1. Introduction
Manganese-arsenosilicates form rare kinds of minerals with ca. 20 species known to date. Most of them are typical products of late-stage mineralization in hydrothermally reworked Mn deposits. Here we present the description of the first nominally Ca and REE-bearing mineral belonging to this category, kesebolite-(Ce), ideally CeCa
2Mn(AsO
4)[SiO
3]
3. The new mineral species and its name were approved by the Commission on New Minerals, Nomenclature and Classification, International Mineralogical Association (No. 2019-097). The name is for the discovery locality, the Kesebol mine, in the Åmål municipality, Västra Götaland, Sweden (lat. 58°59.06′ N, long. 12°31.79′ E, elevation 130 m a.s.l.). Kesebol, pronounced [ɕe:sebu:l] with a “soft” Swedish k, is also the name of a nearby farm. The name “kesebolit” had previously been used for a variety of jacobsite, MnFe
2O
4; it occurs in an unpublished report [
1] and is thus not entrenched in the scientific literature.
The holotype specimen of kesebolite-(Ce) is preserved in the type mineral collection of the Department of Geosciences, Swedish Museum of Natural History, Box 50007, SE-10405 Stockholm, Sweden, under collection no. GEO-NRM 20100343.
2. Occurrence
The Kesebol Mn-(Fe-Cu) deposit belongs to a group of mostly sub-economic Mn occurrences along the SW shore of Lake Vänern, Sweden and the EU’s largest lake. More than 40,000 metric tonnes of Mn ore were, however, produced in the period 1917–1944 from a single deposit, the Kesebol mine, along with lesser amounts of Cu [
2]. Mining efforts occurred down to 110 m depth [
3]. Felsic metavolcanic rocks of the Åmål formation, comprising 1.63–1.59 Ga supracrustal units of the Fennoscandian Shield [
4] host the ores. Deformational, metamorphic and metallogenic processes have affected the region during the Sveconorwegian (1.1–0.9 Ga, commonly grouped with the Grenville) orogeny [
5,
6]. Lead isotope data obtained from sulfide mineralization in the Kesebol deposit indicate a link to this orogenic event [
7].
The Kesebol ores are breccia- and fracture-filling mineralizations bound to a narrow line along a N–S brittle-deformation zone in a fine-grained rhyolitic rock with quartz phenocrysts [
2]. The Mn ores consist of two essentially spatially separated units: fine-grained oxide mineralization (hausmannite ± jacobsite) and carbonate-silicate mineralization (rhodochrosite + manganoan calcite + rhodonite + manganoan garnet). Late-stage alteration formed Mn
4+-bearing oxides and hydrous Mn-silicates that occur in veins. A few rare Ce- and As-bearing minerals have been identified in recent times, from assemblages associated with Mn ore: gasparite-(Ce), chernovite-(Y), ferriakasakaite-(Ce), retzian-(Ce), sarkinite and flinkite (e.g., [
8,
9,
10]). Minor Fe mineralization, consisting of almost pure hematite-magnetite, was found in the periphery of the Mn ores, close to the wall rock at Kesebol. The Cu ore, known since 1718 [
11] consists of mainly chalcocite and chalcopyrite, and is restricted to a few sulfide-rich zones in the deposit [
2].
There are different views on the formation of this kind of Mn occurrences in the region. Geijer [
2] advocated a primarily epigenetic origin, whereas Boström et al. [
12] suggested a genetic relationship to the synsedimentary exhalative Långban-type Mn-Fe-(Ba-Pb-As) deposits in Värmland, ca. 140 km NE of Kesebol (in the ~1.9 Ga Bergslagen ore region).
We consider kesebolite-(Ce) very rare at the deposit; all known samples are from the same piece of rock. It was spotted and collected by one of us (A.P.) in 1991 from a mine dump. It occurs with rhodonite, baryte, quartz, calcite, talc, andradite, rhodochrosite, K-feldspar, hematite, gasparite-(Ce), chernovite-(Y), ferriakasakaite-(Ce) and a possible Mn-analogue to cerite-(Ce). To some extent, kesebolite-(Ce) has also been altered, forming a very fine-grained, porcelain-like light brown-beige aggregate of gasparite-(Ce), calcite, a talc-like mineral and an unknown solid phase, mainly consisting of O, Si, As, Ce and Y as seen from EDS spectra and with low analytical totals.
