Pathogenic Role of Adipose Tissue-Derived Mesenchymal Stem Cells in Obesity and Obesity-Related Inflammatory Diseases
Abstract
:1. Introduction
2. Characteristics of Healthy Mesenchymal Stem Cells (MSCs)
2.1. MSCs Discovery
2.2. MSCs Housing
2.3. MSCs’ Tissue Repair Properties
2.4. MSCs’ Immunomodulatory Properties
2.4.1. In Vitro Experiments
2.4.2. In Vivo Studies
3. Mechanisms Involved in the Induction of MSCs’ Functions
3.1. Immunomodulatory Properties
3.1.1. Soluble Factors
3.1.2. Cell–Cell Contact
Adhesion Molecules
Galectin-1
3.1.3. Immune Check Point Expression
3.2. Pro-Inflammatory Properties
3.2.1. Mechanisms Involved in the Promotion of Anti- or Pro-Inflammatory MSCs
Inflammatory or Anti-Inflammatory Cytokine Effects
Role of Toll-Like Receptors
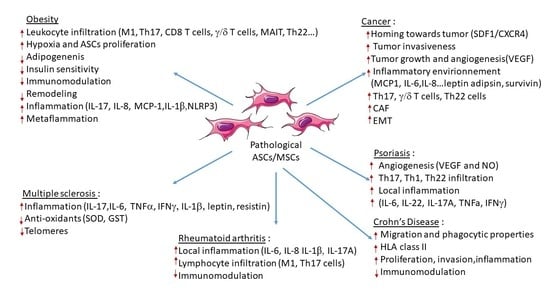
4. Contribution of ASCs in Obesity and Obesity-Related Inflammatory Diseases
4.1. Obesity
4.1.1. Infiltration of Inflammatory Cells
4.1.2. Modification of the Secretome Profile
4.1.3. Alteration of ASC Properties
4.1.4. Metaflammation
4.2. Cancer
4.2.1. Role of Obese ASCs in Increased Vascularization and Tumor Growth
4.2.2. Differentiation of Obese ASCs into Carcinoma-Associated Fibroblasts
4.2.3. Role of the Obese ASC Secretome
4.2.4. Role of Obese ASCs in the Attraction and Polarization of Pathogenic IL-17 and IL-22 Secreting Cells
4.2.5. Role of Obese ASCs in Epithelial Mesenchymal Transition of Cancer Cells
4.3. Chronic Inflammatory and Autoimmune Diseases
4.3.1. Multiple Sclerosis
Role of IL-17
Association with Obesity
Contribution of ASCs and MSCs
4.3.2. Psoriasis
Role of Inflammatory Mediators
Association with Obesity
Role of MSCs
4.3.3. Rheumatoid Arthritis
Association with Obesity
Role of Th17 and MSCs/ASCs
4.3.4. Crohn’s Disease (CD)
Association with Obesity
Role of ASCs
5. Conclusions
5.1. Lean Versus Obese ASCs/MSCs
5.2. Cell Therapy Programs
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Available online: World_Obesity_Atlas_2022_WEB.Pdf (accessed on 26 December 2022).
- Available online: 9789289057738-Eng.Pdf (accessed on 26 December 2022).
- Chehimi, M.; Vidal, H.; Eljaafari, A. Pathogenic Role of IL-17-Producing Immune Cells in Obesity, and Related Inflammatory Diseases. J. Clin. Med. 2017, 6, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berger, N.A. Young Adult Cancer: Influence of the Obesity Pandemic. Obesity 2018, 26, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Winer, S.; Chan, Y.; Paltser, G.; Truong, D.; Tsui, H.; Bahrami, J.; Dorfman, R.; Wang, Y.; Zielenski, J.; Mastronardi, F.; et al. Normalization of Obesity-Associated Insulin Resistance through Immunotherapy. Nat. Med. 2009, 15, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, S.; Manabe, I.; Nagasaki, M.; Eto, K.; Yamashita, H.; Ohsugi, M.; Otsu, M.; Hara, K.; Ueki, K.; Sugiura, S.; et al. CD8 + Effector T Cells Contribute to Macrophage Recruitment and Adipose Tissue Inflammation in Obesity. Nat. Med. 2009, 15, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in Inflammation and Metabolic Disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef]
- Eljaafari, A.; Robert, M.; Chehimi, M.; Chanon, S.; Durand, C.; Vial, G.; Bendridi, N.; Madec, A.-M.; Disse, E.; Laville, M.; et al. Adipose Tissue–Derived Stem Cells From Obese Subjects Contribute to Inflammation and Reduced Insulin Response in Adipocytes Through Differential Regulation of the Th1/Th17 Balance and Monocyte Activation. Diabetes 2015, 64, 2477–2488. [Google Scholar] [CrossRef] [Green Version]
- Pestel, J.; Chehimi, M.; Bonhomme, M.; Robert, M.; Vidal, H.; Eljaafari, A. IL-17A Contributes to Propagation of Inflammation but Does Not Impair Adipogenesis and/or Insulin Response, in Adipose Tissue of Obese Individuals. Cytokine 2020, 126, 154865. [Google Scholar] [CrossRef]
