↑ expression of the anti-apoptotic Bcl-2 protein | [59] |
| EUG & breast adenocarcinoma | Swiss albino rat with Ehrlich Ascites Carcinoma (EAC) ascetic and tumor xenograft models | 25, 50, 100, and 125 mg/kg b.w. for consecutive 14 days | ↑ Sub G1 from 4.80% to 11.54%; ↓ CSC markers expression | [38] |
| EUG & Melanoma | B16 melanoma xenograft female B6D2F1 Mice model | 125 mg/kg BW twice a week intraperitoneally | ↓ Tumor growth by 2.4-day; ↓ tumor size on day 15 (62%); ┴ tumor metastasis | [72] |
| EUG + cisplatin & triple-negative breast cancer | MDA-MB-231 induced nude mice humanized tumor xenografts and ALDH positive BT-20 induced orthotopic breast tumors | 50 mg EUG + 2 mg cisplatin | Cisplatin alone showed a 60% inhibitory effect and combination treatment ↓ tumor growth by 95% | [30] |
| EUG & ovarian carcinoma | female Nu/J mice xenograft model induced by SKOV3 and OV2774 cells injected as single inoculums | Intramuscular injection daily with eugenol (cat # E51719; Sigma, MO, USA) (50 mg/Kg), cisplatin (cat # 1134357, Sigma, MO, USA) (2 mg/Kg), and a combination of both drugs for 21 days | ┴ Ovarian cell growth, ↑ apoptosis, ↑ Hes1 promoted stemness, ↓ drug resistance ABC transporter genes | [84] |
| EUG & lung cancer | N-nitrosodiethyl- amine induced mouse lung carcinogenesis model | EUG 50 mg/kg body weight of the mouse | Targeted tiny, drug-resistant, and most virulent cancer stem cells, targeting β-catenin, ↑ apoptosis ↓ cell proliferation | [64] |
| EUG & non-small cell lung cancer | NSG immunodeficient mice xenograft model by inoculated subcutaneously TRIM59-deficient H1975 cells into the lower flank | EUG 50 mg/kg b.w. intraperitoneal injection 3 times per week | ↓ TRIM59 and p65 expression after treatment, ↑ antitumor effect | [63] |
| EUG & skin cancer | 7,12-dimethylbenz[a] anthracene (DMBA) induced and 12-O-tetra decanoylphorbol-13-acetate (TPA) promoted skin cancer in Swiss albino mice | 30 mL twice a week for 28 weeks | ↑ Apoptosis, ↑ p53 and p21WAF1, ↓ iNOS, COX-2, ↓ TNF-α, IL-6, PGE-2 | [80] |
| EUG & skin cancer | DMBA croton oil-induced skin tumor in Swiss mice | 1.25 mg/kg body weight orally twice a week | ↓ H-ras, c-Myc, and Bcl-2 expression, ↑ Bax, P53 ↑ Caspase-3 expression | [79] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padhy, I.; Paul, P.; Sharma, T.; Banerjee, S.; Mondal, A. Molecular Mechanisms of Action of Eugenol in Cancer: Recent Trends and Advancement. Life 2022, 12, 1795. https://doi.org/10.3390/life12111795
Padhy I, Paul P, Sharma T, Banerjee S, Mondal A. Molecular Mechanisms of Action of Eugenol in Cancer: Recent Trends and Advancement. Life. 2022; 12(11):1795. https://doi.org/10.3390/life12111795
Chicago/Turabian StylePadhy, Ipsa, Paramita Paul, Tripti Sharma, Sabyasachi Banerjee, and Arijit Mondal. 2022. "Molecular Mechanisms of Action of Eugenol in Cancer: Recent Trends and Advancement" Life 12, no. 11: 1795. https://doi.org/10.3390/life12111795