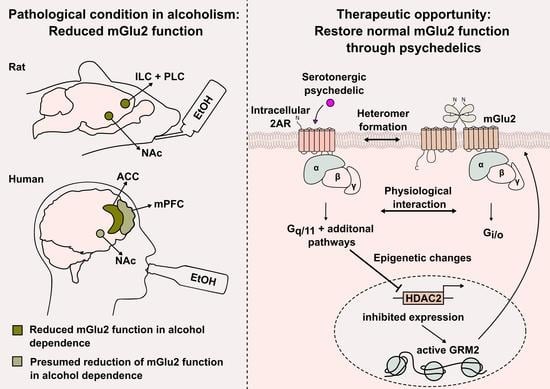
Psychedelic Targeting of Metabotropic Glutamate Receptor 2 and Its Implications for the Treatment of Alcoholism
Abstract
:1. The Use of Serotonergic Psychedelics in the Treatment of Alcoholism
2. The Role of the Metabotropic Glutamate Receptor 2 in the Pathology of AUD

3. Molecular Pharmacology of Serotonergic Psychedelics

4. Physiological Interaction between 2AR and mGlu2 Signaling and Its Implication for Psychedelics
5. Cross-Signaling of 2AR and mGlu2 through the Formation of a GPCR Heteromer
6. The Role of Epigenetic Mechanisms in the Crosstalk between 2AR and mGlu2
7. Future Directions and Therapeutic Implications
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nutt, D.J.; King, L.A.; Phillips, L.D. Drug Harms in the UK: A Multicriteria Decision Analysis. Lancet 2010, 376, 1558–1565. [Google Scholar] [CrossRef] [PubMed]
- Manthey, J.; Hassan, S.A.; Carr, S.; Kilian, C.; Kuitunen-Paul, S.; Rehm, J. What Are the Economic Costs to Society Attributable to Alcohol Use? A Systematic Review and Modelling Study. Pharmacoeconomics 2021, 39, 809–822. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Status Report on Alcohol and Health 2018; WHO: Geneva, Switzerland, 2018; p. 478. ISBN 978-92-4-156563-9. Available online: https://www.who.int/publications/i/item/9789241565639.
- Ritchie, H.; Roser, M. Alcohol Consumption. In Our World Data; 2018; Available online: https://ourworldindata.org/alcohol-consumption (accessed on 21 March 2023).
- Rehm, J.; Allamani, A.; Elekes, Z.; Jakubczyk, A.; Manthey, J.; Probst, C.; Struzzo, P.; della Vedova, R.; Gual, A.; Wojnar, M. Alcohol Dependence and Treatment Utilization in Europe—A Representative Cross-Sectional Study in Primary Care. BMC Fam. Pract 2015, 16, 90. [Google Scholar] [CrossRef] [Green Version]
- Rehm, J.; Guiraud, J.; Poulnais, R.; Shield, K.D. Alcohol Dependence and Very High Risk Level of Alcohol Consumption: A Life-Threatening and Debilitating Disease. Addict. Biol. 2018, 23, 961–968. [Google Scholar] [CrossRef]
- Witkiewitz, K.; Litten, R.Z.; Leggio, L. Advances in the Science and Treatment of Alcohol Use Disorder. Sci. Adv. 2019, 5, eaax4043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Amsterdam, J.; Van Den Brink, W. Reduced-Risk Drinking as a Viable Treatment Goal in Problematic Alcohol Use and Alcohol Dependence. J. Psychopharmacol. 2013, 27, 987–997. [Google Scholar] [CrossRef]
- Litten, R.Z.; Falk, D.E.; Ryan, M.L.; Fertig, J.; Leggio, L. Advances in Pharmacotherapy Development: Human Clinical Studies. Handb. Exp. Pharmacol. 2018, 248, 579–613. [Google Scholar] [CrossRef]
- Heilig, M.; Augier, E.; Pfarr, S.; Sommer, W.H. Developing Neuroscience-Based Treatments for Alcohol Addiction: A Matter of Choice? Transl. Psychiatry 2019, 9, 255. [Google Scholar] [CrossRef] [Green Version]
- Mendes, F.R.; Costa, C.D.S.; Wiltenburg, V.D.; Morales-Lima, G.; Fernandes, J.A.B.; Filev, R. Classic and Non-classic Psychedelics for Substance Use Disorder: A Review of Their Historic, Past and Current Research. Addict. Neurosci. 2022, 3, 100025. [Google Scholar] [CrossRef]
- Reiff, C.M.; Richman, E.E.; Nemeroff, C.B.; Carpenter, L.L.; Widge, A.S.; Rodriguez, C.I.; Kalin, N.H.; McDonald, W.M. Psychedelics and Psychedelic-Assisted Psychotherapy. Am. J. Psychiatry 2020, 177, 391–410. [Google Scholar] [CrossRef]
- Krebs, T.S.; Johansen, P. Lysergic Acid Diethylamide (LSD) for Alcoholism: Meta-Analysis of Randomized Controlled Trials. J. Psychopharmacol. 2012, 26, 994–1002. [Google Scholar] [CrossRef]
- Bogenschutz, M.P.; Forcehimes, A.A.; Pommy, J.A.; Wilcox, C.E.; Barbosa, P.; Strassman, R.J. Psilocybin-Assisted Treatment for Alcohol Dependence: A Proof-of-Concept Study. J. Psychopharmacol. 2015, 29, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Bogenschutz, M.P.; Ross, S.; Bhatt, S.; Baron, T.; Forcehimes, A.A.; Laska, E.; Mennenga, S.E.; O’Donnell, K.; Owens, L.T.; Podrebarac, S.; et al. Percentage of Heavy Drinking Days Following Psilocybin-Assisted Psychotherapy vs Placebo in the Treatment of Adult Patients with Alcohol Use Disorder: A Randomized Clinical Trial. JAMA Psychiatry 2022, 79, 953–962. [Google Scholar] [CrossRef] [PubMed]
- van Elk, M.; Yaden, D.B. Pharmacological, Neural, and Psychological Mechanisms Underlying Psychedelics: A Critical Review. Neurosci. Biobehav. Rev. 2022, 140, 104793. [Google Scholar] [CrossRef] [PubMed]
- Vollenweider, F.X.; Vollenweider-Scherpenhuyzen, M.F.I.; Bäbler, A.; Vogel, H.; Hell, D. Psilocybin Induces Schizophrenia-like Psychosis in Humans via a Serotonin-2 Agonist Action. Neuroreport 1998, 9, 3897–3902. [Google Scholar] [CrossRef] [Green Version]