- Cohnheim, J. Ueber Entzündung und Eiterung. Arch. Pathol. Anat. Physiol. Klin. Med. 1867, 40, 1–79. [Google Scholar] [CrossRef] [Green Version]
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The Development of Fibroblast Colonies in Monolayer Cultures of Guinea-Pig Bone Marrow and Spleen Cells. Cell Prolif. 1970, 3, 393–403. [Google Scholar] [CrossRef]
- Prockop, D.J. Marrow Stromal Cells as Stem Cells for Nonhematopoietic Tissues. Science 1997, 276, 71–74. [Google Scholar] [CrossRef]
- da Silva Meirelles, L.; Chagastelles, P.C.; Nardi, N.B. Mesenchymal Stem Cells Reside in Virtually All Post-Natal Organs and Tissues. J. Cell Sci. 2006, 119, 2204–2213. [Google Scholar] [CrossRef] [Green Version]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.T.-J.; Gronthos, S.; Shi, S. Mesenchymal Stem Cells Derived from Dental Tissues vs. Those from Other Sources: Their Biology and Role in Regenerative Medicine. J. Dent. Res. 2009, 88, 792–806. [Google Scholar] [CrossRef]
- Toma, J.G.; Akhavan, M.; Fernandes, K.J.; Barnabé-Heider, F.; Sadikot, A.; Kaplan, D.R.; Miller, F.D. Isolation of Multipotent Adult Stem Cells from the Dermis of Mammalian Skin. Nat. Cell Biol. 2001, 3, 778–784. [Google Scholar] [CrossRef]
- Lee, O.K.; Kuo, T.K.; Chen, W.-M.; Lee, K.-D.; Hsieh, S.-L.; Chen, T.-H. Isolation of Multipotent Mesenchymal Stem Cells from Umbilical Cord Blood. Blood 2004, 103, 1669–1675. [Google Scholar] [CrossRef] [Green Version]
- Schofield, R. The Relationship between the Spleen Colony-Forming Cell and the Haemopoietic Stem Cell. Blood Cells 1978, 4, 7–25. [Google Scholar]
- Jones, D.L.; Wagers, A.J. No Place like Home: Anatomy and Function of the Stem Cell Niche. Nat. Rev. Mol. Cell Biol. 2008, 9, 11–21. [Google Scholar] [CrossRef]
- Pérez, L.M.; de Lucas, B.; Gálvez, B.G. Unhealthy Stem Cells: When Health Conditions Upset Stem Cell Properties. Cell. Physiol. Biochem. 2018, 46, 1999–2016. [Google Scholar] [CrossRef] [Green Version]
- Zaragosi, L.-E.; Ailhaud, G.; Dani, C. Autocrine Fibroblast Growth Factor 2 Signaling Is Critical for Self-Renewal of Human Multipotent Adipose-Derived Stem Cells. Stem Cells 2006, 24, 2412–2419. [Google Scholar] [CrossRef]
- Rider, D.A.; Dombrowski, C.; Sawyer, A.A.; Ng, G.H.B.; Leong, D.; Hutmacher, D.W.; Nurcombe, V.; Cool, S.M. Autocrine Fibroblast Growth Factor 2 Increases the Multipotentiality of Human Adipose-Derived Mesenchymal Stem Cells. Stem Cells 2008, 26, 1598–1608. [Google Scholar] [CrossRef]
- Westhauser, F.; Senger, A.-S.; Reible, B.; Moghaddam, A. In Vivo Models for the Evaluation of the Osteogenic Potency of Bone Substitutes Seeded with Mesenchymal Stem Cells of Human Origin: A Concise Review. Tissue Eng. Part C Methods 2017, 23, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Satué, M.; Schüler, C.; Ginner, N.; Erben, R.G. Intra-Articularly Injected Mesenchymal Stem Cells Promote Cartilage Regeneration, but Do Not Permanently Engraft in Distant Organs. Sci. Rep. 2019, 9, 10153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sid-Otmane, C.; Perrault, L.P.; Ly, H.Q. Mesenchymal Stem Cell Mediates Cardiac Repair through Autocrine, Paracrine and Endocrine Axes. J. Transl. Med. 2020, 18, 336. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shen, J.; Ke, K.; Gu, X. Clinical potential and current progress of mesenchymal stem cells for Parkinson’s disease: A systematic review. Neurol. Sci. 2020, 41, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, A.; Kojima, Y.; Ikarashi, S.; Seino, S.; Watanabe, Y.; Kawata, Y.; Terai, S. Clinical Trials Using Mesenchymal Stem Cells in Liver Diseases and Inflammatory Bowel Diseases. Inflamm. Regen. 2017, 37, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Meng, Y.; Han, Z.; Ye, F.; Wei, L.; Zong, C. Mesenchymal Stem Cell Therapy for Liver Disease: Full of Chances and Challenges. Cell Biosci. 2020, 10, 123. [Google Scholar] [CrossRef]
