Monkeypox: An Emerging Global Public Health Emergency
Abstract
:1. Introduction
2. Global Scenario
3. Classification
3.1. CDC Case Definitions
3.1.1. Suspect Case
- A new rash resembling monkeypox or
- meets one epidemiologic requirement within 21 days of the onset of symptoms and has a strong clinical suspicion of having monkeypox.
3.1.2. Probable Case
- Criteria for a suspect case have been met, and there are no known recent cases of exposure to the orthopoxvirus (such as through vaccination against smallpox).
- Orthopoxvirus presence confirmed through immunohistochemistry, electron microscopy, polymerase chain reaction testing of a clinical specimen, or
- anti-orthopoxvirus IgM antibody detection between 4 and 56 days after the start of the rash.
- According to the CDC, all confirmed cases of orthopoxvirus infection are assumed to be cases of monkeypox until proven otherwise for the ongoing 2022 outbreak.
3.1.3. Confirmed Case
- Criteria were met for a possible case, and one of the following indicated a monkeypox infection:
3.2. WHO Case Definitions for the Current Multi-Country Monkeypox Outbreak Event (2022) Are Slightly Different
3.2.1. Suspect Case
- Unexplained acute rash in a person of any age in a monkeypox-nonendemic country and
- One or more of the following:
- ⚬
- Headache.
- ⚬
- Acute onset of fever.
- ⚬
- Lymphadenopathy.
- ⚬
- Myalgia.
- ⚬
- Back pain.
- ⚬
- Profound weakness.
- Additionally, other common causes of acute rash deemed incompatible with the clinical presentation.
3.2.2. Probable Case
- Suspected case criteria met and at least one of the following:
- Within 21 days of the onset of symptoms, a confirmed epidemiologic link to a probable or confirmed case of monkeypox through direct physical contact, face-to-face exposure, or contact with contaminated objects.
- Travel within 21 days of the onset of symptoms to a nation where monkeypox is endemic.
- Within 21 days of the onset of symptoms, multiple or anonymous sexual partners, positive orthopoxvirus serology, and hospitalization for this illness.
3.2.3. Confirmed Case
4. Pathogenesis
4.1. Causes
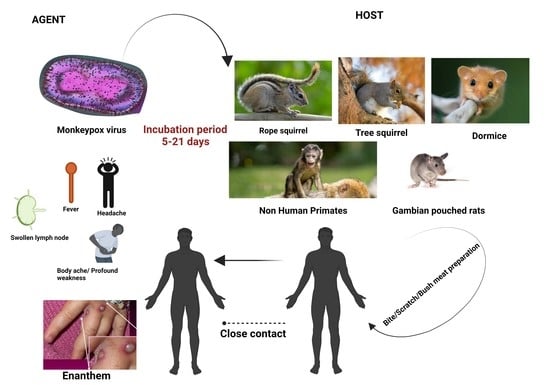
4.1.1. Agent
4.1.2. Host
4.1.3. Incubation Period
4.1.4. Period of Communicability
4.2. Risk Factors and/or Associations
4.2.1. Age
4.2.2. Sex
- ▪
- Touching a patient’s skin, skin lesions, or bodily fluids;
- ▪
- Touching contaminated materials such as linens or clothing;
- ▪
- Allowing their own unprotected clothing to touch patient skin, skin lesions, bodily fluids, or contaminated materials such as linens or clothing;
- ▪
- Being inside a patient’s room or near a patient during aerosol-generating procedures
- ▪
- Close, prolonged presence near a patient;
- ▪
- Personal and household contacts are also at risk of monkeypox infection via direct and indirect contact with monkeypox lesions, lesion material, respiratory secretions, other bodily fluids, and contaminated surfaces or materials (e.g., dishes, utensils, clothing, bedding, linens).
4.3. Clinical Presentation
4.4. Clinical History
5. Diagnosis
- Swabs of the lesion’s surface or fluid are acceptable when dry.
- Two swabs should be taken from each lesion, and lesions from various body regions or with various morphological characteristics should be sampled in particular.
- Within an hour of collection, samples should be frozen (at 20 degrees Celsius or lower); frozen samples can be kept for up to 60 days.
- You should not include any more transport media.
- For CDC testing, the turnaround time is 5 days.
6. Management
- Respiratory tract: bronchodilators, antibiotics for secondary respiratory infections, chest physical therapy, nebulizer treatments, suctioning, incentive spirometry, bronchoscopy, noninvasive ventilation, and intubation/ventilation.