- Aghajanian, G.K.; Marek, G.J. Serotonin and Hallucinogens. Neuropsychopharmacology 1999, 21, 16S–23S. [Google Scholar] [CrossRef] [Green Version]
- Madsen, M.K.; Fisher, P.M.; Burmester, D.; Dyssegaard, A.; Stenbæk, D.S.; Kristiansen, S.; Johansen, S.S.; Lehel, S.; Linnet, K.; Svarer, C.; et al. Psychedelic Effects of Psilocybin Correlate with Serotonin 2A Receptor Occupancy and Plasma Psilocin Levels. Neuropsychopharmacology 2019, 44, 1328–1334. [Google Scholar] [CrossRef] [Green Version]
- Halberstadt, A.L.; Geyer, M.A. Multiple Receptors Contribute to the Behavioral Effects of Indoleamine Hallucinogens. Neuropharmacology 2011, 61, 364–381. [Google Scholar] [CrossRef] [Green Version]
- Pokorny, T.; Preller, K.H.; Kraehenmann, R.; Vollenweider, F.X. Modulatory Effect of the 5-HT1A Agonist Buspirone and the Mixed Non-Hallucinogenic 5-HT1A/2A Agonist Ergotamine on Psilocybin-Induced Psychedelic Experience. Eur. Neuropsychopharmacol. 2016, 26, 756–766. [Google Scholar] [CrossRef]
- Meinhardt, M.W.; Sommer, W.H. Schrooms against Booze: Potential of Mycotherapy for the Treatment of AUD. Neuropsychopharmacology 2023, 48, 211–212. [Google Scholar] [CrossRef]
- Moreno, J.L.; Holloway, T.; Albizu, L.; Sealfon, S.C.; González-Maeso, J. Metabotropic Glutamate MGlu2 Receptor Is Necessary for the Pharmacological and Behavioral Effects Induced by Hallucinogenic 5-HT2A Receptor Agonists. Neurosci. Lett. 2011, 493, 76–79. [Google Scholar] [CrossRef] [Green Version]
- González-Maeso, J.; Ang, R.L.; Yuen, T.; Chan, P.; Weisstaub, N.V.; López-Giménez, J.F.; Zhou, M.; Okawa, Y.; Callado, L.F.; Milligan, G.; et al. Identification of a Serotonin/Glutamate Receptor Complex Implicated in Psychosis. Nature 2008, 452, 93–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodwani, S.; Saternos, H.; Alasmari, F.; Sari, Y. Metabotropic and Ionotropic Glutamate Receptors as Potential Targets for the Treatment of Alcohol Use Disorder. Neurosci. Biobehav. Rev. 2017, 77, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Vengeliene, V.; Spanagel, R. MGlu2 Mechanism-Based Interventions to Treat Alcohol Relapse. Front. Pharmacol. 2022, 13, 3776. [Google Scholar] [CrossRef] [PubMed]
- Heilig, M.; MacKillop, J.; Martinez, D.; Rehm, J.; Leggio, L.; Vanderschuren, L.J.M.J. Addiction as a Brain Disease Revised: Why It Still Matters, and the Need for Consilience. Neuropsychopharmacology 2021, 46, 1715–1723. [Google Scholar] [CrossRef]
- Carvalho, A.F.; Heilig, M.; Perez, A.; Probst, C.; Rehm, J. Alcohol Use Disorders. Lancet 2019, 394, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Kalivas, P.W.; O’Brien, C. Drug Addiction as a Pathology of Staged Neuroplasticity. Neuropsychopharmacology 2007, 33, 166–180. [Google Scholar] [CrossRef] [Green Version]
- Gass, J.T.; Olive, M.F. Glutamatergic Substrates of Drug Addiction and Alcoholism. Biochem. Pharmacol. 2008, 75, 218–265. [Google Scholar] [CrossRef] [Green Version]
- Chiamulera, C.; Piva, A.; Abraham, W.C. Glutamate Receptors and Metaplasticity in Addiction. Curr. Opin. Pharmacol. 2021, 56, 39–45. [Google Scholar] [CrossRef]
- Holmes, A.; Spanagel, R.; Krystal, J.H.; Holmes, A.; Spanagel, R.; Krystal, J.H. Glutamatergic Targets for New Alcohol Medications. Psychopharmacology 2013, 229, 539–554. [Google Scholar] [CrossRef] [Green Version]
- Moussawi, K.; Kalivas, P.W. Group II Metabotropic Glutamate Receptors (MGlu2/3) in Drug Addiction. Eur. J. Pharmacol. 2010, 639, 115–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meinhardt, M.W.; Hansson, A.C.; Perreau-Lenz, S.; Bauder-Wenz, C.; Stählin, O.; Heilig, M.; Harper, C.; Drescher, K.U.; Spanagel, R.; Sommer, W.H. Rescue of Infralimbic MGluR2 Deficit Restores Control Over Drug-Seeking Behavior in Alcohol Dependence. J. Neurosci. 2013, 33, 2794–2806. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Karlsson, C.; Liang, T.; Xiong, W.; Kimura, M.; Tapocik, J.D.; Yuan, Q.; Barbier, E.; Feng, A.; Flanigan, M.; et al. Loss of Metabotropic Glutamate Receptor 2 Escalates Alcohol Consumption. Proc. Natl. Acad. Sci. USA 2013, 110, 16963–16968. [Google Scholar] [CrossRef] [Green Version]
- Wood, C.M.; Nicolas, C.S.; Choi, S.L.; Roman, E.; Nylander, I.; Fernandez-Teruel, A.; Kiianmaa, K.; Bienkowski, P.; de Jong, T.R.; Colombo, G.; et al. Prevalence and Influence of Cys407* Grm2 Mutation in Hannover-Derived Wistar Rats: MGlu2 Receptor Loss Links to Alcohol Intake, Risk Taking and Emotional Behaviour. Neuropharmacology 2017, 115, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.M.; Ingraham, C.M.; Hauser, S.R.; Lasek, A.W.; Bell, R.L.; McBride, W.J. Reduced Levels of MGlu2 Receptors within the Prelimbic Cortex Are Not Associated with Elevated Glutamate Transmission or High Alcohol Drinking. Alcohol. Clin. Exp. Res. 2017, 41, 1896–1906. [Google Scholar] [CrossRef] [Green Version]