- Shin, K.O.; Ha, D.H.; Kim, J.O.; Crumrine, D.A.; Meyer, J.M.; Wakefield, J.S.; Lee, Y.; Kim, B.; Kim, S.; Kim, H.K.; et al. Exosomes from Human Adipose Tissue-Derived Mesenchymal Stem Cells Promote Epidermal Barrier Repair by Inducing de Novo Synthesis of Ceramides in Atopic Dermatitis. Cells 2020, 9, 680. [Google Scholar] [CrossRef] [Green Version]
- Di Nicola, M.; Carlo-Stella, C.; Magni, M.; Milanesi, M.; Longoni, P.D.; Matteucci, P.; Grisanti, S.; Gianni, A.M. Human Bone Marrow Stromal Cells Suppress T-Lymphocyte Proliferation Induced by Cellular or Nonspecific Mitogenic Stimuli. Blood 2002, 99, 3838–3843. [Google Scholar] [CrossRef]
- Angoulvant, D.; Clerc, A.; Benchalal, S.; Galambrun, C.; Farre, A.; Bertrand, Y.; Eljaafari, A. Human Mesenchymal Stem Cells Suppress Induction of Cytotoxic Response to Alloantigens. Biorheology 2004, 41, 469–476. [Google Scholar]
- Aggarwal, S.; Pittenger, M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005, 105, 1815–1822. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.-P.; Paczesny, S.; Lauret, E.; Poirault, S.; Bordigoni, P.; Mekhloufi, F.; Hequet, O.; Bertrand, Y.; Ou-Yang, J.-P.; Stoltz, J.-F.; et al. Human Mesenchymal Stem Cells License Adult CD34+ Hemopoietic Progenitor Cells to Differentiate into Regulatory Dendritic Cells through Activation of the Notch Pathway. J. Immunol. 2008, 180, 1598–1608. [Google Scholar] [CrossRef] [Green Version]
- Corcione, A.; Benvenuto, F.; Ferretti, E.; Giunti, D.; Cappiello, V.; Cazzanti, F.; Risso, M.; Gualandi, F.; Mancardi, G.L.; Pistoia, V.; et al. Human Mesenchymal Stem Cells Modulate B-Cell Functions. Blood 2006, 107, 367–372. [Google Scholar] [CrossRef]
- Bartholomew, A.; Sturgeon, C.; Siatskas, M.; Ferrer, K.; McIntosh, K.; Patil, S.; Hardy, W.; Devine, S.; Ucker, D.; Deans, R.; et al. Mesenchymal Stem Cells Suppress Lymphocyte Proliferation in Vitro and Prolong Skin Graft Survival in Vivo. Exp. Hematol. 2002, 30, 42–48. [Google Scholar] [CrossRef]
- Cohen, J.A. Mesenchymal Stem Cell Transplantation in Multiple Sclerosis. J. Neurol. Sci. 2013, 333, 43–49. [Google Scholar] [CrossRef] [Green Version]
- González, M.A.; Gonzalez–Rey, E.; Rico, L.; Büscher, D.; Delgado, M. Adipose-Derived Mesenchymal Stem Cells Alleviate Experimental Colitis by Inhibiting Inflammatory and Autoimmune Responses. Gastroenterology 2009, 136, 978–989. [Google Scholar] [CrossRef]
- Baron, F.; Storb, R. Mesenchymal Stromal Cells: A New Tool against Graft-versus-Host Disease? Biol. Blood Marrow Transpl. 2012, 18, 822–840. [Google Scholar] [CrossRef] [Green Version]
- Madec, A.M.; Mallone, R.; Afonso, G.; Abou Mrad, E.; Mesnier, A.; Eljaafari, A.; Thivolet, C. Mesenchymal Stem Cells Protect NOD Mice from Diabetes by Inducing Regulatory T Cells. Diabetologia 2009, 52, 1391–1399. [Google Scholar] [CrossRef] [Green Version]
- Le Blanc, K.; Rasmusson, I.; Sundberg, B.; Götherström, C.; Hassan, M.; Uzunel, M.; Ringdén, O. Treatment of Severe Acute Graft-versus-Host Disease with Third Party Haploidentical Mesenchymal Stem Cells. Lancet 2004, 363, 1439–1441. [Google Scholar] [CrossRef]
- Ringden, O.; Le Blanc, K. Mesenchymal Stem Cells for Treatment of Acute and Chronic Graft-versus-Host Disease, Tissue Toxicity and Hemorrhages. Best Pract. Res. Clin. Haematol. 2011, 24, 65–72. [Google Scholar] [CrossRef]
- Carlsson, P.-O.; Schwarcz, E.; Korsgren, O.; Le Blanc, K. Preserved β-Cell Function in Type 1 Diabetes by Mesenchymal Stromal Cells. Diabetes 2015, 64, 587–592. [Google Scholar] [CrossRef] [Green Version]
