1. Introduction
Intestinal wound healing is a tightly regulated and complex process, encompassing superficial re-epithelialization (epithelial restitution), mucosal wound healing of deeper defects (e.g., ulcers formed in inflammatory bowel disease (IBD)) as well as anastomotic healing (AH) following intestinal resection. Disruptive AH consequently leads to anastomotic leakage (AL), a common but feared postoperative complication, associated with significant morbidity and mortality. However, despite the high prevalence (1–19%) of AL, the current understanding of intestinal wound healing remains remarkably limited [
1]. Regarding the clinical impact of AL, there is an urgent unmet need to further unravel fundamental and mechanistic pathways in AH to understand, prevent and treat impaired intestinal wound healing [
2].
In humans and mice, AH proceeds via an overlapping pattern of events, classically divided into three merging stages: (I) an inflammatory, (II) a proliferative, and (III) a reparative phase [
3]. Each phase is temporally defined and characterized by specific hallmark events, elicited by dynamic interactions between resident immune, endothelial, stroma, and epithelial cells (EC), as well as infiltrated leukocytes [
4]. Among them, macrophages (MΦ) represent a key subset. The intestinal mucosa represents human’s largest reservoir for MΦ [
5], which are particularly abundant in the lamina propria (LP) of the colon. Most intestinal MΦ are constantly replenished by monocytes, which enter the gut and differentiate locally into mature, anti-inflammatory MΦ [
6]. However, MΦ precursors display immense plasticity and can give rise to MΦ with various phenotypes depending on tissue-specific signals [
7]. They can differentiate into a spectrum of classically activated pro-inflammatory (M1-like) or alternatively activated, tissue-protective, and pro-resolving (M2-like) MΦ. This niche model of monocyte-to-intestinal-MΦ differentiation demonstrates that the fate and function of MΦ depend on their micro-environment [
8], especially during inflammation and tissue trauma. Since each phase of intestinal AH is characterized by a set of specific events, and consequently, vast changes in the intestinal micro-environment, MΦ play a crucial yet dichotomous role, as they display an immense phase-specific function and plasticity [
9].
The origin of intestinal wound healing is a robust inflammatory response. Tissue-resident and especially migrated monocyte-derived MΦ elicit an inflammatory state to destroy and remove pathogens, necrotic tissue as well as debris [
10,
11]. Having acquired an inflammatory (M1-like) phenotype, MΦ produce reactive oxygen species (ROS), interleukins (IL-1β-6, -8, -12, and -23), and tumor necrosis factor (TNF) to induce inflammation and antimicrobial immunity. In addition, MΦ secrete proteases (e.g., matrix metalloproteinases (MMPs-2 and MMPs-9) to degrade the extracellular matrix (ECM), thereby facilitating the recruitment of inflammatory cells to the site of tissue injury, attracted by cytokines and growth factors [
12].
Beginning in the proliferative phase, MΦ undergo a phenotype switch to acquire a pro-resolving and wound-healing phenotype [
13,
14]. Upon efferocytosis of apoptotic neutrophils and in response to IL-4 or -13 signaling [
15], MΦ switch their phenotype from pro-inflammatory (M1-like) to pro-resolving (M2-like) [
15]. In addition, apoptosis [
16], phagocytosis [
17] and the changes in the microenvironment that favor the differentiation of infiltrating monocytes into resident MΦ with non-inflammatory gene expression profiles further transform the intestinal MΦ pool during the proliferative phase of intestinal AH [
18,
19]. Another critical MΦ-elicited mechanism is the induction of angiogenesis via the secretion of vascular endothelial growth factor A (VEGF-A) to establish a microvascular network throughout the granulation tissue and to accelerate wound healing as well as enhance anastomotic strength [
20,
21].
During the reparative phase, MΦ comprise the dominant cell line for collagen synthesis and ECM remodeling, elicited by soluble growth factors [
4] including transforming growth factor beta 1 (
TGF-β1), platelet-derived growth factor (PDGF), and epidermal growth factor (EGF), which also induce intestinal epithelial cell proliferation and myofibroblast activation [
22]. The composition and arrangement of collagen are further adjusted to reach typical tearing strength accompanied by the transition from provisional wound closure to a colonic wall continuum [
3].
