Combined Toxicity of Glyphosate (Faena®) and Copper to the American Cladoceran Daphnia exilis—A Two-Generation Analysis
Abstract
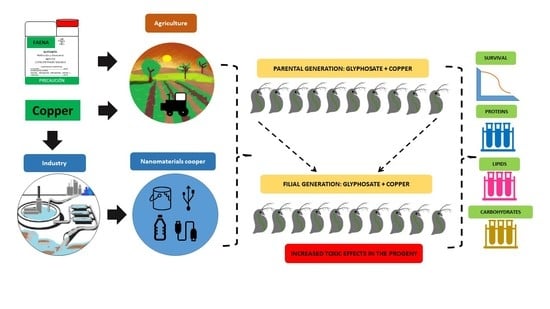
:1. Introduction
2. Materials and Methods
2.1. Tested Chemicals
2.2. Test Organisms
2.3. Acute Toxicity Tests
2.4. Sub-Chronic Test
2.4.1. Population Parameters
2.4.2. Macromolecule Biomarkers
2.4.3. Neonates’ Size
2.5. Data Analysis
3. Results
3.1. Acute Toxicity of Glyphosate and Copper to Daphnia Exilis
3.2. Survival in Chronic Exposure to Glyphosate and Copper
3.3. Reproductive Effects in D. exilis Sub-Chronically Exposed to Glyphosate and Copper
3.4. Macromolecules Content in D. exilis Exposed to Glyphosate and Copper Mixtures
3.5. Effects of Glyphosate and Copper Mixtures on the D. exilis Neonate Size
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uwizeyimana, H.; Wang, M.; Chen, W.; Khan, K. The eco-toxic effect of pesticide and heavy metal mixtures towards earthworms in soil. Environ. Toxicol. Pharm. 2017, 55, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Altenburger, R.; Walter, H.; Grote, M. What contributes to combined effect of a complex mixture? Environ. Sci. Technol. 2004, 38, 6353–6362. [Google Scholar] [CrossRef] [PubMed]
- Hernández, A.F.; Gil, F.; Lacasaña, M. Toxicological interactions of pesticides mixtures: An update. Arch. Toxicol. 2017, 91, 3211–3223. [Google Scholar] [CrossRef] [PubMed]
- Tamsyn, M.; Webster, U.; Laing, L.V.; Florance, H.; Santos, E.M. Effects of glyphosate and its formulation, roundup, on reproduction in zebrafish (Danio rerio). Environ. Sci. Technol. 2014, 48, 1271–1279. [Google Scholar] [CrossRef]
- Domínguez-Cortinas, G.; Mejía-Saavedra, J.; Santos-Medrano, G.E.; Rico-Martínez, R. Analysis of the toxicity of glyphosate and Faena® using the freshwater invertebrates Daphnia magna and Lecane quadritentata. Toxicol. Environ. Chem. 2008, 90, 377–384. [Google Scholar] [CrossRef]
- Defarge, N.; Takács, E.; Lozano, V.L.; Mesnage, R.; Spiroux de Vendomois, J.; Serálini, G.E.; Székács, A. Co-formulants in glyphosate-based herbicides disrupt aromatase activity in human cells below toxic levels. Int. J. Environ. Res. Public Health 2016, 13, 264. [Google Scholar] [CrossRef] [PubMed]
- Vanlaeys, A.; Dubuisson, F.; Seralini, G.E.; Travert, C. Formulants of glyphosate-based herbicides have more deleterious impact than glyphosate on TM4 Sertoli cells. Toxicol. In Vitro 2018, 52, 14–22. [Google Scholar] [CrossRef]
- Lajmanovich, R.C.; Junges, C.M.; Attademo, A.M.; Peltzer, P.M.; Cabagna-Zenklusen, M.C.; Basso, A. Individual and mixture toxicity of commercial formulations containing glyphosate, metsulfuron-methyl, bispyribac-sodium, and picloram on Rhinella arenarum tadpoles. Water Air Soil Pollut. 2013, 224, 1404. [Google Scholar] [CrossRef]
- Roustan, A.; Aye, M.; De Meo, M.; Di Giorgio, C. Genotoxicity of mixtures of glyphosate and atrazine and their environmental transformation products before and after photoactivation. Chemosphere 2014, 108, 93–100. [Google Scholar] [CrossRef]
- Hansen, L.R.; Roslev, P. Behavioral responses of juvenile Daphnia magna after exposure to glyphosate and glyphosate-copper complexes. Aquat. Toxicol. 2016, 179, 36–43. [Google Scholar] [CrossRef]
- La Torre, A.; Iovino, V.; Caradonia, F. Copper in plant protection: Current situation and prospects. Phytopathol. Mediterr. 2018, 57, 201–236. [Google Scholar]
- Tamm, L.; Thuerig, B.; Apostolov, S.; Blogg, H.; Borgo, E.; Corneo, P.E.; Fittje, S.; de Palma, M.; Donko, A.; Experton, C.; et al. Use of Copper-Based Fungicides in Organic Agriculture in Twelve European Countries. Agronomy 2022, 12, 673. [Google Scholar] [CrossRef]