- Meinhardt, M.W.; Sommer, W.H. Postdependent State in Rats as a Model for Medication Development in Alcoholism. Addict. Biol. 2015, 20, 1–21. [Google Scholar] [CrossRef]
- Meinhardt, M.W.; Pfarr, S.; Fouquet, G.; Rohleder, C.; Meinhardt, M.L.; Barroso-Flores, J.; Hoffmann, R.; Jeanblanc, J.; Paul, E.; Wagner, K.; et al. Psilocybin Targets a Common Molecular Mechanism for Cognitive Impairment and Increased Craving in Alcoholism. Sci. Adv. 2021, 7, 2399. [Google Scholar] [CrossRef]
- Zhou, Y.; Danbolt, N.C. Glutamate as a Neurotransmitter in the Healthy Brain. J. Neural Transm. 2014, 121, 799. [Google Scholar] [CrossRef] [Green Version]
- Abraham, W.C. Metaplasticity: Tuning Synapses and Networks for Plasticity. Nat. Rev. Neurosci. 2008, 9, 387. [Google Scholar] [CrossRef]
- Reiner, A.; Levitz, J. Glutamatergic Signaling in the Central Nervous System: Ionotropic and Metabotropic Receptors in Concert. Neuron 2018, 98, 1080–1098. [Google Scholar] [CrossRef] [Green Version]
- Li, S.H.; Abd-Elrahman, K.S.; Ferguson, S.S.G. Targeting MGluR2/3 for Treatment of Neurodegenerative and Neuropsychiatric Diseases. Pharmacol. Ther. 2022, 239, 108275. [Google Scholar] [CrossRef] [PubMed]
- Bowie, D. Ionotropic Glutamate Receptors & CNS Disorders. CNS Neurol. Disord. Drug Targets 2008, 7, 129. [Google Scholar] [CrossRef] [Green Version]
- Niswender, C.M.; Conn, P.J. Metabotropic Glutamate Receptors: Physiology, Pharmacology, and Disease. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 295–322. [Google Scholar] [CrossRef] [Green Version]
- Bodzęta, A.; Berger, F.; MacGillavry, H.D. Subsynaptic Mobility of Presynaptic MGluR Types Is Differentially Regulated by Intra- and Extracellular Interactions. Mol. Biol. Cell 2022, 33, ar66. [Google Scholar] [CrossRef] [PubMed]
- Petralia, R.S.; Wang, Y.X.; Niedzielski, A.S.; Wenthold, R.J. The Metabotropic Glutamate Receptors, MGLUR2 and MGLUR3, Show Unique Postsynaptic, Presynaptic and Glial Localizations. Neuroscience 1996, 71, 949–976. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.; Wendler, O.; Frischknecht, R.; Shigemoto, R.; Schulze, H.; Enz, R. Localization of Group II and III Metabotropic Glutamate Receptors at Pre- and Postsynaptic Sites of Inner Hair Cell Ribbon Synapses. FASEB J. 2019, 33, 13734–13746. [Google Scholar] [CrossRef] [Green Version]
- Moreno, J.L.; Miranda-Azpiazu, P.; García-Bea, A.; Younkin, J.; Cui, M.; Kozlenkov, A.; Ben-Ezra, A.; Voloudakis, G.; Fakira, A.K.; Baki, L.; et al. Allosteric Signaling through an MGlu2 and 5-HT2A Heteromeric Receptor Complex and Its Potential Contribution to Schizophrenia. Sci. Signal. 2016, 9, ra5. [Google Scholar] [CrossRef] [Green Version]
- Jin, L.E.; Wang, M.; Galvin, V.C.; Lightbourne, T.C.; Conn, P.J.; Arnsten, A.F.T.; Paspalas, C.D. MGluR2 versus MGluR3 Metabotropic Glutamate Receptors in Primate Dorsolateral Prefrontal Cortex: Postsynaptic MGluR3 Strengthen Working Memory Networks. Cereb. Cortex 2018, 28, 974–987. [Google Scholar] [CrossRef] [Green Version]
- Bodzęta, A.; Scheefhals, N.; MacGillavry, H.D. Membrane Trafficking and Positioning of MGluRs at Presynaptic and Postsynaptic Sites of Excitatory Synapses. Neuropharmacology 2021, 200, 108799. [Google Scholar] [CrossRef]
- Woo, E.; Datta, D.; Arnsten, A.F.T. Glutamate Metabotropic Receptor Type 3 (MGlu3) Localization in the Rat Prelimbic Medial Prefrontal Cortex. Front. Neuroanat. 2022, 16, 23. [Google Scholar] [CrossRef]
- Tanabe, Y.; Masu, M.; Ishii, T.; Shigemoto, R.; Nakanishi, S. A Family of Metabotropic Glutamate Receptors. Neuron 1992, 8, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Sharon, D.; Vorobiov, D.; Dascal, N. Positive and Negative Coupling of the Metabotropic Glutamate Receptors to a G Protein–Activated K+ Channel, GIRK, in Xenopus Oocytes. J. Gen. Physiol. 1997, 109, 477–490. [Google Scholar] [CrossRef] [Green Version]
- Chavis, P.; Shinozaki, H.; Bockaert, J.; Fagni, L. The Metabotropic Glutamate Receptor Types 2/3 Inhibit L-Type Calcium Channels via a Pertussis Toxin-Sensitive G-Protein in Cultured Cerebellar Granule Cells. J. Neurosci. 1994, 14, 7067–7076. [Google Scholar] [CrossRef] [Green Version]