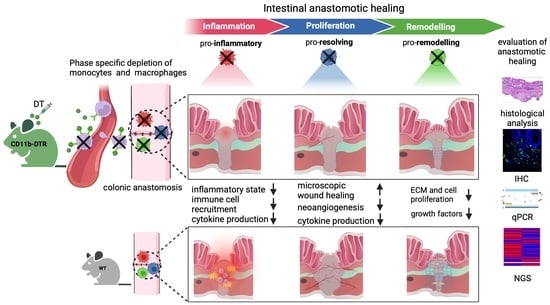
Considering the dramatic changes in local intestinal signaling pathways associated with AH, it is reasonable to suggest a distinct plasticity in MΦ function during the three phases of intestinal AH. Thus, this study aims to define phase-specific effects of MΦ in intestinal AH by using an animal model of colorectal anastomosis in combination with innovative on-demand MΦ ablation, which allows us to specifically study phase- and cell-specific pathways (
Figure 1). It was hypothesized that MΦ possess diverse roles during different phases of intestinal AH, and that MΦ depletion elicits distinct and phase-specific effects on inflammation, proliferation, and regeneration during intestinal AH (
Figure 1).
2. Materials and Methods
2.1. Mice
All procedures were approved by the State Office for Nature, Environment and Consumer Protection of North Rhine-Westphalia (reference number 81-02.04. 2020.A227) and conducted in accordance with German Animal Welfare Law. All experiments were performed using male (20 to 30 g) wild-type (WT) C57BL/6 (Charles River Laboratories, Sulzfeld, Germany) and transgenic CD11b-DTR (B6.FVB-Tg (ITGAM-DTR/EGFP)34 Lan/J, Jackson Laboratory, stock #006000, Bar Harbor, ME, USA, [
23]) mice. Mice were maintained at the local animal facility for at least one week for acclimatization before undergoing treatment. Animals had ad
libitum access to food and water and were kept under pathogen-free conditions and at a constant temperature (22 ± 2 °C) and air humidity (45–65%), with a 12 h light/dark cycle.
2.2. CD11b-DTR Mouse Model and Monocyte and MΦ Ablation
The CD11b-DTR mouse carries a transgene insert, which codes for a simian diphtheria toxin receptor (DTR) regulated by the human integrin alpha M (ITGAM) promoter (CD11b). While WT mice are naturally resistant to diphtheria toxin (DT), the “knock-in” of DTR allows for the specific systemic and local ablation of monocytes and MΦ by injecting DT from
Corynebacterium diphtheriae. Other immune cells, e.g., neutrophils, eosinophils, and lymphocytes, remain unaffected by DT [
23]. All CD11b-DTR mice received DT (25 ng/g body weight; intraperitoneally (i.p.)), Sigma-Aldrich, St. Louis, MO, USA) suspended in phosphate-buffered saline (PBS, Sigma-Aldrich), while WT mice were injected i.p. with vehicle control (PBS, 0.01 mL/g body weight).
2.3. Experimental Design
In the first set of experiments, the efficacy of DT in inducing monocyte depletion was tested in mice without any additional surgical treatment. Injections (PBS for WT mice (
Figure 2A), DT for CD11b-DTR mice (
Figure 2B)) were administered every other day for a total of 3, 6, or 9 days (
Figure 2C), mimicking phase-specific treatment in later experiments, followed by terminal blood collection. Next, a murine model of distal colonic anastomosis was established and performed in CD11b-DTR and WT mice. After surgery, phase-specific DT-induced monocyte and MΦ ablation was conducted during the inflammatory (days 0–3), proliferative (days 4–10), or reparative (days 11–20) phases of intestinal AH. On the postoperative day (POD) 3, 9, or 20, mice were sacrificed for tissue and blood examination to study phase-specific MΦ-associated changes in intestinal AH.