- Dantas, J.R.; Araújo, D.B.; Silva, K.R.; Souto, D.L.; de Fátima Carvalho Pereira, M.; Luiz, R.R.; Dos Santos Mantuano, M.; Claudio-da-Silva, C.; Gabbay, M.A.L.; Dib, S.A.; et al. Adipose Tissue-Derived Stromal/Stem Cells + Cholecalciferol: A Pilot Study in Recent-Onset Type 1 Diabetes Patients. Arch. Endocrinol. Metab. 2021, 65, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Shen, S.-M.; Ling, Q.; Wang, B.; Li, L.-R.; Zhang, W.; Qu, D.-D.; Bi, Y.; Zhu, D.-L. One Repeated Transplantation of Allogeneic Umbilical Cord Mesenchymal Stromal Cells in Type 1 Diabetes: An Open Parallel Controlled Clinical Study. Stem Cell Res. Ther. 2021, 12, 340. [Google Scholar] [CrossRef] [PubMed]
- Shadmanfar, S.; Labibzadeh, N.; Emadedin, M.; Jaroughi, N.; Azimian, V.; Mardpour, S.; Kakroodi, F.A.; Bolurieh, T.; Hosseini, S.E.; Chehrazi, M.; et al. Intra-Articular Knee Implantation of Autologous Bone Marrow-Derived Mesenchymal Stromal Cells in Rheumatoid Arthritis Patients with Knee Involvement: Results of a Randomized, Triple-Blind, Placebo-Controlled Phase 1/2 Clinical Trial. Cytotherapy 2018, 20, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Álvaro-Gracia, J.M.; Jover, J.A.; García-Vicuña, R.; Carreño, L.; Alonso, A.; Marsal, S.; Blanco, F.; Martínez-Taboada, V.M.; Taylor, P.; Martín-Martín, C.; et al. Intravenous Administration of Expanded Allogeneic Adipose-Derived Mesenchymal Stem Cells in Refractory Rheumatoid Arthritis (Cx611): Results of a Multicentre, Dose Escalation, Randomised, Single-Blind, Placebo-Controlled Phase Ib/IIa Clinical Trial. Ann. Rheum. Dis. 2017, 76, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Kamen, D.L.; Wallace, C.; Li, Z.; Wyatt, M.; Paulos, C.; Wei, C.; Wang, H.; Wolf, B.J.; Nietert, P.J.; Gilkeson, G. Safety, Immunological Effects and Clinical Response in a Phase I Trial of Umbilical Cord Mesenchymal Stromal Cells in Patients with Treatment Refractory SLE. Lupus Sci. Med. 2022, 9, e000704. [Google Scholar] [CrossRef]
- Wang, D.; Li, J.; Zhang, Y.; Zhang, M.; Chen, J.; Li, X.; Hu, X.; Jiang, S.; Shi, S.; Sun, L. Umbilical Cord Mesenchymal Stem Cell Transplantation in Active and Refractory Systemic Lupus Erythematosus: A Multicenter Clinical Study. Arthritis Res. Ther. 2014, 16, R79. [Google Scholar] [CrossRef] [Green Version]
- Zang, L.; Li, Y.; Hao, H.; Liu, J.; Cheng, Y.; Li, B.; Yin, Y.; Zhang, Q.; Gao, F.; Wang, H.; et al. Efficacy and Safety of Umbilical Cord-Derived Mesenchymal Stem Cells in Chinese Adults with Type 2 Diabetes: A Single-Center, Double-Blinded, Randomized, Placebo-Controlled Phase II Trial. Stem Cell Res. Ther. 2022, 13, 180. [Google Scholar] [CrossRef]
- Wang, H.; Strange, C.; Nietert, P.J.; Wang, J.; Turnbull, T.L.; Cloud, C.; Owczarski, S.; Shuford, B.; Duke, T.; Gilkeson, G.; et al. Autologous Mesenchymal Stem Cell and Islet Cotransplantation: Safety and Efficacy. Stem Cells Transl. Med. 2018, 7, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Lonardi, R.; Leone, N.; Gennai, S.; Trevisi Borsari, G.; Covic, T.; Silingardi, R. Autologous Micro-Fragmented Adipose Tissue for the Treatment of Diabetic Foot Minor Amputations: A Randomized Controlled Single-Center Clinical Trial (MiFrAADiF). Stem Cell Res. Ther. 2019, 10, 223. [Google Scholar] [CrossRef] [Green Version]
- Skyler, J.S.; Fonseca, V.A.; Segal, K.R.; Rosenstock, J. MSB-DM003 Investigators Allogeneic Mesenchymal Precursor Cells in Type 2 Diabetes: A Randomized, Placebo-Controlled, Dose-Escalation Safety and Tolerability Pilot Study. Diabetes Care 2015, 38, 1742–1749. [Google Scholar] [CrossRef] [Green Version]
