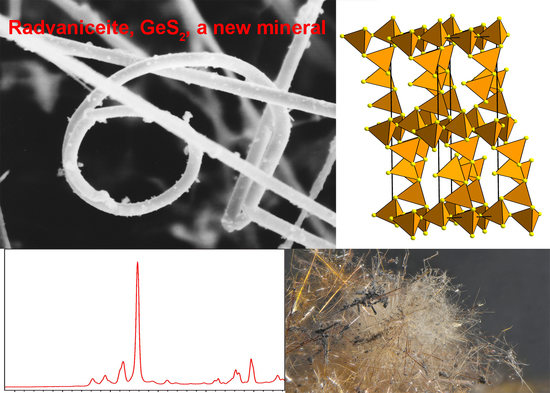
Radvaniceite, GeS2, a New Germanium Sulphide, from the Kateřina Mine, Radvanice near Trutnov, Czech Republic
Abstract
:1. Introduction
2. Occurrence
3. Physical and Optical Properties
4. Chemical Composition
5. Raman Spectroscopy
6. Powder X-ray Diffraction and Crystal Structure
7. Note on the Origin of Radvaniceite
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MacLachlan, M.J.; Petrov, S.; Bedard, R.L.; Manners, I.; Ozin, G.A. Synthesis and crystal structure of δ-GeS2, the first germanium sulfide with an expanded framework structure. Angewand. Chem. Int. Ed. 1998, 37, 2075–2079. [Google Scholar] [CrossRef]
- Viaene, W.; Moh, G.H. Das binare System Germanium-Schwefel und pT Relation im Druckbereich bis 5 kb. J. Miner. Abh. 1973, 119, 113–144. [Google Scholar]
- Dittmar, G.; Schäfer, H. Die Kristallstruktur von HT-GeS2. Acta Crystallogr. 1975, B31, 2060–2064. [Google Scholar] [CrossRef]
- Bletskan, D.I.; Kabacij, V.N.; Sakal, T.A.; Stefanovych, V.A. Structure and vibrational spectra of MIIAIVB3VI-type crystalline and glassy semiconductors. J. Non-Cryst. Solids 2003, 326, 77–82. [Google Scholar] [CrossRef]
- Bletskan, D.I. Phase equilibrium in binary systems AIV–BVI. Part. II Systems Germanium-Chalcogen. J. Ovonic Res. 2005, 1, 47–52. [Google Scholar]
- Dittmar, G.; Schäfer, H. Die Kristallstruktur von LT-GeS2. Acta Crystallogr. 1976, B32, 1188–1192. [Google Scholar] [CrossRef]
- Zachariasen, W.H. The crystal structure of germanium disulphide. J. Chem. Phys. 1936, 4, 618–619. [Google Scholar] [CrossRef]
- Wang, N.; Horn, E.E. β-GeS2, synthesis and crystal data. Neues Jahrb. Mineral. 1975, 41–44. [Google Scholar]
- Málek, J. The thermal stability of chalcogenide glasses. J. Therm. Anal. Calorim. 1993, 40, 159–170. [Google Scholar] [CrossRef]
- Prewitt, C.T.; Young, H.S. Germanium and silicon disulfides: Structure and synthesis. Science 1965, 149, 535–537. [Google Scholar] [CrossRef]
- Wang, N.; Horn, E.E. Synthesis and crystal data of a high pressure modification of GeS2. Neues Jahrb. Mineral.-Mon. 1973, 413–416. [Google Scholar] [CrossRef]
- Finkelman, R.B.; Larson, R.R.; Dwornik, E.J. Naturally occurring vapor-liquid-solid (VLS) whisker growth of germanium sulfide. J. Cryst. Growth 1974, 22, 159–160. [Google Scholar] [CrossRef]
- Lapham, D.M.; Barnes, J.H.; Downey, W.F.; Finkelman, R. Mineralogy Associated with Burning Anthracite Deposits of Eastern Pennsylvania; Mineral Resource Report 78; Pennsylvania Geological Survey: Harrisburg, PA, USA, 1980; 82p.