- Tyszkiewicz, J.P.; Gu, Z.; Wang, X.; Cai, X.; Yan, Z. Group II Metabotropic Glutamate Receptors Enhance NMDA Receptor Currents via a Protein Kinase C-Dependent Mechanism in Pyramidal Neurones of Rat Prefrontal Cortex. J. Physiol. 2004, 554, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Trepanier, C.; Lei, G.; Xie, Y.F.; MacDonald, J.F. Group II Metabotropic Glutamate Receptors Modify N-Methyl-D-Aspartate Receptors via Src Kinase. Sci. Rep. 2013, 3, srep00926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xi, D.; Li, Y.C.; Snyder, M.A.; Gao, R.Y.; Adelman, A.E.; Zhang, W.; Shumsky, J.S.; Gao, W.J. Group II Metabotropic Glutamate Receptor Agonist Ameliorates MK801-Induced Dysfunction of NMDA Receptors via the Akt/GSK-3β Pathway in Adult Rat Prefrontal Cortex. Neuropsychopharmacology 2011, 36, 1260–1274. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, N.; Gerber, U.; Ster, J. Activation of Group II Metabotropic Glutamate Receptors Promotes LTP Induction at Schaffer Collateral-CA1 Pyramidal Cell Synapses by Priming NMDA Receptors. J. Neurosci. 2016, 36, 11521–11531. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Liu, W.; Duffney, L.J.; Yan, Z. SNARE Proteins Are Essential in the Potentiation of NMDA Receptors by Group II Metabotropic Glutamate Receptors. J. Physiol. 2013, 591, 3935–3947. [Google Scholar] [CrossRef]
- Wang, M.J.; Li, Y.C.; Snyder, M.A.; Wang, H.; Li, F.; Gao, W.J. Group II Metabotropic Glutamate Receptor Agonist LY379268 Regulates AMPA Receptor Trafficking in Prefrontal Cortical Neurons. PLoS ONE 2013, 8, e61787. [Google Scholar] [CrossRef] [Green Version]
- Augier, E.; Dulman, R.S.; Rauffenbart, C.; Augier, G.; Cross, A.J.; Heilig, M. The MGluR2 Positive Allosteric Modulator, AZD8529, and Cue-Induced Relapse to Alcohol Seeking in Rats. Neuropsychopharmacology 2016, 41, 2932–2940. [Google Scholar] [CrossRef] [Green Version]
- Rodd, Z.A.; McKinzie, D.L.; Bell, R.L.; McQueen, V.K.; Murphy, J.M.; Schoepp, D.D.; McBride, W.J. The Metabotropic Glutamate 2/3 Receptor Agonist LY404039 Reduces Alcohol-Seeking but Not Alcohol Self-Administration in Alcohol-Preferring (P) Rats. Behav. Brain Res. 2006, 171, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Ray, T.S. Psychedelics and the Human Receptorome. PLoS ONE 2010, 5, e9019. [Google Scholar] [CrossRef]
- Nichols, D.E. Psychedelics. Pharmacol. Rev. 2016, 68, 264–355. [Google Scholar] [CrossRef] [Green Version]
- Vollenweider, F.X.; Preller, K.H. Psychedelic Drugs: Neurobiology and Potential for Treatment of Psychiatric Disorders. Nat. Rev. Neurosci. 2020, 21, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Carhart-Harris, R.L. How Do Psychedelics Work? Curr. Opin. Psychiatry 2019, 32, 16–21. [Google Scholar] [CrossRef]
- González-Maeso, J.; Weisstaub, N.V.; Zhou, M.; Chan, P.; Ivic, L.; Ang, R.; Lira, A.; Bradley-Moore, M.; Ge, Y.; Zhou, Q.; et al. Hallucinogens Recruit Specific Cortical 5-HT2A Receptor-Mediated Signaling Pathways to Affect Behavior. Neuron 2007, 53, 439–452. [Google Scholar] [CrossRef] [Green Version]
- Vargas, M.V.; Dunlap, L.E.; Dong, C.; Carter, S.J.; Tombari, R.J.; Jami, S.A.; Cameron, L.P.; Patel, S.D.; Hennessey, J.J.; Saeger, H.N.; et al. Psychedelics Promote Neuroplasticity through the Activation of Intracellular 5-HT2A Receptors. Science 2023, 379, 700–706. [Google Scholar] [CrossRef]
- Barnes, N.M.; Ahern, G.P.; Becamel, C.; Bockaert, J.; Camilleri, M.; Chaumont-Dubel, S.; Claeysen, S.; Cunningham, K.A.; Fone, K.C.; Gershon, M.; et al. International Union of Basic and Clinical Pharmacology. CX. Classification of Receptors for 5-Hydroxytryptamine; Pharmacology and Function. Pharmacol. Rev. 2021, 73, 310–520. [Google Scholar] [CrossRef] [PubMed]
- Jefsen, O.H.; Elfving, B.; Wegener, G.; Müller, H.K. Transcriptional Regulation in the Rat Prefrontal Cortex and Hippocampus after a Single Administration of Psilocybin. J. Psychopharmacol. 2020, 35, 483–493. [Google Scholar] [CrossRef]
- Vaidya, V.A.; Marek, G.J.; Aghajanian, G.K.; Duman, R.S. 5-HT(2A) Receptor-Mediated Regulation of Brain-Derived Neurotrophic Factor MRNA in the Hippocampus and the Neocortex. J. Neurosci. 1997, 17, 2785–2795. [Google Scholar] [CrossRef] [Green Version]
- Trajkovska, V.; Santini, M.A.; Marcussen, A.B.; Thomsen, M.S.; Hansen, H.H.; Mikkelsen, J.D.; Arneberg, L.; Kokaia, M.; Knudsen, G.M.; Aznar, S. BDNF Downregulates 5-HT2A Receptor Protein Levels in Hippocampal Cultures. Neurochem. Int. 2009, 55, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Gewirtz, J.C.; Chen, A.C.; Terwilliger, R.; Duman, R.C.; Marek, G.J. Modulation of DOI-Induced Increases in Cortical BDNF Expression by Group II MGlu Receptors. Pharmacol. Biochem. Behav. 2002, 73, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Duclot, F.; Kabbaj, M. The Role of Early Growth Response 1 (EGR1) in Brain Plasticity and Neuropsychiatric Disorders. Front. Behav. Neurosci. 2017, 11, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, D.; Gonzales, B.J.; Ashwal-Fluss, R.; Turm, H.; Groysman, M.; Citri, A. Egr2 Induction in Spiny Projection Neurons of the Ventrolateral Striatum Contributes to Cocaine Place Preference in Mice. eLife 2021, 10, e65228. [Google Scholar] [CrossRef] [PubMed]