- Bhansali, S.; Dutta, P.; Yadav, M.K.; Jain, A.; Mudaliar, S.; Hawkins, M.; Kurpad, A.V.; Pahwa, D.; Yadav, A.K.; Sharma, R.R.; et al. Autologous Bone Marrow-Derived Mononuclear Cells Transplantation in Type 2 Diabetes Mellitus: Effect on β-Cell Function and Insulin Sensitivity. Diabetol. Metab. Syndr. 2017, 9, 50. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-W.; Lee, S.-H.; Youn, Y.-J.; Ahn, M.-S.; Kim, J.-Y.; Yoo, B.-S.; Yoon, J.; Kwon, W.; Hong, I.-S.; Lee, K.; et al. A Randomized, Open-Label, Multicenter Trial for the Safety and Efficacy of Adult Mesenchymal Stem Cells after Acute Myocardial Infarction. J. Korean Med. Sci. 2014, 29, 23–31. [Google Scholar] [CrossRef]
- Zhang, R.; Yu, J.; Zhang, N.; Li, W.; Wang, J.; Cai, G.; Chen, Y.; Yang, Y.; Liu, Z. Bone Marrow Mesenchymal Stem Cells Transfer in Patients with ST-Segment Elevation Myocardial Infarction: Single-Blind, Multicenter, Randomized Controlled Trial. Stem Cell Res. Ther. 2021, 12, 33. [Google Scholar] [CrossRef]
- Guijarro, D.; Lebrin, M.; Lairez, O.; Bourin, P.; Piriou, N.; Pozzo, J.; Lande, G.; Berry, M.; Le Tourneau, T.; Cussac, D.; et al. Intramyocardial Transplantation of Mesenchymal Stromal Cells for Chronic Myocardial Ischemia and Impaired Left Ventricular Function: Results of the MESAMI 1 Pilot Trial. Int. J. Cardiol. 2016, 209, 258–265. [Google Scholar] [CrossRef] [Green Version]
- Chullikana, A.; Majumdar, A.S.; Gottipamula, S.; Krishnamurthy, S.; Kumar, A.S.; Prakash, V.S.; Gupta, P.K. Randomized, Double-Blind, Phase I/II Study of Intravenous Allogeneic Mesenchymal Stromal Cells in Acute Myocardial Infarction. Cytotherapy 2015, 17, 250–261. [Google Scholar] [CrossRef]
- Florea, V.; Rieger, A.C.; DiFede, D.L.; El-Khorazaty, J.; Natsumeda, M.; Banerjee, M.N.; Tompkins, B.A.; Khan, A.; Schulman, I.H.; Landin, A.M.; et al. Dose Comparison Study of Allogeneic Mesenchymal Stem Cells in Patients With Ischemic Cardiomyopathy (The TRIDENT Study). Circ. Res. 2017, 121, 1279–1290. [Google Scholar] [CrossRef]
- Hess, D.C.; Wechsler, L.R.; Clark, W.M.; Savitz, S.I.; Ford, G.A.; Chiu, D.; Yavagal, D.R.; Uchino, K.; Liebeskind, D.S.; Auchus, A.P.; et al. Safety and Efficacy of Multipotent Adult Progenitor Cells in Acute Ischaemic Stroke (MASTERS): A Randomised, Double-Blind, Placebo-Controlled, Phase 2 Trial. Lancet Neurol. 2017, 16, 360–368. [Google Scholar] [CrossRef]
- Bartolucci, J.; Verdugo, F.J.; González, P.L.; Larrea, R.E.; Abarzua, E.; Goset, C.; Rojo, P.; Palma, I.; Lamich, R.; Pedreros, P.A.; et al. Safety and Efficacy of the Intravenous Infusion of Umbilical Cord Mesenchymal Stem Cells in Patients With Heart Failure: A Phase 1/2 Randomized Controlled Trial (RIMECARD Trial [Randomized Clinical Trial of Intravenous Infusion Umbilical Cord Mesenchymal Stem Cells on Cardiopathy]). Circ. Res. 2017, 121, 1192–1204. [Google Scholar] [CrossRef]
- Jaillard, A.; Hommel, M.; Moisan, A.; Zeffiro, T.A.; Favre-Wiki, I.M.; Barbieux-Guillot, M.; Vadot, W.; Marcel, S.; Lamalle, L.; Grand, S.; et al. Autologous Mesenchymal Stem Cells Improve Motor Recovery in Subacute Ischemic Stroke: A Randomized Clinical Trial. Transl. Stroke Res. 2020, 11, 910–923. [Google Scholar] [CrossRef]
- Kebriaei, P.; Hayes, J.; Daly, A.; Uberti, J.; Marks, D.I.; Soiffer, R.; Waller, E.K.; Burke, E.; Skerrett, D.; Shpall, E.; et al. A Phase 3 Randomized Study of Remestemcel-L versus Placebo Added to Second-Line Therapy in Patients with Steroid-Refractory Acute Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2020, 26, 835–844. [Google Scholar] [CrossRef]