- Sepsis: IV fluids, vasopressors, antibiotics, and extra oxygen.
- Oral lesions: oral hygiene and topical or oral analgesics.
- Vomiting and diarrhea: Hydration via oral or IV as well as antiemetic and antidiarrheal medications.
- Fever: Antipyretic medications and external cooling techniques.
- Skin compromise: Cleaning, moist dressings, topical antibiotics, surgical debridement, and skin grafts.
- Secondary skin infection: Antibiotics, wound incision and drainage, and advanced wound management.
- Lymphadenopathy and inflammation: Anti-inflammatory and analgesic drugs.
- Ocular infection: Slit lamp examination, comprehensive ophthalmic evaluation, and use of ophthalmic antibiotics, antivirals, and corticosteroids.
- (a)
- Tecovirimat: Endorsed for the treatment of human smallpox in adults and children 3 kg or more. Available as both capsules and injections, Tecovirimat demonstrated effectiveness in both in vitro and in vivo animal studies against a wide variety of orthopoxviruses. The CDC has a protocol known as EA-IND (expanded access-investigational new drug) that permits use in treating monkeypox during an outbreak. In patients with severe renal impairment, injection is not recommended [53,54,55,56].
- (b)
- Cidofovir: Has been used to treat other viral infections since being initially approved for the treatment of cytomegalovirus in AIDS patients. The EA-IND protocol is available from the CDC, and it can be used to treat monkeypox during an outbreak. To minimize the risk of nephrotoxicity, oral probenecid is taken with each cidofovir dose. Probenecid Oral Tablet; Adults: 2 g administered orally 3 h before each cidofovir infusion, then 1 g administered orally 2 and 8 h after the infusion. For neutropenia and renal impairment, there are BLACK BOX WARNINGS. Patients receiving potentially nephrotoxic medications, those who have severe cidofovir hypersensitivity, and those who have severe sensitivity to probenecid or other sulfa-containing drugs should not take this medication [11,12,57].
- (c)
- VIGIV (vaccinia immune globulin intravenous): Approved for the management of vaccine-related side effects. The EA-IND protocol is available from the CDC, and it can be used to treat monkeypox during an outbreak. only for IV use. Adults: 6,000 units/kg/dose IV as soon as symptoms arise; repeat dosing may be considered depending on symptom severity and response to treatment; and 9000 units/kg/dose in patients who do not react to the initial dosage. For interactions with glucose monitoring systems, see BLACK BOX WARNING. During VIGIV therapy, blood sugar should be checked using a glucose-specific technique because VIGIV contains maltose, which can cause falsely elevated glucose readings on some glucose monitoring devices. Patients with a history of anaphylaxis or another severe reaction to IV immune globulin, as well as IgA-deficient individuals with anti-IgA antibodies and a history of IgA hypersensitivity, should not receive this medication [58,59].
- (d)
- Brincidofovir: Approved for the treatment of adult, pediatric, and neonatal cases of human smallpox. Monkeypox treatment is made easier by the EA-IND protocol that the CDC is currently developing. Brincidofovir is not yet accessible from the Strategic National Stockpile. Although the CDC does not presently have an EA-IND protocol for brincidofovir for monkeypox, one is being developed to facilitate its usage. While fasting, administer Brincidofovir oral suspension; newborns: 6 mg/kg/dose administered orally once a week for two weeks (on days 1 and 8). BLACK BOX WARNING: Prolonged use may increase the risk of mortality due to the drug overdosage. The prescribing information contains no contraindications [8,10,52].
7. Prevention
7.1. Infection Prevention in Medical Facilities
7.2. Preventing Infections in Domestic Settings
7.2.1. Isolation and Personal Protective Equipment
7.2.2. Hand Hygiene and Household Disinfection
7.3. Management Recommendations Depend on the Degree of Exposure
7.3.1. High Risk
7.3.2. Intermediate Risk
7.3.3. Low/Uncertain Risk
7.3.4. No Risk
8. Vaccination
8.1. Pre-Exposure Prophylaxis
8.2. Post-Exposure Prophylaxis
8.3. Vaccinia Virus Vaccine
8.4. Jynneos
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaler, J.; Hussain, A.; Flores, G.; Kheiri, S.; Desrosiers, D. Monkeypox: A Comprehensive Review of Transmission, Pathogenesis, and Manifestation. Cureus 2022, 14, e26531. [Google Scholar] [CrossRef] [PubMed]
- Multi-Country Monkeypox Outbreak in Non-Endemic Countries. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON385 (accessed on 24 August 2022).