2.4. Surgical Procedure
All mice received buprenorphine (0.1 mg/kg; Temgesic
®, Indivior, Berkshire, UK) subcutaneously for pre- and postoperative analgesia. Anesthesia was performed using a mixture of N
2O/O
2 = 2:1 (Westfalen AG, Münster, Germany) and 4% isoflurane (Forene
®, AbbVie, Wiesbaden, Germany), and subsequently was maintained by a mixture of N
2O/O
2 = 2:1 and 1.5% isoflurane. Spontaneous breathing of mice was maintained during surgery and a body temperature of 37 °C was secured by a heating pad (ThermoLux, Murrhardt, Germany). For surgery, mice were fixated in a supine position and their fur in the surgical area was removed. After median laparotomy, the rectosigmoid junction was detected, and small bowel tissue was mobilized to the paracolic space. In the microsurgical technique, using an operating microscope (Carl Zeiss, Oberkochen, Germany), a transection of the distal colon was performed under preservation of the vasculature. A standardized end-to-end anastomosis was conducted (
Supplementary Figure S1), consisting of 12 seromuscular single button stitches of non-absorbable monofilament ETHILON™ 9-0 (Polyamide 6,6, Ethicon
®, Norderstedt, Germany). Anchor stitches were set next and opposite to the mesocolon, followed by 5 stitches on the anterior and after a 180° rotation of the tissue, 5 stitches on the posterior wall. The colon was constantly moisturized by preheated PBS. The peritoneum was closed by means of single button stitches of absorbable monofilament PDS*II 5-0 (Polydioxanone, Ethicon
®, Ethicon, Norderstedt, Germany) and the skin was closed by means of single button stitches of absorbable braided Coated VICRYL™ 4-0 (Polyglactin 910, Ethicon
®, Ethicon, Norderstedt, Germany). Post-operative care comprised an immediate supply of heat, water, and food.
2.5. Tissue Harvest and Evaluation of Anastomotic Bursting Pressure
At the end of the respective observation period, blood was extracted via cardiac puncture under general anesthesia and analgesia, followed by cervical dislocation. Tissue samples were rinsed and cleaned with cold PBS, then quick-frozen or stored in 4% phosphate-buffered formalin (Langenbrinck, Emmendingen, Germany). The anastomosis-bearing colon segment was carefully exposed to perform the anastomotic bursting pressure (ABP) test. Briefly, a measurement catheter (Neurovent-P, Raumedic®, Helmbrechts, Germany) connected through a pressure-cable (Raumedic®) to a Zero-Point Stimulator (NSP2; Raumedic®) was introduced aborally, whereas a perfusor line (B. Braun, Melsungen, Germany) was placed orally to the anastomosis. Both ends were tightly ligated with braided silk (Resorba®, Nürnberg, Germany), and natrium chloride 0.9% (B. Braun) was steadily infused (2 mL/min). The intraluminal pressure leading to tissue bursting was recorded. After testing the ABP, the anastomosis-carrying colon segment (±1 cm) was halved lengthwise, with one half fixated on a panel in formalin and the other one quick-frozen for further analysis. After preservation in paraffin (Histosec™ pastilles; Sigma-Aldrich), the tissue was cut in consecutive sections (5 µm), which were subjected to hematoxylin (Sigma-Aldrich) and eosin (Morphisto®, Offenbach am Main, Germany) or immunofluorescent staining.
2.6. Fluorescence-Activated Cell Sorting (FACS)
FACS analysis was used for the assessment of systemic monocyte ablation and was performed on full blood samples using 100 µL of whole blood per specimen. Whole blood was collected in lithium heparin tubes and immediately used for FACS staining. First, blood was incubated with 5 µg/mL Fc-block (TruStain FcXTM, Biolegend, San Diego, CA, USA) for 15 min at room temperature (RT; 20–22 °C) before 1 µg/mL antibodies were added as follows: anti-CD11b (M1/70)-BV421, anti-Ly6C (HK1.4)-APC, anti-Ly6G (1A8)-PE (all Biolegend, San Diego, CA, USA). After thorough vortexing, blood was incubated for 30 min at 4 °C. Then, 100 µL of one-step Fix/lyse solution was added to each tube and incubated for another 15 min at RT to allow for the lysis of erythrocytes and fixation of the stained cells. Tubes were filled with FACS buffer (PBS, 1% vol/vol BSA) and centrifuged at 350× g (RT) for 10 min. The supernatant was discarded, and cells were resuspended with 1 mL of FACS buffer and washed. After the final centrifugation, cells were resuspended in 300 µL of FACS buffer. Measurement was performed in Flow Cytometer CytoFlex S (Beckman Coulter, Krefeld, Germany), and the acquired data were analyzed using FloJo software 10.8.1. The relative abundance of monocytes (defined as CD11b+Ly6GlowLy6C+cells) in the blood was expressed as a percentage of CD11b+ cells.