- Kurtzberg, J.; Abdel-Azim, H.; Carpenter, P.; Chaudhury, S.; Horn, B.; Mahadeo, K.; Nemecek, E.; Neudorf, S.; Prasad, V.; Prockop, S.; et al. A Phase 3, Single-Arm, Prospective Study of Remestemcel-L, Ex Vivo Culture-Expanded Adult Human Mesenchymal Stromal Cells for the Treatment of Pediatric Patients Who Failed to Respond to Steroid Treatment for Acute Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2020, 26, 845–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, F.; Battiwalla, M.; Ito, S.; Feng, X.; Chinian, F.; Melenhorst, J.J.; Koklanaris, E.; Sabatino, M.; Stroncek, D.; Samsel, L.; et al. Bone Marrow Mesenchymal Stromal Cells to Treat Tissue Damage in Allogeneic Stem Cell Transplant Recipients: Correlation of Biological Markers with Clinical Responses. Stem Cells 2014, 32, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Molendijk, I.; Bonsing, B.A.; Roelofs, H.; Peeters, K.C.M.J.; Wasser, M.N.J.M.; Dijkstra, G.; van der Woude, C.J.; Duijvestein, M.; Veenendaal, R.A.; Zwaginga, J.-J.; et al. Allogeneic Bone Marrow-Derived Mesenchymal Stromal Cells Promote Healing of Refractory Perianal Fistulas in Patients With Crohn’s Disease. Gastroenterology 2015, 149, 918–927.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Olmo, D.; Gilaberte, I.; Binek, M.; D Hoore, A.J.L.; Lindner, D.; Selvaggi, F.; Spinelli, A.; Panés, J. Follow-up Study to Evaluate the Long-Term Safety and Efficacy of Darvadstrocel (Mesenchymal Stem Cell Treatment) in Patients With Perianal Fistulizing Crohn’s Disease: ADMIRE-CD Phase 3 Randomized Controlled Trial. Dis. Colon Rectum 2022, 65, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Reinders, M.E.J.; de Fijter, J.W.; Roelofs, H.; Bajema, I.M.; de Vries, D.K.; Schaapherder, A.F.; Claas, F.H.J.; van Miert, P.P.M.C.; Roelen, D.L.; van Kooten, C.; et al. Autologous Bone Marrow-Derived Mesenchymal Stromal Cells for the Treatment of Allograft Rejection after Renal Transplantation: Results of a Phase I Study. Stem Cells Transl. Med. 2013, 2, 107–111. [Google Scholar] [CrossRef]
- Tan, J.; Wu, W.; Xu, X.; Liao, L.; Zheng, F.; Messinger, S.; Sun, X.; Chen, J.; Yang, S.; Cai, J.; et al. Induction Therapy with Autologous Mesenchymal Stem Cells in Living-Related Kidney Transplants: A Randomized Controlled Trial. JAMA 2012, 307, 1169–1177. [Google Scholar] [CrossRef] [Green Version]
- Petrou, P.; Kassis, I.; Yaghmour, N.E.; Ginzberg, A.; Karussis, D. A Phase II Clinical Trial with Repeated Intrathecal Injections of Autologous Mesenchymal Stem Cells in Patients with Amyotrophic Lateral Sclerosis. Front. Biosci. Landmark 2021, 26, 693–706. [Google Scholar] [CrossRef]
- Nabavi, S.M.; Arab, L.; Jarooghi, N.; Bolurieh, T.; Abbasi, F.; Mardpour, S.; Azimyian, V.; Moeininia, F.; Maroufizadeh, S.; Sanjari, L.; et al. Safety, Feasibility of Intravenous and Intrathecal Injection of Autologous Bone Marrow Derived Mesenchymal Stromal Cells in Patients with Amyotrophic Lateral Sclerosis: An Open Label Phase I Clinical Trial. Cell J. 2019, 20, 592–598. [Google Scholar] [CrossRef]
- Petrou, P.; Kassis, I.; Ginzberg, A.; Halimi, M.; Yaghmour, N.; Abramsky, O.; Karussis, D. Long-Term Clinical and Immunological Effects of Repeated Mesenchymal Stem Cell Injections in Patients With Progressive Forms of Multiple Sclerosis. Front. Neurol. 2021, 12, 639315. [Google Scholar] [CrossRef]
- Cohen, J.A.; Imrey, P.B.; Planchon, S.M.; Bermel, R.A.; Fisher, E.; Fox, R.J.; Bar-Or, A.; Sharp, S.L.; Skaramagas, T.T.; Jagodnik, P.; et al. Pilot Trial of Intravenous Autologous Culture-Expanded Mesenchymal Stem Cell Transplantation in Multiple Sclerosis. Mult. Scler. J. 2018, 24, 501–511. [Google Scholar] [CrossRef] [Green Version]
- Riordan, N.H.; Morales, I.; Fernández, G.; Allen, N.; Fearnot, N.E.; Leckrone, M.E.; Markovich, D.J.; Mansfield, D.; Avila, D.; Patel, A.N.; et al. Clinical Feasibility of Umbilical Cord Tissue-Derived Mesenchymal Stem Cells in the Treatment of Multiple Sclerosis. J. Transl. Med. 2018, 16, 57. [Google Scholar] [CrossRef] [Green Version]