- Kurita, M.; Moreno, J.L.; Holloway, T.; Kozlenkov, A.; Mocci, G.; García-Bea, A.; Hanks, J.B.; Neve, R.; Nestler, E.J.; Russo, S.J.; et al. Repressive Epigenetic Changes at the MGlu2 Promoter in Frontal Cortex of 5-HT2A Knockout Mice. Mol. Pharmacol. 2013, 83, 1166–1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urban, J.D.; Clarke, W.P.; von Zastrow, M.; Nichols, D.E.; Kobilka, B.; Weinstein, H.; Javitch, J.A.; Roth, B.L.; Christopoulos, A.; Sexton, P.M.; et al. Functional Selectivity and Classical Concepts of Quantitative Pharmacology. J. Pharmacol. Exp. Ther. 2007, 320, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Raote, I.; Bhattacharya, A.; Panicker, M.M. Serotonin 2A (5-HT2A) Receptor Function: Ligand-Dependent Mechanisms and Pathways. Serotonin Recept. Neurobiol. 2007, 6, 123–150. [Google Scholar] [CrossRef]
- Garcia, E.E.; Smith, R.L.; Sanders-Bush, E. Role of Gq Protein in Behavioral Effects of the Hallucinogenic Drug 1-(2,5-Dimethoxy-4-Iodophenyl)-2-Aminopropane. Neuropharmacology 2007, 52, 1671–1677. [Google Scholar] [CrossRef] [Green Version]
- Canal, C.E.; Morgan, D. Head-Twitch Response in Rodents Induced by the Hallucinogen 2,5-Dimethoxy-4-Iodoamphetamine: A Comprehensive History, a Re-Evaluation of Mechanisms, and Its Utility as a Model. Drug Test. Anal. 2012, 4, 556–576. [Google Scholar] [CrossRef]
- Inoue, A.; Raimondi, F.; Kadji, F.M.N.; Singh, G.; Kishi, T.; Uwamizu, A.; Ono, Y.; Shinjo, Y.; Ishida, S.; Arang, N.; et al. Illuminating G-Protein-Coupling Selectivity of GPCRs. Cell 2019, 177, 1933–1947.e25. [Google Scholar] [CrossRef]
- Kim, K.; Che, T.; Panova, O.; DiBerto, J.F.; Lyu, J.; Krumm, B.E.; Wacker, D.; Robertson, M.J.; Seven, A.B.; Nichols, D.E.; et al. Structure of a Hallucinogen-Activated Gq-Coupled 5-HT2A Serotonin Receptor. Cell 2020, 182, 1574–1588.e19. [Google Scholar] [CrossRef]
- Banerjee, A.A.; Vaidya, V.A. Differential Signaling Signatures Evoked by DOI versus Lisuride Stimulation of the 5-HT2A Receptor. Biochem. Biophys Res. Commun. 2020, 531, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.I.; Yadav, P.N.; Yao, W.D.; Arbuckle, M.I.; Grant, S.G.N.; Caron, M.G.; Roth, B.L. PSD-95 Is Essential for Hallucinogen and Atypical Antipsychotic Drug Actions at Serotonin Receptors. J. Neurosci. 2009, 29, 7124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, C.D.; Kim, M.J.; Hsin, H.; Chen, Y.; Sheng, M. Phosphorylation of Threonine-19 of PSD-95 by GSK-3β Is Required for PSD-95 Mobilization and Long-Term Depression. J. Neurosci. 2013, 33, 12122–12135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Zhu, W.; Roh, M.S.; Friedman, A.B.; Rosborough, K.; Jope, R.S. In Vivo Regulation of Glycogen Synthase Kinase-3β (GSK3β) by Serotonergic Activity in Mouse Brain. Neuropsychopharmacology 2004, 29, 1426–1431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polter, A.M.; Li, X. Glycogen Synthase Kinase-3 Is an Intermediate Modulator of Serotonin Neurotransmission. Front. Mol. Neurosci. 2011, 4, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmid, C.L.; Raehal, K.M.; Bohn, L.M. Agonist-Directed Signaling of the Serotonin 2A Receptor Depends on β-Arrestin-2 Interactions in Vivo. Proc. Natl. Acad. Sci. USA 2008, 105, 1079–1084. [Google Scholar] [CrossRef] [Green Version]
- Schmid, C.L.; Bohn, L.M. Serotonin, But Not N-Methyltryptamines, Activates the Serotonin 2A Receptor Via a β-Arrestin2/Src/Akt Signaling Complex In Vivo. J. Neurosci. 2010, 30, 13513–13524. [Google Scholar] [CrossRef] [Green Version]
- de La Fuente Revenga, M.; Jaster, A.M.; McGinn, J.; Silva, G.; Saha, S.; González-Maeso, J. Tolerance and Cross-Tolerance among Psychedelic and Nonpsychedelic 5-HT2A Receptor Agonists in Mice. ACS Chem. Neurosci. 2022, 13, 2436–2448. [Google Scholar] [CrossRef]
- Rodriguiz, R.M.; Nadkarni, V.; Means, C.R.; Pogorelov, V.M.; Chiu, Y.T.; Roth, B.L.; Wetsel, W.C. LSD-Stimulated Behaviors in Mice Require β-Arrestin 2 but Not β-Arrestin 1. Sci. Rep. 2021, 11, 17690. [Google Scholar] [CrossRef]
- Karaki, S.; Becamel, C.; Murat, S.; La Cour, C.M.; Millan, M.J.; Prezeau, L.; Bockaert, J.; Marin, P.; Vandermoere, F. Quantitative Phosphoproteomics Unravels Biased Phosphorylation of Serotonin 2A Receptor at Ser280 by Hallucinogenic versus Nonhallucinogenic Agonists. Mol. Cell. Proteom. 2014, 13, 1273–1285. [Google Scholar] [CrossRef] [Green Version]