2.7. Histological Analysis
Three nonconsecutive sections of the anastomosis were stained with hematoxylin and eosin (H&E) and analyzed by a board-certified and experienced pathologist, blinded to treatment modalities. The phase-specific AH was evaluated using a standardized histological anastomotic healing score (AHS) as previously described [
24], including the following parameters: blood vessel ingrowth (0–4 points); fibroblasts (0–4 points); collagen formation (0–4 points), inflammatory cells (0–4 points, inversed); first layer in which continuity has been restored (counted from the mucosa outwards towards serosa; 0–4); the number of healed layers (0–4); epithelium closed (0 = no, 1 = yes); crypt architecture restored (0 = no, 1 = yes) and overall healing quality (1–3 points). In addition, quantification of the perianastomotic inflammatory state (defined as the severity of inflammatory cell infiltration) was conducted using a standardized semiquantitative anastomotic inflammation score (AIS) consisting of 4 parameters (0 = absent; 1 = mild; 2 = moderate, 3 = dense) as previously described [
25]. Hypertrophic scar tissue (defined as granulation tissue outside the physiological colonic structure exclusive of ectopic organs and fat adhesions) and re-epithelialization (defined as the distance between epithelial edges) were measured with ImageJ (NIH, Bethesda, MD, USA). The collagen density of scar tissue was further investigated using picrosirius red (Morphisto
®, Offenbach am Main, Germany) staining and the abundance of collagen I and III was expressed as a fraction of the scar area. The architecture of the colon in the perianastomotic region was further determined by measuring the thickness of the mucosa, submucosa, and muscularis on the oral and aboral edge using ImageJ.
2.8. Immunofluorescence (IF)
Three nonconsecutive sections of the anastomosis were treated as described above, then deparaffinized and rehydrated in a descending ethanol series. After heat-induced epitope retrieval with citrate concentrated solution (100×; Santa Cruz, Dallas, TX, USA) and Tween 20® (Sigma), a blocking solution was applied for 1 h, containing 5% goat, 5% horse (VWR™ International GmbH, Darmstadt, Germany) and 5% fetal calf serum (Sigma) as well as 0.1% Triton® X100 (Carl Roth®, Karlsruhe, Germany). The sections were then incubated overnight at 4 °C with primary antibodies against either F4/80 (1:100; Bio-Rad, Hercules, CA, USA) or CD31 (1:100; dianova, Eching, Germany). The sections were then stained for 1 h with anti-rat IgG2a secondary antibody (1:300; Novus, Wiesbaden, Germany), 4,6-diamidino-2-phenylindole (DAPI, 1:100,000; ThermoScientific™, Waltham, MA, USA) for nuclear marking and 5% goat, 5% horse and 5% fetal calf serum against the non-specific binding. After being washed in PBS, slides were mounted (Shandon™, Immu-Mount™, Epredia™, Kalamazoo, MI, USA). The local intestinal MΦ burden was analyzed in three pictures at ×10 magnification in three nonconsecutive slides per animal. First, the anastomosis-carrying colon segment (exclusive adhesions) was defined as a region of interest (ROI). Using automated cell counting software, adjusting the threshold, and selecting ROI individually, the number of MΦ, defined as F4/80-positive cells, was expressed in relation to the number of nuclei, defined as DAPI-positive cells. The perianastomotic neoangiogenesis was expressed as blood vessels per square millimeter (mm2). Using an ×20 magnification, three pictures of three nonconsecutive slides per animal were analyzed and blood vessels were defined as CD31 (PECAM)-positive structures with the lumen. Blood vessels were counted manually and expressed per mm2. All visualization and evaluation processes were realized with a BZ-X800 fluorescence microscope (Keyence, Neu-Insenburg, Germany) and its associated analyzer software.
2.9. Real-Time Quantitative PCR (qPCR)
Quick-frozen tissue was treated with RLT buffer (Qiagen, Venlo, The Netherlands) and 1% β-mercaptoethanol (Sigma), then homogenized and lysed (TissueLyser LT and stainless-steel beans 7 mm, Qiagen). Total RNA was extracted from the lysate, using the QiaCube protocol and AllPrep DNA/RNA/Protein Mini Kit (Qiagen). Additionally, the RNA material was treated with the Turbo DNA-free Kit (ThermoScientific™). The First Strand cDNA Synthesis Kit (ThermoScientific™) was used for cDNA synthesis according to the manufacturer’s instructions. The dilution for real-time qPCR was prepared by adding SsoAdvanced™ Universal SYBR® Green Supermix (Bio-Rad) and primers (metabion international AG, Planegg/Steinkirchen, Germany): EGF (for 5′-CGG ACA GCT ACA CGG AAT G-3′, rev 5′-CGA GGC AGA CAC AAA TAA CCC-3′), PDGF-A (for 5′-GAT CCA CCT CGC ATC ATC TT-3′, rev 5′-GTT CCC GAC AGG AAA ACT CA-3′), PDGF-B (for 5′-GAT CTC TCG GAA CCT CAT CG-3′, rev 5′-GGC TTC TTT CGC ACA ATC TC-3′), VEGF (for 5′-GCT GTA ACG ATG AAG CCC TG-3′, rev 5′-TCG TCT TCT CAC CCT CAA CC-3′), TGF-β1 (for 5′-AGA CAT CTC ACA CAG TAT-3′, rev 5′-CCA GGA ATT GTT GCT ATA-3′), eukaryotic translation elongation factor 2 (Eef2) (for 5′-TGT CAG TCA TCG CCC ATG TG-3′, rev 5′-CAT CCT TGC GAG TGT CAG TGA-3′). qPCR was realized using the CFX96™ Touch™ Real-Time System (Bio-Rad). The expression rates, normalized to the housekeeping gene Eef2, were evaluated by applying the 2−∆∆Ct method.