- Liu, N.; Zhong, G.; Zhou, J.; Liu, Y.; Pang, Y.; Cai, H.; Wu, Z. Separate and combined effects of glyphosate and coper on growth and antioxidative enzymes in Salvina natans (L.) All. Sci. Total Environ. 2019, 655, 1448–1456. [Google Scholar] [CrossRef] [PubMed]
- Palza, H.; Nuñez, M.; Bastías, R.; Delgado, K. In situ antimicrobial behavior of materials with copper-based additives in a hospital environment. Int. J. Antimicrob. Agents 2018, 51, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Huo, D.; Hou, C.; Zhao, Y.; Bao, J.; Yang, M.; Fa, H. A regenerative and selective electrochemical aptasensor based on copper oxide nano flowers-single walled carbon nanotubes nanocomposite for chlorpyrifos detection. Talanta 2018, 178, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Arratia, F.; Olivares-Ferretti, P.; García-Rodríguez, A.; Marcos, E.; Carmona, E.R. Comparative toxic effects of copper-based nanoparticles and their microparticles in Daphnia magna by using natural freshwater media. N. Z. J. Mar. Freshw. Res. 2019, 53, 460–469. [Google Scholar] [CrossRef]
- Griffitt, R.J.; Weil, R.; Hyndman, K.A.; Denslow, N.D.; Powers, K.; Taylor, D.; Barber, D.S. Exposure to copper nanoparticles causes gill injury and acute lethality in zebrafish (Danio rerio). Environ. Sci. Technol. 2007, 41, 8178–8186. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Vijver, M.G.; Chen, G.; Peijnenburg, W.J.G.M. Toxicity and accumulation of Cu and ZnO nanoparticles in Daphnia magna. Environ. Sci. Technol. 2015, 49, 4657–4664. [Google Scholar] [CrossRef]
- Gilliom, R.J.; Barbash, J.E.; Crawford, C.G.; Hamilton, P.A.; Martin, J.D.; Nakagaki, N.; Nowell, L.H.; Scott, J.C.; Stackelberg, P.E.; Thelin, G.P.; et al. Pesticides in the nation’s streams and ground water, 1992–2001. In The Quality of Our Nation’s Waters; U.S. Geological Survey: Reston, VA, USA, 2006; p. 184. [Google Scholar]
- Bal, N.; Kumar, A.; Du, J.; Nugegoda, D. Multi-generational effects of two glucocorticoids (prednisolone and dexamethasone) on life-history parameters of crustacean Ceriodaphnia dubia (Cladocera). Environ. Pollut. 2017, 225, 569–578. [Google Scholar] [CrossRef]
- Rodríguez-Miguel, A.; Hernández-Zamora, M.; Martínez-Jerónimo, L.; Martínez-Jerónimo, F. Exposure to sublethal concentrations of the glyphosate- based herbicide Faena increases sensitivity in the progeny of the American cladoceran Daphnia exilis (Herrick, 1895). Enviromental Sci. Pollut. Res. 2021, 28, 38094–38105. [Google Scholar] [CrossRef]
- Li, Z.; Al, F.; Zhang, J.; Yu, Z.; Yin, D. Using Caenorhabditis elegans for studying trans- and multi-generational effects of toxicants. J. Vis. Exp. 2019, 29, e59367. [Google Scholar] [CrossRef]
- Barata, C.; Campos, B.; Rivetti, C.; LeBlanc, G.A.; Eytcheson, S.; McKnight, S.; Tobor-Kaplon, M.; de Vries, B.S.; Choi, S.; Choi, J.; et al. Validation of a two-generational reproduction test in Daphnia magna: An interlaboratory exercise. Sci. Total Environ. 2017, 579, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test, No. 202: Daphnia sp., acute immobilisation test. In OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2004; p. 12. [Google Scholar] [CrossRef]
- Hairston, N.G.; Perry, L.J., Jr.; Bohonak, A.J.; Fellows, M.Q.; Kearns, C.M. Population biology of a failed invasion: Paleolimnology of Daphnia exilis in upstate New York. Limnol. Oceanogr. 1999, 44, 477–486. [Google Scholar] [CrossRef]