- Delille, H.K.; Mezler, M.; Marek, G.J. The Two Faces of the Pharmacological Interaction of MGlu2 and 5-HT2A-Relevance of Receptor Heterocomplexes and Interaction through Functional Brain Pathways. Neuropharmacology 2013, 70, 296–305. [Google Scholar] [CrossRef]
- Marek, G.J.; Wright, R.A.; Schoepp, D.D.; Monn, J.A.; Aghajanian, G.K. Physiological Antagonism between 5-Hydroxytryptamine2A and Group II Metabotropic Glutamate Receptors in Prefrontal Cortex. J. Pharmacol. Exp. Ther. 2000, 292, 76–87. [Google Scholar] [PubMed]
- Aghajanian, G.K.; Marek, G.J. Serotonin Induces Excitatory Postsynaptic Potentials in Apical Dendrites of Neocortical Pyramidal Cells. Neuropharmacology 1997, 36, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Delille, H.K.; Becker, J.M.; Burkhardt, S.; Bleher, B.; Terstappen, G.C.; Schmidt, M.; Meyer, A.H.; Unger, L.; Marek, G.J.; Mezler, M. Heterocomplex Formation of 5-HT2A-MGlu2 and Its Relevance for Cellular Signaling Cascades. Neuropharmacology 2012, 62, 2184–2191. [Google Scholar] [CrossRef]
- Marek, G.J. Interactions of Hallucinogens with the Glutamatergic System: Permissive Network Effects Mediated through Cortical Layer V Pyramidal Neurons. In Current Topics in Behavioral Neurosciences; Springer: Heidelberg, Germany, 2018; Volume 36, pp. 107–135. [Google Scholar]
- Gewirtz, J.C.; Marek, G.J. Behavioral Evidence for Interactions between a Hallucinogenic Drug and Group II Metabotropic Glutamate Receptors. Neuropsychopharmacology 2000, 23, 569–576. [Google Scholar] [CrossRef]
- Klodzinska, A.; Bijak, M.; Tokarski, K.; Pilc, A. Group II MGlu Receptor Agonists Inhibit Behavioural and Electrophysiological Effects of DOI in Mice. Pharmacol. Biochem. Behav. 2002, 73, 327–332. [Google Scholar] [CrossRef]
- Benneyworth, M.A.; Xiang, Z.; Smith, R.L.; Garcia, E.E.; Conn, P.J.; Sanders-Bush, E. A Selective Positive Allosteric Modulator of Metabotropic Glutamate Receptor Subtype 2 Blocks a Hallucinogenic Drug Model of Psychosis. Mol. Pharmacol. 2007, 72, 477–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taddeucci, A.; Olivero, G.; Roggeri, A.; Milanese, C.; Di Giorgio, F.P.; Grilli, M.; Marchi, M.; Garrone, B.; Pittaluga, A. Presynaptic 5-HT2A-mGlu2/3 Receptor–Receptor Crosstalk in the Prefrontal Cortex: Metamodulation of Glutamate Exocytosis. Cells 2022, 11, 3035. [Google Scholar] [CrossRef] [PubMed]
- Olivero, G.; Grilli, M.; Vergassola, M.; Bonfiglio, T.; Padolecchia, C.; Garrone, B.; Di Giorgio, F.P.; Tongiani, S.; Usai, C.; Marchi, M.; et al. 5-HT2A-MGlu2/3 Receptor Complex in Rat Spinal Cord Glutamatergic Nerve Endings: A 5-HT2A to MGlu2/3 Signalling to Amplify Presynaptic Mechanism of Auto-Control of Glutamate Exocytosis. Neuropharmacology 2018, 133, 429–439. [Google Scholar] [CrossRef]
- Benvenga, M.J.; Chaney, S.F.; Baez, M.; Britton, T.C.; Hornback, W.J.; Monn, J.A.; Marek, G.J. Metabotropic Glutamate2 Receptors Play a Key Role in Modulating Head Twitches Induced by a Serotonergic Hallucinogen in Mice. Front. Pharmacol. 2018, 9, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hideshima, K.S.; Hojati, A.; Saunders, J.M.; On, D.M.; de la Fuente Revenga, M.; Shin, J.M.; Sánchez-González, A.; Dunn, C.M.; Pais, A.B.; Pais, A.C.; et al. Role of MGlu2 in the 5-HT 2A Receptor-Dependent Antipsychotic Activity of Clozapine in Mice. Psychopharmacology 2018, 235, 3149–3165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Marek, G.J. AMPA Receptor Involvement in 5-Hydroxytryptamine2A Receptor-Mediated Pre-Frontal Cortical Excitatory Synaptic Currents and DOI-Induced Head Shakes. Prog. Neuropsycho-Pharmacol. Biol. Psychiatry 2008, 32, 62–71. [Google Scholar] [CrossRef]
- Fribourg, M.; Moreno, J.L.; Holloway, T.; Provasi, D.; Baki, L.; Mahajan, R.; Park, G.; Adney, S.K.; Hatcher, C.; Eltit, J.M.; et al. Decoding the Signaling of a GPCR Heteromeric Complex Reveals a Unifying Mechanism of Action of Antipsychotic Drugs. Cell 2011, 147, 1011–1023. [Google Scholar] [CrossRef] [Green Version]
- Ferré, S.; Casadó, V.; Devi, L.A.; Filizola, M.; Jockers, R.; Lohse, M.J.; Milligan, G.; Pin, J.P.; Guitart, X. G Protein-Coupled Receptor Oligomerization Revisited: Functional and Pharmacological Perspectives. Pharmacol. Rev. 2014, 66, 413–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuxe, K.; Marcellino, D.; Guidolin, D.; Woods, A.S.; Agnati, L.F. Heterodimers and Receptor Mosaics of Different Types of G-Protein-Coupled Receptors. Physiology 2008, 23, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Albizu, L.; Moreno, J.L.; González-Maeso, J.; Sealfon, S.C. Heteromerization of G Protein-Coupled Receptors: Relevance to Neurological Disorders and Neurotherapeutics. CNS Neurol. Disord. Drug Targets 2012, 9, 636–650. [Google Scholar] [CrossRef] [Green Version]