2.10. Next Generation Sequencing and RNAseq Analysis
The library preparation of total RNA (see above) was performed with the NEBNext Ultra II RNA directional Kit and single read sequencing was performed using a NextSeq
® 2000 System with a read length of 72 bp. The samples were demultiplexed with the Illumina
® DRAGEN™ Bio-IT Platform. Quality control was performed using FastQC version 0.11.9 [
26]. Trimmomatic version 0.39 [
27] was used for an adapter and low-quality end trimming as well as for general quality trimming utilizing a sliding window of 4 bp with a minimal average base quality of 15. Reads below a minimum read length of 15 bp were discarded. The resulting reads were aligned to the Ensembl GRCm39 reference genome using HISAT2 version 2.1.0 [
28] and were sorted using SAMtools version 1.9 [
29]. Gene-based read counting was performed using HTSeq version 0.12.4 [
30] with the Ensembl annotation version 107. Ensembl IDs were converted to mgi symbols with the R package biomaRt [
31]. Differential expression analysis was performed using the R package DESeq2 version 1.32.0 [
32]. Gene set enrichment analysis (GSEA) was performed using the R package fgsea [
33] with Gene Ontology (GO) terms from the Molecular Signatures Database (MSigDB) msigdbr [
34]. For pathway analysis, the GO terms inflammatory response (GO:0006954), wound healing (GO:0042060), collagen metabolic process (GO:0032963), cytokine production (GO:0001816), and cell population proliferation (GO:0085029) were analyzed, including plotting of all direct descendants. A significance threshold of 0.05 was used for the FDR-corrected p-values to determine significantly expressed genes and gene sets. Heatmaps were created using the R package gplots [
35] and scatterplots, volcano plots, and bar plots using ggplot2 [
36]. A total of 422 genes (
Supplementary Table S1) related to wound healing were identified from msigdbr using GO term 0042060 (“wound healing”); of these, the top 50 differently regulated (lowest
p value) genes per timepoint were plotted as a heatmap.
2.11. Statistical Analysis
The purpose of this study was to test the null hypothesis that MΦ depletion would not interfere with phase-specific intestinal AH. The sample size was analyzed by G*Power analysis to ensure adequate power to detect a prespecified effect size to possibly reject the null hypothesis. All statistical analyses were performed using GraphPad Prism 9 (GraphPad Software, San Diego, CA, USA). Groups were compared using an unpaired two-tailored t-test, considering p < 0.05 as statistically significant. Results are presented as mean ± SEM and experiment-specific n values are indicated in the corresponding figure.
4. Discussion
This study provides evidence demonstrating that intestinal MΦ elicit phase-specific key tasks during colonic AH and that temporal MΦ depletion attenuates pro-inflammatory as well as pro-resolving pathways during intestinal AH. It is convincingly demonstrated that in the early postoperative phase, intestinal MΦ greatly contribute to the inflammatory response, collagen hypertrophy as well as neoangiogenesis in the perianastomotic scar region and that MΦ depletion significantly improves microscopic AH. In the later postoperative phase, MΦ constitute a source of growth factors and are critically linked to tissue repair including ECM assembly and cell proliferation; however, later MΦ depletion had no effect on microscopic AH.