- Martínez-Jerónimo, F.; Rodríguez-Estrada, J.; Martínez-Jerónimo, L. Daphnia exilis Herrick, 1985 (Crustacea: Cladocera). Una especie zooplanctónica potencialmente utilizable como organismo de prueba en bioensayos de toxicidad aguda en ambientes tropicales y subtropicales. Rev. Int. Contam. Ambient. 2008, 24, 153–159. [Google Scholar]
- Fontoura, N.F.; Agostinho, A.A. Growth with seasonally varying temperatures: An expansion of the von Bertalanffy growth model. J. Fish Biol. 1996, 48, 569–584. [Google Scholar] [CrossRef]
- Martínez-Jerónimo, F. Description of the individual growth of Daphnia magna (Crustacea: Cladocera) through the von Bertalanffy growth equation. Effect of photoperiod and temperature. Lymnology 2012, 13, 65–71. [Google Scholar] [CrossRef]
- US Environmental Protection Agency. Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms, 5th ed.; Office of Research and Development: Cincinnati, OH, USA, 2002.
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Dubois, M.; Gille, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugar and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Zöllner, N.; Kirsch, K. Microdetermination of lipids by sulphophosphovanillin reaction. Z. Für Die Gesamte Exp. Med. 1962, 135, 545–561. [Google Scholar] [CrossRef]
- Guyton, K.; Loomis, D.; Grosse, Y.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Scoccianti, C.; Mattoc, H.; Straif, K. Carcinogenicity of tetrachlorvinphos, parathion, malathion, diazinon, and glyphosate. Lancet Oncol. 2015, 16, 490–491. [Google Scholar] [CrossRef]
- Lares, B.A.; Vignatti, A.M.; Echaniz, S.A.; Gutiérrez, M.F. Effects of glyphosate on Cladocera: A synthetic Review. Aquat. Toxicol. 2022, 249, 106232. [Google Scholar] [CrossRef] [PubMed]
- Reno, U.; Doyle, S.R.; Momo, F.R.; Regaldo, L.; Gagneten, A.M. Effects of glyphosate formulations on the population dynamics of two freshwater cladoceran species. Ecotoxicology 2018, 27, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Reno, U.; Gutierrez, M.F.; Longo, M.; Vidal, E.; Regaldo, L.; Negro, A.; Mariani, M.; Zalazar, C.; Gagneten, A.M. Microcrustaceans: Biological models to evaluate a remediation process of glyphosate-based formulations. Water Air Soil Pollut. 2015, 226, 226–349. [Google Scholar] [CrossRef]
- Tsui, M.T.K.; Chu, L.M. Aquatic toxicity of glyphosate-based formulations: Comparison between different organisms and the effects of environmental factors. Chemosphere 2003, 52, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Bondarenko, O.; Juganson, K.; Ivask, A.; Kasemets, K.; Mortimer, M.; Kahru, A. Toxicity of Ag, CuO and ZnO nanoparticles to select environmentally relevant test organism and mammalian cells in vitro: A critical review. Arch. Toxicol. 2013, 87, 1181–1200. [Google Scholar] [CrossRef]
- Fan, W.; Zhang, Y.; Liu, S.; Li, X.; Li, J. Alleviation of copper toxicity in Daphnia magna by hydrogen nanobubble water. J. Hazard. Mater. 2020, 389, 122155. [Google Scholar] [CrossRef]
- Dong, D.T.; Miranda, A.; Trestrail, C.; De Souza, H.; Dinh, K.V.; Nugegoda, D. Antagonistic effects of copper and microplastics in single and binary mixtures on development and reproduction in the freshwater cladoceran Daphnia carinata. Environ. Technol. Innov. 2021, 24, 102045. [Google Scholar] [CrossRef]
- Cooper, N.L.; Bidwell, J.R.; Kumar, A. Toxicity of copper, lead, and zinc mixtures to Ceriodaphnia dubia and Daphnia carinata. Ecotoxicol. Environ. Saf. 2009, 72, 1523–1528. [Google Scholar] [CrossRef]
- Banks, K.E.; Wood, S.H.; Matthews, C.; Thuesen, K. Joint acute toxicity of diazinon and copper to Ceriodaphnia dubia. Environ. Toxicol. Chem. 2003, 22, 1562–1567. [Google Scholar] [CrossRef]