- Borroto-Escuela, D.O.; Wydra, K.; Filip, M.; Fuxe, K. A2AR-D2R Heteroreceptor Complexes in Cocaine Reward and Addiction. Trends Pharmacol. Sci. 2018, 39, 1008–1020. [Google Scholar] [CrossRef]
- Gomes, I.; Ayoub, M.A.; Fujita, W.; Jaeger, W.C.; Pfleger, K.D.G.; Devi, L.A. G Protein-Coupled Receptor Heteromers. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 403–425. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; An, S.; Ward, R.; Yang, Y.; Liu, Y.; Guo, X.X.; Hao, Q.; Xu, T.R. Methods Used to Study the Oligomeric Structure of G-Protein-Coupled Receptors. Biosci. Rep. 2017, 37, BSR20160547. [Google Scholar] [CrossRef] [Green Version]
- Moreno, J.L.; Muguruza, C.; Umali, A.; Mortillo, S.; Holloway, T.; Pilar-Cuéllar, F.; Mocci, G.; Seto, J.; Callado, L.F.; Neve, R.L.; et al. Identification of Three Residues Essential for 5-Hydroxytryptamine 2A-Metabotropic Glutamate 2 (5-HT2A·mGlu2) Receptor Heteromerization and Its Psychoactive Behavioral Function. J. Biol. Chem. 2012, 287, 44301–44319. [Google Scholar] [CrossRef] [Green Version]
- Rives, M.-L.; Vol, C.; Fukazawa, Y.; Tinel, N.; Trinquet, E.; Ayoub, M.A.; Shigemoto, R.; Pin, J.-P.; Prézeau, L. Crosstalk between GABAB and MGlu1a Receptors Reveals New Insight into GPCR Signal Integration. EMBO J. 2009, 28, 2195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hámor, P.U.; Šírová, J.; Páleníček, T.; Zaniewska, M.; Bubeníková-Valešová, V.; Schwendt, M. Chronic Methamphetamine Self-Administration Dysregulates 5-HT2A and MGlu2 Receptor Expression in the Rat Prefrontal and Perirhinal Cortex: Comparison to Chronic Phencyclidine and MK-801. Pharmacol. Biochem. Behav. 2018, 175, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Dueñas, V.; Gómez-Soler, M.; Valle-León, M.; Watanabe, M.; Ferrer, I.; Ciruela, F. Revealing Adenosine A2A-Dopamine D2 Receptor Heteromers in Parkinson’s Disease Post-Mortem Brain through a New AlphaScreen-Based Assay. Int. J. Mol. Sci. 2019, 20, 3600. [Google Scholar] [CrossRef] [Green Version]
- Toneatti, R.; Shin, J.M.; Shah, U.H.; Mayer, C.R.; Saunders, J.M.; Fribourg, M.; Arsenovic, P.T.; Janssen, W.G.; Sealfon, S.C.; López-Giménez, J.F.; et al. Interclass GPCR Heteromerization Affects Localization and Trafficking. Sci. Signal. 2020, 13, eaaw3122. [Google Scholar] [CrossRef] [PubMed]
- Bécamel, C.; Berthoux, C.; Barre, A.; Marin, P. Growing Evidence for Heterogeneous Synaptic Localization of 5-HT2A Receptors. ACS Chem. Neurosci. 2017, 8, 897–899. [Google Scholar] [CrossRef] [Green Version]
- Barre, A.; Berthoux, C.; De Bundel, D.; Valjent, E.; Bockaert, J.; Marin, P.; Bécamel, C. Presynaptic Serotonin 2A Receptors Modulate Thalamocortical Plasticity and Associative Learning. Proc. Natl. Acad. Sci. USA 2016, 113, E1382–E1391. [Google Scholar] [CrossRef] [Green Version]
- Alasmari, F.; Goodwani, S.; McCullumsmith, R.E.; Sari, Y. Role of Glutamatergic System and Mesocorticolimbic Circuits in Alcohol Dependence. Prog. Neurobiol. 2018, 171, 32–49. [Google Scholar] [CrossRef]
- Müller, C.P.; Homberg, J.R. The Role of Serotonin in Drug Use and Addiction. Behav. Brain Res. 2015, 277, 146–192. [Google Scholar] [CrossRef]
- Domi, E.; Barchiesi, R.; Barbier, E. Epigenetic Dysregulation in Alcohol-Associated Behaviors: Preclinical and Clinical Evidence. Curr. Top Behav. Neurosci. 2023. Online ahead of print. [Google Scholar] [CrossRef]
- Kurita, M.; Holloway, T.; García-Bea, A.; Kozlenkov, A.; Friedman, A.K.; Moreno, J.L.; Heshmati, M.; Golden, S.A.; Kennedy, P.J.; Takahashi, N.; et al. HDAC2 Regulates Atypical Antipsychotic Responses through the Modulation of MGlu2 Promoter Activity. Nat. Neurosci. 2012, 15, 1245–1254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de la Fuente Revenga, M.; Ibi, D.; Cuddy, T.; Toneatti, R.; Kurita, M.; Ijaz, M.K.; Miles, M.F.; Wolstenholme, J.T.; González-Maeso, J. Chronic Clozapine Treatment Restrains via HDAC2 the Performance of MGlu2 Receptor Agonism in a Rodent Model of Antipsychotic Activity. Neuropsychopharmacology 2019, 44, 443–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibi, D.; De La Fuente Revenga, M.; Kezunovic, N.; Muguruza, C.; Saunders, J.M.; Gaitonde, S.A.; Moreno, J.L.; Ijaz, M.K.; Santosh, V.; Kozlenkov, A.; et al. Antipsychotic-Induced Hdac2 Transcription via NF-ΚB Leads to Synaptic and Cognitive Side Effects. Nat. Neurosci. 2017, 20, 1247–1259. [Google Scholar] [CrossRef]