3. Physical Properties
Kesebolite-(Ce) occurs mostly as euhedral crystals up to 3 mm in the type specimen, in close contact with manganoan calcite, rhodonite, gasparite-(Ce), ferriakasakaite-(Ce) and the possible Mn-analogue of cerite-(Ce) (
Figure 1 and
Figure 2), in a brecciated, fine-grained andradite skarn. The crystals are short-prismatic along
c, with lengthwise striation. The major forms are {001}, {100} and {110}. The color of kesebolite-(Ce) is dark brown to grayish brown, with a vitreous luster and a light brown streak. No fluorescence effects were detected in UV light.
The Mohs hardness is estimated to be 5–6 from scratch tests. From micro-indentation (VHN100) measurements using a Shimadzu type-M tester we obtained a mean of 825 (the complete range for 11 indentations is 711–974). The mineral is brittle with irregular fractures; a fair cleavage is observed on {100}. The density was not measured, because of safety regulations concerning heaviest liquids; the calculated value is 3.998(5) g·cm−3 using the empirical formula and unit-cell volume from powder X-ray diffraction data (see below).
Optically, kesebolite-(Ce) is biaxial positive. Pleochroism is weak, in brownish-yellow shades. Refractive indices were not determined directly; the calculated value is 1.740 from Gladstone–Dale constants [
13]. Polarized reflectance spectra were measured in air with an AVASPEC-ULS2048×16 spectrometer (Avantes BV, Apeldoorn, The Netherlands) attached to a Zeiss Axiotron UV-microscope (Zeiss, Oberkochen, Germany) (10×/0.20 ultrafluar objective), using a halogen lamp (100 W) and a SiC (Zeiss no. 846) standard, with a circular sample measurement field of 120 µm in diameter. The results from the 400–700 nm range (average of 600 scans, 100 ms integration time) are given in
Table 1. Calculated
n values from the reflectance data using Fresnel’s equation are
n2 = 1.76(2),
n1 = 1.74(2) at 589 nm.
4. Mineral Chemistry
The chemical composition of kesebolite-(Ce), with respect to the major oxides (SiO
2, SO
3, CaO, MnO and As
2O
5), was determined using an FEI Quanta 650 field-emission scanning electron microscope (hosted by the Swedish Museum of Natural History), fitted with an 80 mm
2 X-MaxN Oxford Instruments EDS detector (Oxford Instruments, High Wycombe, UK) (20 kV, beam size 1 μm, working distance 10 mm). Beam current was calibrated on Co metal, with the instrument calibrated against metal and mineral standards for each element. Three separate crystal fragments were analyzed and the total number of point analyses was 9 (
Table 2). A slight chemical zonation indicated by BSE images (
Figure 2) is obviously related to variations in Ca and REE (darker and lighter areas, respectively).
All other elements reported including the REE were measured with LA-ICP-MS/MS by an Agilent 8800 instrument coupled to a ESI NWR 213 nm Nd:YAG laser (at the Department of Earth Sciences, University of Gothenburg). Laser spots were set to 30 µm in diameter (close to the points analyzed by EDS) with a laser fluence of ca. 4.9 J/cm
2 at a repetition frequency of 10 Hz. Standardization was achieved by repeatedly analyzing NIST SRM 610 glass as a primary standard, normalizing by taking SiO
2 concentration values based on EDS from the closest analyzed spot. Quantification of the REE in kesebolite-(Ce) with this approach was monitored by analyzing natural xenotime-(Y) and monazite-(Ce) with well-established concentrations. Trace element concentrations are reported in
Table 3.