- Cuhra, M.; Traavik, T.; Bøhn, T. Clone- and age-dependent toxicity of a glyphosate commercial formulation and its active ingredient in Daphnia magna. Ecotoxicology 2013, 22, 251–262. [Google Scholar] [CrossRef]
- Koivisto, S.; Ketola, M.; Wall, M. Comparison of five cladoceran species in short- and log-term cooper exposure. Hydrobiologia 1992, 248, 125–136. [Google Scholar] [CrossRef]
- Mahar, A.M.; Watzin, M.C. Effects of metal and organophospate mixtures on Ceriodaphnia dubia survival and reproduction. Environ. Toxicol. Chem. 2005, 24, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- Agra, A.R.; Soares, A.M.V.M.; Barata, C. Life-history consequences of adaptation to pollution. Daphnia longispina clones historically exposed to copper. Ecotoxicology 2011, 20, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.M.S.; Deruytter, D.; Blust, R.; De Schamphelaere, K.A.C. Effect of temperature on chronic toxicity of copper, zinc, and nickel to Daphnia magna. Environ. Toxicol. Chem. 2017, 36, 1909–1916. [Google Scholar] [CrossRef]
- Taylor, N.S.; Kirwan, J.A.; Johnson, C.; Yan, N.D.; Viant, M.R.; Gunn, J.M.; McGeer, J.C. Predicting chronic copper and nickel reproductive toxicity to Daphnia pulex-pulicaria from whole-animal metabolic profiles. Environ. Pollut. 2016, 212, 325–329. [Google Scholar] [CrossRef]
- Silva, M.L.N.; Nogueira, D.J.; Köerich, J.S.; Vaz, V.P.; Justino, N.M.; Schmidt, J.R.A.; Matias, W.G. Multi-generational toxic effects on Daphnia magna induced by silver nanoparticles and glyphosate mixture. Environ. Toxicol. Chem. 2020, 39, 28–29. [Google Scholar] [CrossRef]
- Papchenkova, G.A.; Golovanova, I.L.; Ushakova, N.V. The parameters of reproduction, sizes, and activities of hydrolases in Daphnia magna Straus of successive generations affected by Roundup herbicide. Inland Water Biol. 2009, 2, 286–291. [Google Scholar] [CrossRef]
- Villarroel, M.J.; Ferrando, M.D.; Sancho, E.; Andreau, E. Effects of tetradifon on Daphnia magna during chronic exposure and alterations in the toxicity to generations pre-exposed to the pesticide. Aquat. Toxicol. 2000, 49, 39–47. [Google Scholar] [CrossRef]
- Campos, B.; Jordao, R.; Rivetti, C.; Lemos, M.F.L.; Soares, A.M.V.M.; Barata, C. Two-generational effects of contaminants in Daphnia magna: Effects of offspring quality. Environ. Toxicol. Chem. 2016, 35, 1470–1477. [Google Scholar] [CrossRef]
- Ingersoll, C.G.; Winner, R.W. Effect on Daphnia pulex (de Geer) of daily pulse exposures to cooper or cadmium. Environmental Toxicol. Chem. 1982, 1, 321–327. [Google Scholar] [CrossRef]
- Winner, R.W. Evaluation of the relative sensitivies of 7-D Daphnia magna and Ceriodaphnia dubia toxicity tests for cadmium and sodium pentachlorophenate. Environ. Toxicol. Chem. 1988, 7, 153–159. [Google Scholar] [CrossRef]
- Kimberly, D.A.; Salice, C.J. Multi-generational contaminant exposures produce non-monotonic transgenerational responses in Daphnia magna. Environ. Pollut. 2015, 207, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.P.K.; Sethi, N.; Mohan, A.; Datta, S.; Girdhar, M. Glyphosate toxicity for animals. Environ. Chem. Lett. 2018, 16, 401–426. [Google Scholar] [CrossRef]
- Zhou, C.F.; Wang, Y.J.; Li, C.C.; Sun, R.J.; Yu, C.; Zhou, D.M. Subacute toxicity of copper and glyphosate and their interaction to earthworm (Eisenia foetida). Environ. Pollut. 2013, 180, 71–77. [Google Scholar] [CrossRef]
- Villarroel, U.M.J. Alteraciones Fisiológicas en el Crustáceo Daphnia magna por Exposición a Plaguicidas. Ph.D. Thesis, Universidad de Valencia, Valencia, Spain, 2004. Available online: https://roderic.uv.es/handle/10550/15079 (accessed on 23 May 2023).