- Sejkora, J.; Berlepsch, P.; Makovicky, E.; Balić-Zunić, T. Natural SnGeS3 from Radvanice near Trutnov (Czech Republic): Its description, crystal structure refinement and solid solution with PbGeS3. Eur. J. Mineral. 2001, 13, 791–800. [Google Scholar] [CrossRef]
- Sejkora, J. Mineral Association of the Burning Coal Mine Dump of the Kateřina Mine at Radvanice near Trutnov and Processes of Its Origin. Ph.D. Thesis, Faculty of Science, Masaryk University, Brno, Czech Republic, 2002; pp. 1–144. (In Czech). [Google Scholar]
- Nickel, E.H.; Grice, J.D. The IMA Commission on New Minerals and Mineral Names: Procedures and guidelines on mineral nomenclature. Mineral. Petrol. 1998, 64, 237–263. [Google Scholar] [CrossRef]
- Miyawaki, R.; Hatert, F.; Pasero, M.; Mills, S.J. IMA Commission on New Minerals, Nomenclature and Classification (CNMNC), NEWSLETTER 50 New minerals and nomenclature modifications approved in 2019. Mineral. Mag. 2019, 83, 615–620. [Google Scholar] [CrossRef] [Green Version]
- Žáček, V.; Ondruš, P. Mineralogy of recently formed sublimates from Kateřina colliery in Radvanice, Eastern Bohemia. Czech Republic. Bull. Czech Geol. Surv. 1997, 72, 289–302. [Google Scholar]
- Tvrdý, J.; Sejkora, J. The burning coal mine dump and redeposition of toxic compounds during spontaneous thermic decomposition of coal matter. EKO—Ekol. A Společnost 1999, 10, 11–15. (In Czech) [Google Scholar]
- Sejkora, J.; Makovicky, E.; Balić-Zunić, T.; Berlepsch, P. Stangersite, a new tin germanium sulfide, from the Kateřina mine, Radvanice near Trutnov, Czech Republic. J. Geosci. 2020, 65, 141–152. [Google Scholar] [CrossRef]
- Žáček, V.; Skála, R. Mineralogy of Burning-Coal Waste Piles in Collieries of the Czech Republic. In Coal and Peat Fires: A Global Perspective, Volume 3 Case Studies-Coal Fires; Stracher, G.B., Prakash, A., Sokol, E.V., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 109–159. [Google Scholar]
- Osner, Z.; Němec, J. Remediation of the Burning Waste Bank of the Mine Kateřina (Radvanice, Bohemia). Energie Kladno Ltd. 2002. Available online: http://slon.diamo.cz/hpvt/2002/sekce/zahlazovani/Z11/P_11.htm (accessed on 9 March 2020). (In Czech).
- Laufek, F.; Veselovský, F.; Drábek, M.; Kříbek, B.; Klementová, M. Experimental formation of Pb, Sn, Ge and Sb sulfides, selenides and chlorides in the presence of sal ammoniac: A contribution to the understanding of the mineral formation processes in coal wastes self-ignition. Int. J. Coal Geol. 2017, 176–177, 1–7. [Google Scholar] [CrossRef]
- Tásler, R.; Čadková, Z.; Dvořák, J.; Fediuk, F.; Chaloupský, J.; Jetel, J.; Kaiserová-Kalibová, M.; Prouza, V.; Schovánková-Hrdličková, D.; Středa, J.; et al. Geology of Czech part of the Intra-Sudetic Basin; Academia: Praha, Czech Republic, 1979; pp. 1–292. (In Czech) [Google Scholar]
- Kudělásek, V. Trace elements of coal of the Intra-Sudetic Basin. Part I. Sbor. Věd. Prac. Vys. Šk. Báň. Ostrava, Ř. Horn.-Geol. 1959, 5, 319–347. (In Czech) [Google Scholar]