- Technical Report: Multi-National Monkeypox Outbreak, United States, 2022 | Monkeypox | Poxvirus | CDC. Available online: https://www.cdc.gov/poxvirus/monkeypox/clinicians/technical-report.html (accessed on 25 August 2022).
- Monkeypox—Symptoms, Diagnosis and Treatment | BMJ Best Practice US. Available online: https://bestpractice.bmj.com/topics/en-us/1611 (accessed on 25 August 2022).
- Cheema, A.Y.; Ogedegbe, O.J.; Munir, M.; Alugba, G.; Ojo, T.K. Monkeypox: A Review of Clinical Features, Diagnosis, and Treatment. Cureus 2022, 14, e26756. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.; Nchasi, G. Monkeypox Virus: A Zoonosis of Concern. J. Med. Virol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Laboratory Guidelines for the Detection and Diagnosis of Monkeypox Virus Infection—PAHO/WHO | Pan American Health Organization. Available online: https://www.paho.org/en/documents/laboratory-guidelines-detection-and-diagnosis-monkeypox-virus-infection (accessed on 25 August 2022).
- Adler, H.; Gould, S.; Hine, P.; Snell, L.B.; Wong, W.; Houlihan, C.F.; Osborne, J.C.; Rampling, T.; Beadsworth, M.B.; Duncan, C.J.; et al. Clinical Features and Management of Human Monkeypox: A Retrospective Observational Study in the UK. Lancet Infect. Dis. 2022, 22, 1153–1162. [Google Scholar] [CrossRef]
- Guidelines for Management of Monkeypox Disease | Ministry of Health and Family Welfare | GOI. Available online: https://main.mohfw.gov.in/diseasealerts-0 (accessed on 25 August 2022).
- Siegrist, E.A.; Sassine, J. Antivirals with Activity Against Monkeypox: A Clinically Oriented Review. Clin. Infect. Dis. 2022, ciac622. [Google Scholar] [CrossRef] [PubMed]
- CDC Provides Interim Guidance for Treatment, Prevention of Monkeypox Among PLWH. Available online: https://www.ajmc.com/view/cdc-provides-interim-guidance-for-treatment-prevention-of-monkeypox-among-plwh (accessed on 25 August 2022).
- Rizk, J.G.; Lippi, G.; Henry, B.M.; Forthal, D.N.; Rizk, Y. Prevention and Treatment of Monkeypox. Drugs 2022, 82, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Key Questions About the Current U.S. Monkeypox Outbreak. Available online: https://www.kff.org/other/issue-brief/key-questions-about-the-current-u-s-monkeypox-outbreak/ (accessed on 1 September 2022).
- Brencic, D.J.; Pinto, M.; Gill, A.; Kinzer, M.H.; Hernandez, L.; Pasi, O.G. CDC Support for Global Public Health Emergency Management. Emerg. Infect. Dis. 2017, 23, S183–S189. [Google Scholar] [CrossRef] [Green Version]
- CDC Emergency Operations Center | CDC. Available online: https://www.cdc.gov/cpr/eoc/eoc.htm (accessed on 25 August 2022).
- Ladnyj, I.D.; Ziegler, P.; Kima, E. A Human Infection Caused by Monkeypox Virus in Basankusu Territory, Democratic Republic of the Congo. Bull. World Health Organ. 1972, 46, 593–597. [Google Scholar]
- Multi-Country Monkeypox Outbreak: Situation Update. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON390 (accessed on 24 August 2022).
- Saxena, S.K.; Ansari, S.; Maurya, V.K.; Kumar, S.; Jain, A.; Paweska, J.T.; Tripathi, A.K.; Abdel-Moneim, A.S. Re-Emerging Human Monkeypox: A Major Public-Health Debacle. J. Med. Virol. [CrossRef]
- Hutin, Y.J.; Williams, R.J.; Malfait, P.; Pebody, R.; Loparev, V.N.; Ropp, S.L.; Rodriguez, M.; Knight, J.C.; Tshioko, F.K.; Khan, A.S.; et al. Outbreak of Human Monkeypox, Democratic Republic of Congo, 1996 to 1997. Emerg. Infect. Dis. 2001, 7, 434–438. [Google Scholar] [CrossRef]
- Alakunle, E.; Moens, U.; Nchinda, G.; Okeke, M.I. Monkeypox Virus in Nigeria: Infection Biology, Epidemiology, and Evolution. Viruses 2020, 12, 1257. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, M. Monkeypox in Africa: The Science the World Ignored. Nature 2022, 607, 17–18. [Google Scholar] [CrossRef]
- Ligon, B.L. Monkeypox: A Review of the History and Emergence in the Western Hemisphere. Semin. Pediatr. Infect. Dis. 2004, 15, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Monkeypox. Available online: https://www.who.int/news-room/fact-sheets/detail/monkeypox (accessed on 24 August 2022).