- Rambabu, J.P.; Rao, M.B. Effects of organochlorine and three organophosphate pesticides on glucose, glycogen, lipids and proteins contents in tissues of the freshwater snail Bellamya dissimilis (Müller). Bull. Environ. Contam. Toxicol. 1994, 53, 142–148. [Google Scholar] [PubMed]
- Beyers, D.W.; Rice, J.A.; Clements, W.H.; Henry, C.J. Estimating physiological cost of chemical exposure: Integrating energetics and stress to quantify toxic effects in fish. Can. J. Fish. Aquat. Sci. 1999, 56, 814–822. [Google Scholar] [CrossRef]
- Calow, P.; Silby, M.M. A physiological basis of population processes: Ecotoxicological implications. Funct. Ecol. 1990, 4, 283–288. [Google Scholar] [CrossRef]
- Green, J. Size reproduction in Daphnia magna (Crustacea: Cladocera). Proc. Zool. Soc. Lond. 1954, 124, 535–545. [Google Scholar] [CrossRef]
- Reno, U.; Regaldo, L.; Vidal, E.; Mariani, M.; Zalazar, C.; Gagneten, A.M. Water polluted with glyphosate formulations: Effectiveness of a decontamination process using Chlorella vulgaris growing as bioindicator. J. Appl. Phycol. 2016, 28, 2279–2286. [Google Scholar] [CrossRef]
- Knops, M.; Altenburger, R.; Segner, H. Alterations of physiological energetics, growth and reproduction of Daphnia magna under toxicant stress. Aquat. Toxicol. 2001, 53, 79–90. [Google Scholar] [CrossRef]




| Lethal Concentration (LC) | Glyphosate (mg L−1) | Copper (g L−1) |
|---|---|---|
| 0.1 | 2.08 (0.76–2.72) | 4.90 (3.03–6.22) |
| 1 | 2.48 (1.02–3.06) | 6.18 (4.10–7.47) |
| 10 | 3.14 (1.63–3.64) | 8.62 (6.28–9.79) |
| 50 | 4.22 (3.47–5.43) | 13.45 (11.48–14.82) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Zamora, M.; Rodríguez-Miguel, A.; Martínez-Jerónimo, L.; Martínez-Jerónimo, F. Combined Toxicity of Glyphosate (Faena®) and Copper to the American Cladoceran Daphnia exilis—A Two-Generation Analysis. Water 2023, 15, 2018. https://doi.org/10.3390/w15112018
Hernández-Zamora M, Rodríguez-Miguel A, Martínez-Jerónimo L, Martínez-Jerónimo F. Combined Toxicity of Glyphosate (Faena®) and Copper to the American Cladoceran Daphnia exilis—A Two-Generation Analysis. Water. 2023; 15(11):2018. https://doi.org/10.3390/w15112018
Chicago/Turabian StyleHernández-Zamora, Miriam, Alma Rodríguez-Miguel, Laura Martínez-Jerónimo, and Fernando Martínez-Jerónimo. 2023. "Combined Toxicity of Glyphosate (Faena®) and Copper to the American Cladoceran Daphnia exilis—A Two-Generation Analysis" Water 15, no. 11: 2018. https://doi.org/10.3390/w15112018