- Harris, V.K.; Stark, J.W.; Yang, S.; Zanker, S.; Tuddenham, J.; Sadiq, S.A. Mesenchymal Stem Cell-Derived Neural Progenitors in Progressive MS: Two-Year Follow-up of a Phase I Study. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e928. [Google Scholar] [CrossRef]
- Karussis, D.; Karageorgiou, C.; Vaknin-Dembinsky, A.; Gowda-Kurkalli, B.; Gomori, J.M.; Kassis, I.; Bulte, J.W.M.; Petrou, P.; Ben-Hur, T.; Abramsky, O.; et al. Safety and Immunological Effects of Mesenchymal Stem Cell Transplantation in Patients with Multiple Sclerosis and Amyotrophic Lateral Sclerosis. Arch. Neurol. 2010, 67, 1187–1194. [Google Scholar] [CrossRef]
- Uccelli, A.; Laroni, A.; Brundin, L.; Clanet, M.; Fernandez, O.; Nabavi, S.M.; Muraro, P.A.; Oliveri, R.S.; Radue, E.W.; Sellner, J.; et al. MEsenchymal StEm Cells for Multiple Sclerosis (MESEMS): A Randomized, Double Blind, Cross-over Phase I/II Clinical Trial with Autologous Mesenchymal Stem Cells for the Therapy of Multiple Sclerosis. Trials 2019, 20, 263. [Google Scholar] [CrossRef] [Green Version]
- Llufriu, S.; Sepúlveda, M.; Blanco, Y.; Marín, P.; Moreno, B.; Berenguer, J.; Gabilondo, I.; Martínez-Heras, E.; Sola-Valls, N.; Arnaiz, J.-A.; et al. Randomized Placebo-Controlled Phase II Trial of Autologous Mesenchymal Stem Cells in Multiple Sclerosis. PLoS ONE 2014, 9, e113936. [Google Scholar] [CrossRef]
- Petrou, P.; Kassis, I.; Ginzberg, A.; Hallimi, M.; Karussis, D. Effects of Mesenchymal Stem Cell Transplantation on Cerebrospinal Fluid Biomarkers in Progressive Multiple Sclerosis. Stem Cells Transl. Med. 2022, 11, 55–58. [Google Scholar] [CrossRef]
- Zhu, Y.-G.; Shi, M.-M.; Monsel, A.; Dai, C.-X.; Dong, X.; Shen, H.; Li, S.-K.; Chang, J.; Xu, C.-L.; Li, P.; et al. Nebulized Exosomes Derived from Allogenic Adipose Tissue Mesenchymal Stromal Cells in Patients with Severe COVID-19: A Pilot Study. Stem Cell Res. Ther. 2022, 13, 220. [Google Scholar] [CrossRef]
- Karyana, M.; Djaharuddin, I.; Rif’ati, L.; Arif, M.; Choi, M.K.; Angginy, N.; Yoon, A.; Han, J.; Josh, F.; Arlinda, D.; et al. Safety of DW-MSC Infusion in Patients with Low Clinical Risk COVID-19 Infection: A Randomized, Double-Blind, Placebo-Controlled Trial. Stem Cell Res. Ther. 2022, 13, 134. [Google Scholar] [CrossRef]
- Fathi-Kazerooni, M.; Fattah-Ghazi, S.; Darzi, M.; Makarem, J.; Nasiri, R.; Salahshour, F.; Dehghan-Manshadi, S.A.; Kazemnejad, S. Safety and Efficacy Study of Allogeneic Human Menstrual Blood Stromal Cells Secretome to Treat Severe COVID-19 Patients: Clinical Trial Phase I & II. Stem Cell Res. Ther. 2022, 13, 96. [Google Scholar] [CrossRef]
- Le Blanc, K.; Tammik, C.; Rosendahl, K.; Zetterberg, E.; Ringdén, O. HLA Expression and Immunologic Properties of Differentiated and Undifferentiated Mesenchymal Stem Cells. Exp. Hematol. 2003, 31, 890–896. [Google Scholar] [CrossRef]
- Jarvinen, L.; Badri, L.; Wettlaufer, S.; Ohtsuka, T.; Standiford, T.J.; Toews, G.B.; Pinsky, D.J.; Peters-Golden, M.; Lama, V.N. Lung Resident Mesenchymal Stem Cells Isolated from Human Lung Allografts Inhibit T Cell Proliferation via a Soluble Mediator. J. Immunol. 2008, 181, 4389–4396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.; Cao, K.; Liu, K.; Xue, Y.; Roberts, A.I.; Li, F.; Han, Y.; Rabson, A.B.; Wang, Y.; Shi, Y. Kynurenic Acid, an IDO Metabolite, Controls TSG-6-Mediated Immunosuppression of Human Mesenchymal Stem Cells. Cell Death Differ. 2018, 25, 1209–1223. [Google Scholar] [CrossRef] [PubMed]