- Kudělásek, V. Trace elements of coal of the Intra-Sudetic Basin. Part II. Sbor. Věd. Prac. Vys. šk. Báň. Ostrava, Ř. Horn.-geol. 1959, 5, 457–479. (In Czech) [Google Scholar]
- Čadková, Z. The metal distribution in sediments of the Radvanice measures. Čas. mineral. geol. 1971, 16, 147–157. (In Czech) [Google Scholar]
- Pouchou, J.L.; Pichoir, F. “PAP” (πρZ) procedure for improved quantitative microanalysis. In Microbeam Analysis; Armstrong, J.T., Ed.; San Francisco Press: San Francisco, CA, USA, 1985; pp. 104–106. [Google Scholar]
- Inoue, K.; Matsuda, O.; Murase, K. Raman spectra of tetrahedral vibrations in crystalline germanium dichalcogenides, GeS2 and GeSe2, in high and low temperature forms. Solid Stat. Comm. 1991, 79, 905–910. [Google Scholar] [CrossRef]
- Černošek, Z.; Černošková, E.; Beneš, L. Raman scattering in GeS2 glass and its crystalline polymorphs compared. J. Mol. Struct. 1997, 435, 193–198. [Google Scholar] [CrossRef]
- Popović, Z.V. Molecular vibration in Sn(Pb)GeS3 and GeS2. Phys. Lett. 1983, A94, 242–246. [Google Scholar] [CrossRef]
- Popović, Z.V.; Holtz, M.; Reimann, K.; Syassen, K. High-pressure Raman scattering and optical absorption study of β-GeS2. Phys. Stat. Solid. 1996, 198, 533–537. [Google Scholar] [CrossRef]
- Jakšić, Z.M. Temperature and pressure dependence of phonon frequencies in GeS2, GeSe2, and SnGeS3. Phys. Stat. Solid. 2003, 239, 131–143. [Google Scholar] [CrossRef]
- Pohl, S.; Schiwy, W.; Weinstock, N.; Krebs, B. Darstellung, Schwingungsspektren und Normalkoordinatenanalyse der Ionen GeS44− und SnS44−. Z. Naturforsch. 1973, B28, 565–569. [Google Scholar] [CrossRef]
- Ondruš, P. A computer program for analysis of X-ray powder diffraction patterns. Mater. Sci. Forum EPDIC-2 Enchede 1993, 133–136, 297–300. [Google Scholar] [CrossRef]
- Kraus, W.; Nolze, G. POWDER CELL—A program for the representation and manipulation of crystal structures and calculation of the resulting X-ray powder patterns. J. Appl. Crystallogr. 1996, 29, 301–303. [Google Scholar] [CrossRef]
- Burnham, C.W. Lattice constant refinement. Carnegie Inst. Wash. Yearb. 1962, 61, 132–135. [Google Scholar]
- Le Page, Y.; Donnay, G. Refinement of the crystal structure of low-quartz. Acta Crystallogr. 1976, B32, 2456–2459. [Google Scholar] [CrossRef]
- Bernstein, L.R. Germanium geochemistry and mineralogy. Geochim. Cosmochim. Acta 1985, 49, 2409–2422. [Google Scholar] [CrossRef]
- Höll, R.; Kling, M.; Schroll, E. Metallogenesis of germanium—A review. Ore Geol. Rev. 2007, 30, 145–180. [Google Scholar] [CrossRef]
- Pešek, J.; Sýkorová, I.; Jelínek, E.; Michna, O.; Forstová, J.; Martínek, K.; Vašíček, M.; Havelcová, M. Major and minor elements in the hard coal from the Czech Upper Paleozoic basins. Czech Geol. Surv. Spec. Pap. 2010, 10, 1–48. [Google Scholar]






| Rmax | Rmin | λ (nm) | Rmax | Rmin | λ (nm) |
|---|---|---|---|---|---|
| 13.6 | 13.1 | 400 | 20.6 | 16.2 | 560 |
| 15.9 | 14.2 | 420 | 20.8 | 16.4 | 580 |