The remaining elements of the periodic table (from Li to U, with the exception of PGE and halogens), not reported in Tables 2 or 3, were analyzed in two points with a 50 µm laser spot diameter in order to evaluate their presence, but they all were found below the detection limit with the current settings. No H2O or CO2 contents are indicated by the structural and spectroscopic data, see below.
The empirical formula calculated from the average analysis (
Table 2), on the basis of 13 O, is: (Ce
0.70La
0.12Nd
0.09Pr
0.03Sm
0.01Y
0.01)
Σ0.96(Ca
2.01Sr
0.01)
Σ2.02(Mn
2+1.01Mg
0.05Fe
2+0.01)
Σ1.07(Si
3.05As
5+0.81P
0.06S
6+0.04V
5+0.02)
Σ3.98O
13. A simplified formula for kesebolite-(Ce) can be written (Ce,La,Nd)Ca
2(Mn,Mg) (As,P,Si,S)Si
3O
13. The ideal formula, CeCa
2Mn(AsO
4)[SiO
3]
3, requires SiO
2 28.06, As
2O
5 17.89, Ce
2O
3 25.55, CaO 17.46, MnO 11.04, total 100 wt.%.
5. X-ray Crystallography
Powder X-ray diffraction data (
Figure 3 and
Table 4) were collected with a PANalytical X’Pert
3 powder diffractometer equipped with an X’celerator silicon-strip detector and operated at 40 mA and 45 kV (Cu
Kα-radiation, λ = 1.5406 Å). Bragg peak positions were determined with the PANalytical HighScorePlus 4.6 software (Malvern Panalytical, Kassel, Germany) and corrected against an external Si standard (NBS 640b). Monoclinic unit-cell parameters refined from the powder data are
a = 6.7408(8) Å,
b = 13.0133(15),
c = 12.0649(20) Å, β = 98.504(13)° and
V = 1046.67(17) Å
3.
Single-crystal X-ray diffraction measurements were carried out at CRIST (Centro di Cristallografia of the University of Florence) on a crystal fragment (size 30 × 40 × 70 μm) of kesebolite-(Ce) using a Bruker D8 Venture equipped with a Photon II CCD detector, with graphite-monochromatized Mo
Kα radiation (λ = 0.71073 Å), working at 60 kV with 15 s exposure time per frame; the detector-to-sample distance was 6 cm. The intensity data were integrated and corrected for standard Lorentz polarization factors with the software package APEX3 [
14]. A total of 4636 unique reflections was collected up to 2θ = 70.08°. The reflection conditions (
h0
l:
h = 2
n; 0
k0:
k = 2
n; 00
l:
l = 2
n) pointed unequivocally to the space group
P2
1/
c, and thus the structure solution was undertaken in this space group. The positions of the majority of the atoms (three
A sites = Ca and REE;
M = Mn and
T1 = Si, As cations) were determined by means of direct methods [
15]. A least squares refinement on
F2 using these heavy-atom positions and isotropic temperature factors produced an
R factor of 0.156. Three-dimensional difference Fourier synthesis yielded the position of the remaining
T cations (Si) and thirteen O atoms.
The program SHELXL [
15] was used for the refinement of the structure. The occupancy of all the sites was left free to vary (Ca vs. Ce at the
A sites; Mn vs. structural vacancy at the Mn site; Si vs. As at the
T sites) and then fixed to the resulting value. Neutral scattering curves were taken from the International Tables for X-ray Crystallography [
16]. At the last stage, with an-isotropic atomic displacement parameters for all atoms and no constraints, the residual value settled at
R = 0.0456 for 3282 observed reflections (2σ(
I) level) and 195 parameters and at
R = 0.0780 for all 4636 independent reflections. A crystallographic information file with the full experimental data is available (see
Supplementary Material). Experimental details and
R indices are given in
Table 5. Fractional atomic coordinates and isotropic displacement parameters are reported in
Table 6. Bond distances are given in
Table 7, and the corresponding bond-valence calculation [
17] is presented in
Table 8.