Intestinal wound healing is a complex process that is essential for the regeneration of epithelial integrity, tissue architecture, and homeostasis, tightly orchestrated by local and recruited immune cells. MΦ play a pivotal role throughout the cascade of inflammatory and resolving events, which has been addressed in multiple studies in various tissues, in particular experimental models of skin wound healing [
37]. Although many of these findings can be applied to the intestine, the aspects of the individual microbiome and gut mobility complicate intestinal AH. In addition, MΦ function is now recognized as a result of spatiotemporal cues received from the local microenvironment, and thus, MΦ-associated pathways in dermal and intestinal wound healing are identified to be different. From an experimental perspective, it is also important to highlight that the underlying mechanisms responsible for undisrupted intestinal AH and wound healing in cutaneous punch biopsy wounds are markedly different, as the latter highly depends on wound contraction. Moreover, collagen subtypes and the cells contributing to collagen formation differ between the skin and the intestine. In addition, the inflammatory response during intestinal AH is of greater importance than it is for the skin [
38]. Accordingly, one of the most important aspects is that impaired wound morphology and delayed healing during intestinal AH have a dramatic functional consequence with a recognized clinical feature, namely AL, which is in broad contrast to complications of impaired cutaneous healing including transformation into a chronic wound or infection. Therefore, additional research on intestinal AH is of paramount importance.
The current results are partially in contrast to previous findings obtained in murine models of dermal wound healing. Using CD11b-DTR mice, Mirza et al. found that MΦ depletion resulted in delayed re-epithelialization, decreased collagen deposition, reduced angiogenesis, diminished cell proliferation as well as increased levels of TNF-α and reduced levels of growth factors [
39]. However, in contrast to the phase-specific depletion strategy used in the present study, Mirza et al. depleted MΦ at the time of wounding as well as 48 h later. Goren et al. used a lysozyme M promoter-driven and Cre-mediated DTR strategy and revealed that MΦ depletion was associated with impaired skin wound healing and reduced neoangiogenesis. Of interest, they also demonstrated an exacerbated inflammatory response in MΦ depleted mice [
40]. Lucas et al. used a similar model but were the first to specifically study phase-dependent MΦ depletion during skin repair [
41]. Their data revealed that MΦ depletion during the inflammatory phase diminished epithelialization and reduced the formation of granulation tissue. During the repair phase, MΦ depletion resulted in impaired angiogenesis, while a later MΦ depletion in the healing process had no further impact on wound repair, which is in line with the current results. In summary, the model of intestinal AH used here and the mentioned models of skin repair have all demonstrated reduced scar formation as well as attenuated angiogenesis following MΦ depletion, while demonstrating conflicting results regarding the inflammatory state following wounding in MΦ-depleted mice.
The currently available data regarding the role of MΦ during intestinal AH are scarce. Wu et al. found the presence of CD206+ anti-inflammatory MΦ to be associated with reduced rates of AL in a murine model of AH during peritonitis [
42]. Cetinkaya et al. reported that granulocyte macrophage-colony stimulating factor (GM-CSF) improved AH complicated by intraperitoneal mitomycin-C application [
43]. On the contrary, Pantelis et al. could demonstrate that genetic or pharmacological (using clodronate) muscularis MΦ depletion did not influence ileal or colonic AH, even when complicated by endotoxemia. However, there are crucial differences in the present study. This study is the first to specifically deplete MΦ during the sequential phases of intestinal AH. In addition, the use of a DT-based on-demand depletion strategy circumvents several problems that arise from conventional knock-out strategies. Furthermore, this study provides in-depth GSEA, which further helps to overcome the problem of morphological descriptions.
The distinct role of MΦ during the inflammatory phase of AH remains elusive [
44]. Some studies suggest that MΦ depletion diminishes phagocytosis of apoptotic cells, bacteria, and foreign debris, leading to prolonged damage-associated molecular patterns (DAMPS, e.g., high-mobility group Box 1 protein (HMGB-1)) release, and thus causing an amplified inflammatory response [
45]. Accordingly, Goren et al. and Mirza et al. found increased levels of TNF-α and a heightened neutrophil presence in skin wounds after MΦ depletion. The initial inflammatory response is also crucial for subsequent AL since inflammatory monocytes and MΦ produce MMP-2 and -9, which has been recently shown to be associated with AL in a murine model of colonic anastomosis [
25]. However, the results of significantly reduced microscopic inflammation as well as the identification of MΦ-dependent inflammatory gene sets are in line with previous reports suggesting that tissue-resident MΦ be crucially involved in neutrophil recruitment and the initiation of the early inflammatory response [
46]. This is further supported by the finding of the reduced expression of genes involved in leucocyte recruitment as well as cytokine and chemokine signaling in CD11b-DTR mice. The reduced inflammatory state (in contrast to previous reports) could be explained by the used DT treatment strategy, which depleted tissue-resident MΦ, thereby blocking the initiation of inflammation as well as circulating monocytes, blocking the subsequent monocyte influx. The data presented here also suggest that despite the significant MΦ depletion, the remaining mucosal immune system in the used model is sufficient to foster an immunological response to the tissue trauma during AH. This is of critical importance, since bacteria in particular are involved in collagen degradation and subsequent AL [
47].