- Suspected Monkeypox Case in Kerala, Samples of Traveller from UAE Sent for Testing—ET HealthWorld. Available online: https://health.economictimes.indiatimes.com/news/industry/suspected-monkeypox-case-in-kerala-samples-of-traveller-from-uae-sent-for-testing/92871068 (accessed on 24 August 2022).
- Epidemiological Data on the 2022 Monkeypox Outbreak. Available online: https://www.ecdc.europa.eu/en/infectious-disease-topics/z-disease-list/monkeypox/epidemiological-data-2022-monkeypox-outbreak (accessed on 11 September 2022).
- Seven Countries in Europe and North America Report Monkeypox Cases. Available online: https://thewire.in/health/seven-countries-in-europe-and-north-america-now-report-monkeypox-cases (accessed on 24 August 2022).
- Reuters WHO: More than 6000 Monkeypox Cases Reported, Emergency Meeting Set. Available online: https://www.reuters.com/business/healthcare-pharmaceuticals/who-more-than-6000-monkeypox-cases-reported-another-emergency-meeting-set-2022-07-06/ (accessed on 1 September 2022).
- Service, T.N. Four Confirmed Cases of Monkeypox in India till Date: Minister. Available online: https://www.tribuneindia.com/news/health/four-confirmed-cases-of-monkeypox-in-india-till-date-minister-416846 (accessed on 24 August 2022).
- Taylor, L. Monkeypox: WHO Declares a Public Health Emergency of International Concern. BMJ 2022, 378, o1874. [Google Scholar] [CrossRef] [PubMed]
- CDC Monkeypox in the U.S. Available online: https://www.cdc.gov/poxvirus/monkeypox/considerations-for-monkeypox-vaccination.html (accessed on 25 August 2022).
- Patient Classification/CDC Case Definitions. Available online: https://www.acep.org/monkeypox-field-guide/patient-classification-cdc-case-definitions/ (accessed on 25 August 2022).
- Monkeypox Outbreak Toolbox. Available online: https://www.who.int/emergencies/outbreak-toolkit/disease-outbreak-toolboxes/monkeypox-outbreak-toolbox (accessed on 25 August 2022).
- Department of Health and Aged Care; Australian Government. Monkeypox Virus Infection—Surveillance Case Definition. Available online: https://www.health.gov.au/resources/publications/monkeypox-virus-infection-surveillance-case-definition (accessed on 25 August 2022).
- Sklenovská, N.; Van Ranst, M. Emergence of Monkeypox as the Most Important Orthopoxvirus Infection in Humans. Front. Public Health 2018, 6, 241. [Google Scholar] [CrossRef] [Green Version]
- Simpson, K.; Heymann, D.; Brown, C.S.; Edmunds, W.J.; Elsgaard, J.; Fine, P.; Hochrein, H.; Hoff, N.A.; Green, A.; Ihekweazu, C.; et al. Human Monkeypox—After 40 Years, an Unintended Consequence of Smallpox Eradication. Vaccine 2020, 38, 5077–5081. [Google Scholar] [CrossRef]
- Likos, A.M.; Sammons, S.A.; Olson, V.A.; Frace, A.M.; Li, Y.; Olsen-Rasmussen, M.; Davidson, W.; Galloway, R.; Khristova, M.L.; Reynolds, M.G.; et al. A Tale of Two Clades: Monkeypox Viruses. J. Gen. Virol. 2005, 86, 2661–2672. [Google Scholar] [CrossRef]
- Kumar, S.; Subramaniam, G.; Karuppanan, K. Human Monkeypox Outbreak in 2022. J. Med. Virol. 2022. [Google Scholar] [CrossRef]
- Kumar, N.; Acharya, A.; Gendelman, H.E.; Byrareddy, S.N. The 2022 Outbreak and the Pathobiology of the Monkeypox Virus. J. Autoimmun. 2022, 131, 102855. [Google Scholar] [CrossRef]
- Moore, M.J.; Rathish, B.; Zahra, F. Monkeypox. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Wilson, M.E. Re-Emerging Diseases: Overview. In International Encyclopedia of Public Health (Second Edition); Quah, S.R., Ed.; Academic Press: Oxford, UK, 2017; pp. 269–277. ISBN 978-0-12-803708-9. [Google Scholar]