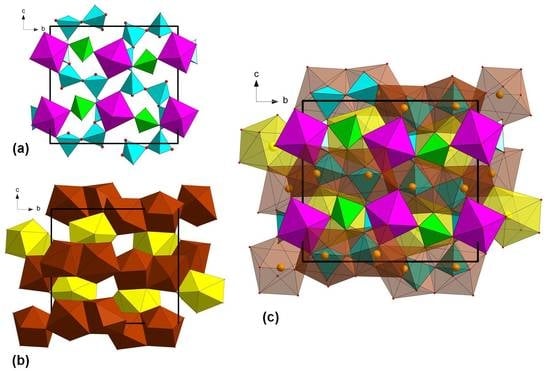
In the crystal structure of kesebolite-(Ce), markedly wavy, 6-periodic single tetrahedral (
T2 +
T3 +
T4) chains run along (001) and are interconnected by corner sharing to infinite (010) strings formed by alternating, corner-sharing
M-octahedra and
T1-tetrahedra. In a projection along the (100) axis, the structure appears as a trellis-like framework (
Figure 4a), with
A1,
A2 and
A3 sites occupying the remaining voids (
Figure 4b).
The
T2,
T3 and
T4 sites are occupied by Si alone, with <Si–O> distances varying between 1.636 and 1.642 Å. As
5+ is completely ordered at
T1, which shows a mean distance of 1.656 Å, longer than the other tetrahedral distances. Indeed, the crystal-structure refinement of
T1 occupancy points to a site scattering of 27.6 electrons, corresponding to a site population (As
0.715Si
0.285). Taking into account the ionic radii established by [
18] for four-fold coordinated As
5+ and Si
4+ (0.335 and 0.26 Å, respectively) and three-fold coordinated O
2− (1.36 Å), the expected <
T1–O> value (1.674 Å) is reasonably in accord with the experimental data.
Mn
2+ at the
M site shows a slightly distorted octahedral coordination, with an average bond distance of 2.216 Å. Such a value is in keeping with those observed in arsenmedaite, Mn
2+6As
5+Si
5O
18OH (range 2.196–2.218 Å; [
19]) and with the ideal <Mn–O> distance (2.19 Å) calculated using the ionic radii of
VIMn
2+ and
IIIO
2− [
17]. Actually, oxygen atoms are three- and four-fold coordinated here, and consequently the ideal calculated average <Mn–O> distance should be slightly larger (~2.20 Å). The
A1 and
A2 sites, dominated by Ca, are eight-fold coordinated (average bond distance = 2.525 and 2.575 Å, respectively), whereas
A3, which hosts REE cations, is nine-fold coordinated (average bond distance = 2.579 Å).
The bond valence sums (BVS in
Table 8) at the
T sites of kesebolite-(Ce) range between 4.11 and 4.16 v.u. for the Si-centered tetrahedra and it is 4.96 v.u. for
T1, which is mainly occupied by As
5+. The BVSs of the oxygen atoms range between 1.91 and 2.12 v.u., which rules out the presence of OH
- groups in the structure.
Kesebolite-(Ce) fits in the Strunz group 9.DM (inosilicates with 6-periodic single chains).
6. Micro-Raman Spectroscopy
Raman-spectroscopy measurements were carried out at room temperature using a Horiba (Jobin Yvon) LabRam HR Evolution, at the Department of Earth Sciences, University of Gothenburg. The samples were excited with an air-cooled, frequency doubled 532 nm Nd-YAG laser utilizing an Olympus 100× objective (numerical aperture = 0.9). Spectra were generated in the range of 25 to 4000 cm
−1 utilizing a 600 grooves/cm grating. The spectral resolution on randomly oriented polished crystals of kesebolite-(Ce) was in the order of 1 cm
−1. The lateral resolution was in the order of 1 μm. The wavenumber calibration was done using the 520.7 cm
−1 Raman band on a polished silicon wafer; it showed a wavenumber accuracy usually better than 0.5 cm
−1. Raman spectra of the sample were collected through 10 acquisition cycles with single counting times of 30 s in a close to back-scattered geometry. Separate spectra were collected in two distinct zones (visible in BSE, see
Figure 2) of the crystals, both of which yield similar Raman spectra (
Figure 5).