Besides sufficient microscopic wound healing, intestinal AH aims to provide anastomotic strength, which is directly associated with collagen metabolism. One common finding in wound healing models coupled with MΦ depletion is that of reduced collagen deposition and granulation tissue formation. The data extend this concept to intestinal AH, demonstrating collagen metabolic processes and extracellular matrix assembly to be highly MΦ-dependent. Although a significantly reduced amount of granulation tissue was found in CD11b-DTR mice, the respective collagen density remained comparable to WT mice. In addition, the breaking strength of colorectal anastomoses was also comparable between CD11b-DTR and WT mice. This points to an apparent discrepancy between the enhanced microscopic AH (improved histological healing, reduced scar area, diminished perianastomotic infiltrate) and the comparable ABP between WT and CD11b-DTR mice at POD9. One possible explanation is that the concept of phase-specific AH is temporally dynamic, and the defined endpoints at POD 3, 9 and 20 may not fully recapitulate all features of the respective phase. Therefore, an improved microscopic AH at POD9 may not directly translate into an improved mechanical stability, or would require later testing, e.g., at POD12 or 15. In line with this, further studies are needed to better define the time frame of the inflammatory, proliferative, and reparative phase of intestinal AH.
Since intestinal MΦ are predominately replenished by a constant monocyte influx (especially under inflammatory conditions, e.g., AH), it is paramount to not only deplete intestinal MΦ but also circulating monocytes when establishing a temporally selective and cell type-specific depletion strategy during intestinal AH. There are three well-defined monocyte subsets in humans when stratified by the expression of CD14 and CD16 surface antigens: classical (CD14
++CD16
−), intermediate (CD14
+CD16
+), and nonclassical (CD14
dimCD16
+) monocytes [
48]. Analogically, murine monocytes are divided based on lymphocyte antigen 6 complex expression, locus C (Ly6C), and can be grouped according to their human counterparts: Ly6C
hi (Ly6
+, classical or inflammatory), Ly6C
int (Ly6
+, intermediate) and Ly6C
low (Ly6
-, non-classical) [
49,
50]. Notably, traditionally, Ly6C
+ monocytes were seen as the main monocyte population to enter the gut; however, there is emerging evidence that suggests a crucial role of intermediate and non-classical monocytes in inflammatory conditions, including wound healing [
51]. Ly6C
hi monocytes were shown to be more dominant in the early inflammatory phase, exhibiting phagocytic and inflammatory functions, whereas Ly6C
low monocytes dominate the later phase, displaying anti-inflammatory properties and promoting healing [
52]. Consistent with earlier studies, this study could demonstrate a highly significant monocyte reduction, irrespective of the DT injection count. In line with other reports, different depletory effects on monocyte subsets were found, with Ly6C
low monocytes being the most affected, corroborating previous reports [
53,
54,
55]. The mature Ly6C
low subset is assumed to derive from Ly6C
hi and consequently requires a longer regeneration time. When analyzing the FACS data, it is important to note that only relative frequencies, but not absolute numbers, were analyzed. Therefore, the apparent minor effect of DT on Ly6C
hi monocytes in CD11b-DTR mice is most likely due to the enormous stimulus elicited by the surgical procedure on the frequency of circulating Ly6C
hi monocytes. Similar to the distinct phenotypes of circulating monocytes, there is compelling evidence demonstrating that various MΦ subsets exist within the intestinal MΦ pool. These subsets are defined by surface markers or a distinct genetic profile and exist within a specific niche [
8]. Among them are LP MΦ, mucosal perivascular macrophages, blood-vessel-associated MΦ or neuron-associated MΦ. Given the heterogeny of specialized macrophage subsets in the intestine, it is important to acknowledge that this study does not provide in-depth information regarding the susceptibility of these subsets towards DT-induced depletion, and it is possible that one subset is more susceptible to DT treatment than another, which could have impacted intestinal AH in our model. Accordingly, further research is needed to better define the subset-specific effect of DT treatment in CD11b-DTR mice.