- Mullendore, N.F.; Lawner, B.J.; Malone, J.D. Chapter 149—Monkeypox Attack. In Ciottone’s Disaster Medicine (Second Edition); Ciottone, G.R., Ed.; Elsevier: Philadelphia, PA, USA, 2016; pp. 774–776. ISBN 978-0-323-28665-7. [Google Scholar]
- Miura, F.; van Ewijk, C.E.; Backer, J.A.; Xiridou, M.; Franz, E.; Op de Coul, E.; Brandwagt, D.; van Cleef, B.; van Rijckevorsel, G.; Swaan, C.; et al. Estimated Incubation Period for Monkeypox Cases Confirmed in the Netherlands, May 2022. Eurosurveillance 2022, 27, 2200448. [Google Scholar] [CrossRef]
- Thornhill, J.P.; Barkati, S.; Walmsley, S.; Rockstroh, J.; Antinori, A.; Harrison, L.B.; Palich, R.; Nori, A.; Reeves, I.; Habibi, M.S.; et al. Monkeypox Virus Infection in Humans across 16 Countries—April–June 2022. N. Engl. J. Med. 2022, 387, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wilkins, K.; McCollum, A.M.; Osadebe, L.; Kabamba, J.; Nguete, B.; Likafi, T.; Balilo, M.P.; Lushima, R.S.; Malekani, J.; et al. Evaluation of the GeneXpert for Human Monkeypox Diagnosis. Am. J. Trop. Med. Hyg. 2017, 96, 405–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CDC Director: Monkeypox May Be Tricky to Diagnose. Available online: https://www.cidrap.umn.edu/news-perspective/2022/06/cdc-director-monkeypox-may-be-tricky-diagnose (accessed on 27 August 2022).
- Monkeypox: When to Get Tested and What to Do If Exposed. Available online: https://asm.org/Articles/2022/August/Monkeypox-When-to-Get-Tested-and-What-to-Do-if-Exp (accessed on 27 August 2022).
- Monkeypox Virus Qualitative PCR | Laboratory Test Guide | Dept. of Laboratory Medicine & Pathology | UW Medicine. Available online: https://dlmp.uw.edu/test-guide/view/MPXQLT (accessed on 27 August 2022).
- Surveillance, Case Investigation and Contact Tracing for Monkeypox: Interim Guidance. Available online: https://www.who.int/publications-detail-redirect/WHO-MPX-Surveillance-2022.3 (accessed on 27 August 2022).
- ECDC Publishes Contact Tracing Guidance for the Current Monkeypox Outbreak. Available online: https://www.ecdc.europa.eu/en/news-events/ecdc-publishes-contact-tracing-guidance-current-monkeypox-outbreak (accessed on 27 August 2022).
- Laboratory Testing for the Monkeypox Virus: Interim Guidance. Available online: https://www.who.int/publications-detail-redirect/WHO-MPX-laboratory-2022.1 (accessed on 27 August 2022).
- FDA Monkeypox Response. Available online: https://www.fda.gov/emergency-preparedness-and-response/mcm-issues/fda-monkeypox-response (accessed on 1 September 2022).
- Harris, E. Global Monkeypox Outbreaks Spur Drug Research for the Neglected Disease. JAMA 2022, 328, 231–233. [Google Scholar] [CrossRef]
- Grosenbach, D.W.; Honeychurch, K.; Rose, E.A.; Chinsangaram, J.; Frimm, A.; Maiti, B.; Lovejoy, C.; Meara, I.; Long, P.; Hruby, D.E. Oral Tecovirimat for the Treatment of Smallpox. N. Engl. J. Med. 2018, 379, 44–53. [Google Scholar] [CrossRef]
- Merchlinsky, M.; Albright, A.; Olson, V.; Schiltz, H.; Merkeley, T.; Hughes, C.; Petersen, B.; Challberg, M. The Development and Approval of Tecoviromat (TPOXX®), the First Antiviral against Smallpox. Antiviral. Res. 2019, 168, 168–174. [Google Scholar] [CrossRef]
- Repurposing Tecovirimat for Monkeypox. Available online: https://www.jwatch.org/na55199/2022/08/04/repurposing-tecovirimat-monkeypox (accessed on 25 August 2022).