| 17.6 | 14.8 | 440 | 20.8 | 16.4 | 589 (COM) |
| 18.5 | 15.2 | 460 | 20.9 | 16.6 | 600 |
| 18.8 | 15.4 | 470 (COM) | 20.9 | 16.8 | 620 |
| 19.1 | 15.5 | 480 | 20.9 | 16.9 | 640 |
| 19.6 | 15.7 | 500 | 20.9 | 16.9 | 650 (COM) |
| 20.0 | 15.9 | 520 | 20.9 | 17.0 | 660 |
| 20.3 | 16.0 | 540 | 20.8 | 17.2 | 680 |
| 20.4 | 16.1 | 546 (COM) | 20.7 | 17.3 | 700 |
| Constituent | Mean | Range | Stand. Dev. (σ) | Reference Material |
|---|---|---|---|---|
| Ge | 51.84 | 50.82–52.80 | 0.54 | Ge |
| Pb | 0.18 | 0.00–0.45 | 0.15 | PbS |
| Sn | 0.21 | 0.00–1.23 | 0.39 | Sn |
| Bi | 0.66 | 0.20–1.00 | 0.21 | Bi |
| Sb | 0.12 | 0.00–0.56 | 0.17 | Sb2Se3 |
| As | 0.12 | 0.00–0.38 | 0.15 | Cu3AsS4 |
| S | 45.65 | 44.42–46.66 | 0.68 | chalcopyrite |
| Se | 1.74 | 0.49–3.42 | 0.91 | Sb2Se3 |
| total | 100.52 |
| Holotype | Other Samples | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 1 | 2 | 3 | 4 | 5 | 6 | ||
| wt. % | Ge | 51.36 | 52.21 | 51.23 | 52.06 | 51.45 | 51.73 | 51.03 | 52.11 | 51.25 | 51.56 | 51.62 | 52.08 |
| Pb | 0.28 | 0.00 | 0.00 | 0.45 | 0.42 | 0.16 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Sn | 0.19 | 0.00 | 0.22 | 0.00 | 1.23 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Bi | 0.79 | 0.75 | 0.54 | 0.20 | 1.00 | 0.56 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Sb | 0.25 | 0.56 | 0.22 | 0.00 | 0.00 | 0.16 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| As | 0.38 | 0.00 | 0.00 | 0.28 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| S | 46.54 | 45.56 | 45.54 | 46.66 | 44.42 | 45.12 | 43.79 | 43.32 | 43.21 | 43.54 | 43.85 | 42.60 | |
| Se | 0.49 | 1.11 | 1.41 | 1.48 | 3.05 | 3.08 | 4.78 | 4.88 | 4.95 | 4.95 | 5.16 | 5.33 | |
| total | 100.28 | 100.19 | 99.16 | 101.13 | 101.57 | 100.81 | 99.61 | 100.31 | 99.40 | 100.05 | 100.63 | 100.00 | |
| apfu | Ge | 0.97 | 1.00 | 0.98 | 0.98 | 0.99 | 0.99 | 0.99 | 1.01 | 1.00 | 1.00 | 0.99 | 1.02 |
| Pb | 0.002 | 0.000 | 0.000 | 0.003 | 0.003 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| Sn | 0.002 | 0.000 | 0.003 | 0.000 | 0.014 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| Bi | 0.005 | 0.005 | 0.004 | 0.001 | 0.007 | 0.004 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| Sb | 0.003 | 0.006 | 0.003 | 0.000 | 0.000 | 0.002 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| As | 0.007 | 0.000 | 0.000 | 0.005 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| Σ | 0.993 | 1.009 | 0.993 | 0.988 | 1.013 | 0.995 | 0.990 | 1.011 | 1.001 | 1.000 | 0.995 | 1.018 | |
| S | 1.998 | 1.971 | 1.982 | 1.986 | 1.933 | 1.951 | 1.924 | 1.902 | 1.910 | 1.912 | 1.914 | 1.886 | |
| Se | 0.009 | 0.020 | 0.025 | 0.026 | 0.054 | 0.054 | 0.085 | 0.087 | 0.089 | 0.088 | 0.091 | 0.096 | |
| Σ | 2.007 | 1.991 | 2.007 | 2.012 | 1.987 | 2.005 | 2.010 | 1.989 | 1.999 | 2.000 | 2.005 | 1.982 | |
| Imeas | dmeas | dcalc | Icalc * | h | k | l | Imeas | dmeas | dcalc | Icalc * | h | k | l | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 100.0 | 5.7395 |  | 5.7422 | 100 | 1 | 1 | −1 | 3.3 | 2.3028 |  | 2.3042 | 6 | 1 | 9 | 0 |