The GSEA results indicate that MΦ function during intestinal AH is highly dynamic, evidenced by the vast changes in MΦ-associated pathways, ranging from orchestrating the inflammatory response and cytokine production as well as eliciting collagen catabolism (inflammatory phase) to regulating ECM assembly and facilitating collagen biosynthesis (reparative phase). The phase-specific and especially context-dependent roles of intestinal MΦ presented here are in line with recent data from Strowitzki et al., showing the positive effects of a decreased infiltration of M1-like MΦ and enhanced numbers of M2-line MΦ on AH under septic conditions [
56]. Similar data have been presented by Neumann et al. in a model of inflammation-associated AH, suggesting the MΦ phenotypic switch from M1 to M2 to be a possible protective mechanism of action for annexin A1 [
57]. The data presented here add to the existing concept of targeting an MΦ phenotype switch to improve intestinal AH by demonstrating that the MΦ function is dynamic, temporally defined, and evolves with the stages during AH. Additionally, the inflammatory and proliferative phases in particular represent a potential therapeutic window to enhance an M2-like phenotype in MΦ without compromising intestinal AH. When interpreting the results in comparison to the data from Strowitzki et al. and Neumann et. al., one must point out that both groups used models of AH under inflammatory, ischemic, or septic conditions. Therefore, the used model of physiological AH might underestimate MΦ-elicited effects and further research is needed to expand the model of phase-dependent MΦ depletion to AH under pathological conditions. This is further supported by recent data showing that unlike under normal conditions, MΦ depletion under chronic inflammatory conditions (diabetes-impaired wound-healing) improved dermal wound healing [
58].
One of the limitations of the current study is the lack of evaluation of colonic motility, since intestinal dysmotility and postoperative ileus are common and clinically relevant complications of gastrointestinal surgery, especially after the creation of an anastomosis. This is of special interest as MΦ have been linked to gastrointestinal motility as well as inflammation and surgery-related intestinal dysmotility [
59,
60]. Neuron-associated MΦ are located in close proximity to the myenteric plexus in the muscularis externa [
8]. Initially, depletion of these muscularis-externa-resident MΦ has been shown to prevent postoperative ileus [
61]. However, a recent study by Farro et al. demonstrated that a genetic reduction in monocyte-derived MΦ (using the CCR2 knockout mouse) impairs the resolution of inflammation in the muscularis externa and thus increases neuronal damage and delays recovery from postoperative ileus [
62]. Therefore, tissue-resident and recruited monocyte-derived MΦ can have opposite roles during the initiation and resolution of inflammation. This in line with results from this study, since it further demonstrates that targeting MΦ must always balance their dual and temporally defined role in inflammation and resolution as well as tissue repair.
This study also adds considerable evidence to the concept that as wounds heal, the local MΦ population transitions from predominantly pro-inflammatory (M1-like) to anti-inflammatory and pro-resolving (M2-like) phenotypes. From a translational perspective, this is of importance since chronic skin wounds are characterized by the prolonged persistence of pro-inflammatory MΦ and diminished wound healing has been associated with a delayed MΦ switch from M1 to M2 [
63]. Impaired intestinal AH is especially frequent in IBD patients, which are at higher risk for AL but also suffer frequently from fibrosis, which also has been identified to be MΦ-dependent. One of the key drivers of intestinal fibrosis is
TGF-β, which is secreted by M2 MΦ. The observed decrease in
TGF-β in CD11b-DTR mice during the reparative phase of AH demonstrates M2-like MΦ to be significantly present during this phase, but also suggests that reducing or antagonizing
TGF-β during the later stages of AH to counteract intestinal fibrosis is possible without compromising AH. However, recent data from Salvador et al. demonstrated that the M2a MΦ subset in particular reduced intestinal fibrosis elicited by chronic intestinal inflammation [
64]. So far, there are insufficient data regarding the exact MΦ subset that elicit profibrotic or fibrolytic effects during intestinal AH. However, the available data suggest that alterations in the intestinal MΦ compartment, regardless of the phenotype, can lead to wound healing complications ranging from AL to fibrosis. Therefore, any therapeutic intervention (irrespective of aiming at preventing AL or intestinal fibrosis) must critically balance the distinct and dual roles that MΦ take on to ensure proper intestinal AH.