- Staff, B. Obtaining Treatment for Eligible Monkeypox Patients. Available online: https://www.uspharmacist.com/article/obtaining-treatment-for-eligible-monkeypox-patients (accessed on 25 August 2022).
- Monkeypox Treatment | NIH: National Institute of Allergy and Infectious Diseases. Available online: https://www.niaid.nih.gov/diseases-conditions/monkeypox-treatment (accessed on 27 August 2022).
- Elsevier—Drug Monograph │Vaccinia Immune Globulin, VIG. Available online: https://elsevier.health/en-US/preview/vaccinia-immune-globulin-vig (accessed on 27 August 2022).
- Titanji, B.K.; Tegomoh, B.; Nematollahi, S.; Konomos, M.; Kulkarni, P.A. Monkeypox: A Contemporary Review for Healthcare Professionals. Open Forum Infect. Dis. 2022, 9, ofac310. [Google Scholar] [CrossRef] [PubMed]
- How to Protect Yourself | Monkeypox | Poxvirus | CDC. Available online: https://www.cdc.gov/poxvirus/monkeypox/prevention/protect-yourself.html (accessed on 25 August 2022).
- Government of Canada. Monkeypox: For Health Professionals. Available online: https://www.canada.ca/en/public-health/services/diseases/monkeypox/health-professionals.html (accessed on 27 August 2022).
- Zachary, K.C.; Shenoy, E.S. Monkeypox Transmission Following Exposure in Healthcare Facilities in Nonendemic Settings: Low Risk but Limited Literature. Infect. Control Hosp. Epidemiol. 2022, 43, 920–924. [Google Scholar] [CrossRef]
- The Implications of Monkeypox for Healthcare Workers. Available online: https://www.news-medical.net/news/20220614/The-implications-of-monkeypox-for-healthcare-workers.aspx (accessed on 28 August 2022).
- Healthcare Provider Monkeypox Health Advisory, May 27, 2022: Monkeypox Virus Infection in the United States and Other Non-Endemic Countries. Available online: https://www.cdph.ca.gov/Programs/CID/DCDC/Pages/Health-Advisory-Monkeypox-Virus-5-27-22.aspx (accessed on 28 August 2022).
- Healthcare Provider Health Advisory: Managing Monkeypox Virus Infection in California, Updated June 23, 2022. Available online: https://www.cdph.ca.gov/Programs/CID/DCDC/Pages/Health-Advisory-Monkeypox-Virus-6-23-22.aspx (accessed on 28 August 2022).
- Petersen, E.; Zumla, A.; Hui, D.S.; Blumberg, L.; Valdoleiros, S.R.; Amao, L.; Ntoumi, F.; Asogun, D.; Simonsen, L.; Haider, N.; et al. Vaccination for Monkeypox Prevention in Persons with High-Risk Sexual Behaviours to Control on-Going Outbreak of Monkeypox Virus Clade 3. Int. J. Infect. Dis. 2022, 122, 569–571. [Google Scholar] [CrossRef]
- Key Facts About Vaccines to Prevent Monkeypox Disease. Available online: https://www.fda.gov/vaccines-blood-biologics/vaccines/key-facts-about-vaccines-prevent-monkeypox-disease (accessed on 1 September 2022).
- FDA. Monkeypox Update: FDA Authorizes Emergency Use of JYNNEOS Vaccine to Increase Vaccine Supply. Available online: https://www.fda.gov/news-events/press-announcements/monkeypox-update-fda-authorizes-emergency-use-jynneos-vaccine-increase-vaccine-supply (accessed on 25 August 2022).
- Petersen, B.W.; Harms, T.J.; Reynolds, M.G.; Harrison, L.H. Use of Vaccinia Virus Smallpox Vaccine in Laboratory and Health Care Personnel at Risk for Occupational Exposure to Orthopoxviruses—Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2015. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 257–262. [Google Scholar] [CrossRef] [Green Version]
- CDC Monkeypox in the U.S. Available online: https://www.cdc.gov/poxvirus/monkeypox/about.html (accessed on 24 August 2022).