The strongest band at 867 cm
−1 could tentatively be assigned to antisymmetric stretching of the AsO
43− groups and the slightly weaker band at 784 cm
−1 to symmetric stretching mode of the same groups [
20]. The strong band at 976 cm
−1 is likely related to the asymmetric vibration mode of the [SiO
3]-groups and the one at 660 cm
−1 to bending mode of the same functional group [
21]. The weaker bands at lower wavenumbers (300 to 450 cm
−1) could be attributed to bending and stretching modes of Mn-O and out of plane bending modes of the AsO
43− group [
22]. The very broad spectral features from 1300 cm
−1 are probably fluorescence effects.
7. Discussion
The chemical composition obtained from structure refinement (
Table 6) of kesebolite-(Ce), Ce
0.96Ca
2.04Mn
1.00(As
0.72Si
0.28)Si
3O
13, is in reasonably good agreement with the above empirical formula. The calculated bond valences (
Table 8) confirm the overall structural model. As discussed above, the average
T1-O bond distance of 1.656 Å is rather short when compared to what is expected from a pure, tetrahedrally coordinated As
5+-site (around 1.69 Å; [
23]), but explained by the incorporation of smaller ions (Si
4+ and minor amounts of P
5+ and S
6+) replacing As
5+. The crystal-chemical role of hexavalent S should be noted. There is a clear antipathetic variation of S vs. REE in kesebolite-(Ce) and sympathetic S vs. Ca (
Figure 6), indicating the occurrence of the heterovalent substitution mechanism REE
3+ + As
5+ → Ca
2+ + S
6+. The zonation pattern seen in the BSE images of kesebolite-(Ce) (
Figure 2) is mainly related to this coupled variation. SO
42−-for-AsO
43− replacement is not very common in minerals but has been documented, e.g., members of the tsumcorite group [
24] and for synthetic jarosite [
25] and ettringite [
26].
The crystal structure of kesebolite-(Ce) is of a novel type, with a unique topology, and it is the only known mineral with essential Ce, Ca, Mn, As and Si. It has the same metal:As:Si:O ratio as tiragalloite, Mn
4[AsSi
3O
12(OH)], but exhibits a different structural topology. In tiragalloite and related arsenmedaite, As-tetrahedra are part of terminated silicate chains [
18,
27]. Braccoite, NaMn
2+5[Si
5AsO
17(OH)](OH), has pyroxenoid-like infinite silicate chains with the As-tetrahedra included [
28].
Kesebolite-(Ce) is rather unique among Mn-As silicate minerals in that it is not protonated. In contrast to the above mentioned related minerals, belonging to late-stage vein systems developed at zeolite to prehnite-pumpellyite facies conditions, kesebolite-(Ce) likely formed at higher temperatures without a significant contribution from mobilization of hydrothermal fluids. Overall, the present paragenesis constitutes a “dry” skarn assemblage.
The chondrite-normalized REE pattern of kesebolite-(Ce) indicates significant Ce-enrichment (
Figure 7) in the deposit. The other REE minerals from the same deposit exhibit the same pattern. The positive Ce-anomaly is typical of hydrogenetic Mn deposits whereas hydrothermal-sedimentary Mn ores normally have negative (or no) Ce anomalies [
29,
30]. Under those conditions, a Ce-enriched REE source formed from oxidation of Ce
3+ to Ce
4+ (with the formation of insoluble CeO
2). The precursors to the Mn ore at Kesebol likely precipitated in an oxic, possibly marine environment, like the Långban-type deposits [
12,
31]. At present it cannot, however, be concluded if co-precipitation of REE and Mn (plus As) actually occurred, or if REE is derived from a detrital source. Subsequent reworking and neo-mineralization in one or more phases of the Sveconorwegian orogeny could lead to the so far unique, essentially anhydrous Mn-As-REE mineral assemblage.