| 5.7422 | 97 | 1 | 1 | 0 | 2.3042 | 6 | 1 | 9 | −1 | ||||||
| 7.7 | 5.6243 | 5.6252 | 27 | 0 | 4 | 0 | 9.5 | 2.2409 |  | 2.2406 | 5 | 3 | 1 | −1 | |
| 15.9 | 5.2067 | 5.2071 | 29 | 0 | 2 | 1 | 2.2406 | 5 | 3 | 1 | −2 | ||||
| 9.1 | 4.6547 |  | 4.6560 | 11 | 1 | 3 | −1 | 3.7 | 2.1914 |  | 2.1913 | 7 | 2 | 2 | 1 |
| 4.6560 | 11 | 1 | 3 | 0 | 2.1913 | 6 | 2 | 2 | −3 | ||||||
| 0.9 | 4.0658 | 4.0628 | 5 | 0 | 4 | 1 | 4.2 | 2.1326 |  | 2.1319 | 7 | 1 | 3 | 2 | |
| 7.3 | 3.5891 |  | 3.5867 | 19 | 1 | 5 | 0 | 2.1319 | 7 | 1 | 3 | −3 | |||
| 3.5867 | 21 | 1 | 5 | −1 | 7.4 | 2.0153 |  | 2.0150 | 4 | 1 | 9 | −2 | |||
| 32.5 | 3.3650 |  | 3.3655 | 18 | 1 | 1 | 1 | 2.0150 | 4 | 1 | 9 | 1 | |||
| 3.3655 | 17 | 1 | 1 | −2 | 10.9 | 1.9932 |  | 1.9936 | 11 | 1 | 5 | 2 | |||
| 6.2 | 3.0996 |  | 3.0995 | 17 | 1 | 3 | 1 | 1.9936 | 11 | 1 | 5 | −3 | |||
| 3.0995 | 15 | 1 | 3 | −2 | 3.0 | 1.9296 | 1.9291 | 5 | 0 | 2 | 3 | ||||
| 11.6 | 2.9707 |  | 2.9694 | 6 | 2 | 0 | 0 | 3.3 | 1.9134 |  | 1.9141 | 8 | 3 | 3 | 0 |
| 2.9694 | 5 | 2 | 0 | −2 | 1.9141 | 8 | 3 | 3 | −3 | ||||||
| 9.9 | 2.9365 | 2.9371 | 5 | 0 | 0 | 2 | 3.5 | 1.8453 |  | 1.8443 | 10 | 3 | 7 | −1 | |
| 8.3 | 2.8702 |  | 2.8711 | 12 | 2 | 2 | 0 | 1.8443 | 9 | 3 | 7 | −2 | |||
| 2.8711 | 11 | 2 | 2 | −2 | 3.0 | 1.8116 |  | 1.8120 | 7 | 3 | 5 | 0 | |||
| 32.9 | 2.8417 | 2.8418 | 17 | 0 | 2 | 2 | 1.8120 | 7 | 3 | 5 | −3 | ||||
| 15.9 | 2.8236 |  | 2.8269 | 21 | 1 | 7 | 0 | 5.7 | 1.7865 | 1.7863 | 11 | 0 | 12 | 1 | |
| 2.8269 | 21 | 1 | 7 | −1 | 5.6 | 1.7216 | 1.7208 | 6 | 4 | 0 | −2 | ||||
| 19.8 | 2.8134 | 2.8126 | 28 | 0 | 8 | 0 | 12.6 | 1.6828 |  | 1.6827 | 11 | 2 | 2 | 2 | |
| 19.0 | 2.6257 |  | 2.6260 | 14 | 2 | 4 | 0 | 1.6827 | 12 | 2 | 2 | −4 | |||
| 2.6260 | 14 | 2 | 4 | −2 | 5.0 | 1.6624 |  | 1.6617 | 8 | 1 | 13 | 0 | |||
| 9.4 | 2.6040 | 2.6036 | 23 | 0 | 4 | 2 | 1.6617 | 8 | 1 | 13 | −1 | ||||
| 11.3 | 2.5340 | 2.5356 | 26 | 2 | 6 | −1 | 3.0 | 1.6454 | 1.6455 | 7 | 4 | 4 | −2 | ||
| 13.4 | 2.3271 |  | 2.3280 | 6 | 2 | 6 | 0 | 4.9 | 1.6262 |  | 1.6263 | 4 | 1 | 1 | 3 |
| 2.3280 | 6 | 2 | 6 | −2 | 1.6263 | 4 | 1 | 1 | −4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sejkora, J.; Žáček, V.; Škoda, R.; Laufek, F.; Dolníček, Z. Radvaniceite, GeS2, a New Germanium Sulphide, from the Kateřina Mine, Radvanice near Trutnov, Czech Republic. Minerals 2022, 12, 222. https://doi.org/10.3390/min12020222
Sejkora J, Žáček V, Škoda R, Laufek F, Dolníček Z. Radvaniceite, GeS2, a New Germanium Sulphide, from the Kateřina Mine, Radvanice near Trutnov, Czech Republic. Minerals. 2022; 12(2):222. https://doi.org/10.3390/min12020222
Chicago/Turabian StyleSejkora, Jiří, Vladimír Žáček, Radek Škoda, František Laufek, and Zdeněk Dolníček. 2022. "Radvaniceite, GeS2, a New Germanium Sulphide, from the Kateřina Mine, Radvanice near Trutnov, Czech Republic" Minerals 12, no. 2: 222. https://doi.org/10.3390/min12020222