- Orthopoxvirus Vaccine Guidance for Persons at Risk for Occupational Exposure. Available online: https://www.acep.org/monkeypox-field-guide/orthopoxvirus-vaccine-guidance-for-persons-at-risk-for-occupational-exposure/ (accessed on 25 August 2022).
- CDC Monkeypox in the U.S. Available online: https://www.cdc.gov/poxvirus/monkeypox/response/2022/index.html (accessed on 24 August 2022).
- Considerations for Expanded Monkeypox Post-Exposure Prophylaxis (PEP++). Available online: https://www.cdph.ca.gov/Programs/CID/DCDC/Pages/Considerations-for-Expanded-Monkeypox-Post-Exposure-Prophylaxis.aspx (accessed on 25 August 2022).
- Jacobs, B.L.; Langland, J.O.; Kibler, K.V.; Denzler, K.L.; White, S.D.; Holechek, S.A.; Wong, S.; Huynh, T.; Baskin, C.R. Vaccinia Virus Vaccines: Past, Present and Future. Antivir. Res. 2009, 84, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Henderson, D.A.; Moss, B. Recombinant Vaccinia Virus Vaccines; Saunders: Philadephia, PA, USA, 1999. [Google Scholar]
- Kaplan, C. Vaccinia Virus: A Suitable Vehicle for Recombinant Vaccines? Arch. Virol. 1989, 106, 127–139. [Google Scholar] [CrossRef] [PubMed]
- JYNNEOS Vaccine for Monkeypox Exposure Q&A. Available online: https://www.cdph.ca.gov/Programs/CID/DCDC/Pages/JYNNEOS-Vaccine-for-Monkeypox-Exposure-QA.aspx (accessed on 28 August 2022).
- Department of Health and Aged Care; Australian Government. Monkeypox (MPX) Vaccines. Available online: https://www.health.gov.au/health-alerts/monkeypox-mpx/monkeypox-mpx-vaccines (accessed on 28 August 2022).
- Department of Health and Aged Care; Australian Government. Monkeypox (MPX)—Information on JYNNEOS® Vaccine. Available online: https://www.health.gov.au/resources/publications/monkeypox-mpx-information-on-jynneosr-vaccine (accessed on 28 August 2022).
- EMA EMA’s Emergency Task Force Advises on Intradermal Use of Imvanex/Jynneos against Monkeypox. Available online: https://www.ema.europa.eu/en/news/emas-emergency-task-force-advises-intradermal-use-imvanex-jynneos-against-monkeypox (accessed on 28 August 2022).

| Country | Cases |
|---|---|
| United States | 25,340 |
| Brazil | 7445 |
| Spain | 7122 |
| France | 3970 |
| Germany | 3607 |
| United Kingdom | 3585 |
| Peru | 2423 |
| Colombia | 1653 |
| Canada | 1389 |
| Mexico | 1367 |
| The Netherlands | 1221 |
| Portugal | 917 |
| Italy | 846 |
| Chile | 842 |
| Belgium | 757 |
| Switzerland | 513 |
| Argentina | 326 |
| Austria | 309 |
| Nigeria | 277 |
| Israel | 250 |
| Democratic Republic of the Congo | 195 |
| Sweden | 186 |
| Denmark | 184 |
| Poland | 182 |
| Ireland | 178 |
| Bolivia | 175 |
| Australia | 135 |
| Ecuador | 120 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeyaraman, M.; Selvaraj, P.; Halesh, M.B.; Jeyaraman, N.; Nallakumarasamy, A.; Gupta, M.; Maffulli, N.; Gupta, A. Monkeypox: An Emerging Global Public Health Emergency. Life 2022, 12, 1590. https://doi.org/10.3390/life12101590
Jeyaraman M, Selvaraj P, Halesh MB, Jeyaraman N, Nallakumarasamy A, Gupta M, Maffulli N, Gupta A. Monkeypox: An Emerging Global Public Health Emergency. Life. 2022; 12(10):1590. https://doi.org/10.3390/life12101590
Chicago/Turabian StyleJeyaraman, Madhan, Preethi Selvaraj, Manjunatha Budihal Halesh, Naveen Jeyaraman, Arulkumar Nallakumarasamy, Manu Gupta, Nicola Maffulli, and Ashim Gupta. 2022. "Monkeypox: An Emerging Global Public Health Emergency" Life 12, no. 10: 1590. https://doi.org/10.3390/life12101590