- Hinz, M.; Scheidereit, C. The IκB Kinase Complex in NF-ΚB Regulation and Beyond. EMBO Rep. 2014, 15, 46–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de la Fuente Revenga, M.; Ibi, D.; Saunders, J.M.; Cuddy, T.; Ijaz, M.K.; Toneatti, R.; Kurita, M.; Holloway, T.; Shen, L.; Seto, J.; et al. HDAC2-Dependent Antipsychotic-like Effects of Chronic Treatment with the HDAC Inhibitor SAHA in Mice. Neuroscience 2018, 388, 102–117. [Google Scholar] [CrossRef]
- de la Fuente Revenga, M.; Zhu, B.; Guevara, C.A.; Naler, L.B.; Saunders, J.M.; Zhou, Z.; Toneatti, R.; Sierra, S.; Wolstenholme, J.T.; Beardsley, P.M.; et al. Prolonged Epigenomic and Synaptic Plasticity Alterations Following Single Exposure to a Psychedelic in Mice. Cell Rep. 2021, 37, 109836. [Google Scholar] [CrossRef]
- Savino, A.; Nichols, C.D. Lysergic Acid Diethylamide Induces Increased Signalling Entropy in Rats’ Prefrontal Cortex. J. Neurochem. 2022, 162, 9–23. [Google Scholar] [CrossRef]
- Koob, G.F.; Volkow, N.D. Neurobiology of Addiction: A Neurocircuitry Analysis. Lancet Psychiatry 2016, 3, 760–773. [Google Scholar] [CrossRef]
- Trabanco, A.A.; Bartolomé, J.M.; Cid, J.M. MGluR2 Positive Allosteric Modulators: An Updated Patent Review (2013–2018). Expert Opin. Ther. Pat. 2019, 29, 497–507. [Google Scholar] [CrossRef]
- Corti, C.; Battaglia, G.; Molinaro, G.; Riozzi, B.; Pittaluga, A.; Corsi, M.; Mugnaini, M.; Nicoletti, F.; Bruno, V. The Use of Knock-Out Mice Unravels Distinct Roles for MGlu2 and MGlu3 Metabotropic Glutamate Receptors in Mechanisms of Neurodegeneration/Neuroprotection. J. Neurosci. 2007, 27, 8297–8308. [Google Scholar] [CrossRef] [Green Version]
- Bespalov, A.; Müller, R.; Relo, A.L.; Hudzik, T. Drug Tolerance: A Known Unknown in Translational Neuroscience. Trends Pharmacol. Sci. 2016, 37, 364–378. [Google Scholar] [CrossRef]
- Calleja-Conde, J.; Morales-García, J.A.; Echeverry-Alzate, V.; Bühler, K.M.; Giné, E.; López-Moreno, J.A. Classic Psychedelics and Alcohol Use Disorders: A Systematic Review of Human and Animal Studies. Addict. Biol. 2022, 27, e13229. [Google Scholar] [CrossRef] [PubMed]
- Andersen, K.A.A.; Carhart-Harris, R.; Nutt, D.J.; Erritzoe, D. Therapeutic Effects of Classic Serotonergic Psychedelics: A Systematic Review of Modern-Era Clinical Studies. Acta Psychiatr. Scand. 2021, 143, 101–118. [Google Scholar] [CrossRef] [PubMed]
- van der Meer, P.B.; Fuentes, J.J.; Kaptein, A.A.; Schoones, J.W.; de Waal, M.M.; Goudriaan, A.E.; Kramers, K.; Schellekens, A.; Somers, M.; Bossong, M.G.; et al. Therapeutic Effect of Psilocybin in Addiction: A Systematic Review. Front. Psychiatry 2023, 14, 129. [Google Scholar] [CrossRef] [PubMed]
- United Nations Convention on Psychotropic Substances, Vienna. 1971. Available online: https://www.unodc.org/pdf/convention_1971_en.pdf (accessed on 21 March 2023).
- Johnson, M.W.; Griffiths, R.R.; Hendricks, P.S.; Henningfield, J.E. The Abuse Potential of Medical Psilocybin According to the 8 Factors of the Controlled Substances Act. Neuropharmacology 2018, 142, 143–166. [Google Scholar] [CrossRef]
- dos Santos, R.G.; Bouso, J.C.; Alcázar-Córcoles, M.Á.; Hallak, J.E.C. Efficacy, Tolerability, and Safety of Serotonergic Psychedelics for the Management of Mood, Anxiety, and Substance-Use Disorders: A Systematic Review of Systematic Reviews. Expert Rev. Clin. Pharmacol. 2018, 11, 889–902. [Google Scholar] [CrossRef]
- Schlag, A.K.; Aday, J.; Salam, I.; Neill, J.C.; Nutt, D.J. Adverse Effects of Psychedelics: From Anecdotes and Misinformation to Systematic Science. Br. Assoc. Psychopharmacol. 2022, 36, 258–272. [Google Scholar] [CrossRef]
- Holze, F.; Becker, A.M.; Kolaczynska, K.E.; Duthaler, U.; Liechti, M.E. Pharmacokinetics and Pharmacodynamics of Oral Psilocybin Administration in Healthy Participants. Clin. Pharmacol. Ther. 2022, 113, 822–831. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domanegg, K.; Sommer, W.H.; Meinhardt, M.W. Psychedelic Targeting of Metabotropic Glutamate Receptor 2 and Its Implications for the Treatment of Alcoholism. Cells 2023, 12, 963. https://doi.org/10.3390/cells12060963
Domanegg K, Sommer WH, Meinhardt MW. Psychedelic Targeting of Metabotropic Glutamate Receptor 2 and Its Implications for the Treatment of Alcoholism. Cells. 2023; 12(6):963. https://doi.org/10.3390/cells12060963
Chicago/Turabian StyleDomanegg, Kevin, Wolfgang H. Sommer, and Marcus W. Meinhardt. 2023. "Psychedelic Targeting of Metabotropic Glutamate Receptor 2 and Its Implications for the Treatment of Alcoholism" Cells 12, no. 6: 963. https://doi.org/10.3390/cells12060963