- Munn, D.H.; Mellor, A.L. Indoleamine 2,3 Dioxygenase and Metabolic Control of Immune Responses. Trends Immunol. 2013, 34, 137–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mezrich, J.D.; Fechner, J.H.; Zhang, X.; Johnson, B.P.; Burlingham, W.J.; Bradfield, C.A. An Interaction between Kynurenine and the Aryl Hydrocarbon Receptor Can Generate Regulatory T Cells. J. Immunol. 2010, 185, 3190–3198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frumento, G.; Rotondo, R.; Tonetti, M.; Damonte, G.; Benatti, U.; Ferrara, G.B. Tryptophan-Derived Catabolites Are Responsible for Inhibition of T and Natural Killer Cell Proliferation Induced by Indoleamine 2,3-Dioxygenase. J. Exp. Med. 2002, 196, 459–468. [Google Scholar] [CrossRef] [Green Version]
- Sato, K.; Ozaki, K.; Oh, I.; Meguro, A.; Hatanaka, K.; Nagai, T.; Muroi, K.; Ozawa, K. Nitric Oxide Plays a Critical Role in Suppression of T-Cell Proliferation by Mesenchymal Stem Cells. Blood 2007, 109, 228–234. [Google Scholar] [CrossRef]
- Chen, P.-M.; Liu, K.-J.; Hsu, P.-J.; Wei, C.-F.; Bai, C.-H.; Ho, L.-J.; Sytwu, H.-K.; Yen, B.L. Induction of Immunomodulatory Monocytes by Human Mesenchymal Stem Cell-Derived Hepatocyte Growth Factor through ERK1/2. J. Leukoc. Biol. 2014, 96, 295–303. [Google Scholar] [CrossRef]
- Nemeth, K.; Keane-Myers, A.; Brown, J.M.; Metcalfe, D.D.; Gorham, J.D.; Gorham, J.D.; Bundoc, V.G.; Bundoc, V.G.; Hodges, M.G.; Jelinek, I.; et al. Bone Marrow Stromal Cells Use TGF-Beta to Suppress Allergic Responses in a Mouse Model of Ragweed-Induced Asthma. Proc. Natl. Acad. Sci. USA 2010, 107, 5652–5657. [Google Scholar] [CrossRef] [Green Version]
- Luz-Crawford, P.; Djouad, F.; Toupet, K.; Bony, C.; Franquesa, M.; Hoogduijn, M.J.; Jorgensen, C.; Noël, D. Mesenchymal Stem Cell-Derived Interleukin 1 Receptor Antagonist Promotes Macrophage Polarization and Inhibits B Cell Differentiation. Stem Cells 2016, 34, 483–492. [Google Scholar] [CrossRef] [Green Version]
- Ren, G.; Zhao, X.; Zhang, L.; Zhang, J.; L’Huillier, A.; Ling, W.; Roberts, A.I.; Le, A.D.; Shi, S.; Shao, C. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J. Immunol. 2010, 184, 2321–2328. [Google Scholar] [CrossRef] [Green Version]
- Gieseke, F.; Böhringer, J.; Bussolari, R.; Dominici, M.; Handgretinger, R.; Müller, I. Human Multipotent Mesenchymal Stromal Cells Use Galectin-1 to Inhibit Immune Effector Cells. Blood 2010, 116, 3770–3779. [Google Scholar] [CrossRef]
- He, J.; Baum, L.G. Presentation of Galectin-1 by Extracellular Matrix Triggers T Cell Death. J. Biol. Chem. 2004, 279, 4705–4712. [Google Scholar] [CrossRef]
- Kim, N.; Kim, H.S. Targeting Checkpoint Receptors and Molecules for Therapeutic Modulation of Natural Killer Cells. Front. Immunol. 2018, 9, 2041. [Google Scholar] [CrossRef]
- Baumeister, S.H.; Freeman, G.J.; Dranoff, G.; Sharpe, A.H. Coinhibitory Pathways in Immunotherapy for Cancer. Annu. Rev. Immunol. 2016, 34, 539–573. [Google Scholar] [CrossRef]
- Eljaafari, A.; Pestel, J.; Le Magueresse-Battistoni, B.; Chanon, S.; Watson, J.; Robert, M.; Disse, E.; Vidal, H. Adipose-Tissue-Derived Mesenchymal Stem Cells Mediate PD-L1 Overexpression in the White Adipose Tissue of Obese Individuals, Resulting in T Cell Dysfunction. Cells 2021, 10, 2645. [Google Scholar] [CrossRef]
- Krampera, M.; Cosmi, L.; Angeli, R.; Pasini, A.; Liotta, F.; Andreini, A.; Santarlasci, V.; Mazzinghi, B.; Pizzolo, G.; Vinante, F.; et al. Role for Interferon-γ in the Immunomodulatory Activity of Human Bone Marrow Mesenchymal Stem Cells. Stem Cells 2006, 24, 386–398. [Google Scholar] [CrossRef]
- Ren, G.; Zhang, L.; Zhao, X.; Xu, G.; Zhang, Y.; Roberts, A.I.; Zhao, R.C.; Shi, Y. Mesenchymal Stem Cell-Mediated Immunosuppression Occurs via Concerted Action of Chemokines and Nitric Oxide. Cell Stem Cell 2008, 2, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Polchert, D.; Sobinsky, J.; Douglas, G.; Kidd, M.; Moadsiri, A.; Reina, E.; Genrich, K.; Mehrotra, S.; Setty, S.; Smith, B.; et al. IFN-Gamma Activation of Mesenchymal Stem Cells for Treatment and Prevention of Graft versus Host Disease. Eur. J. Immunol. 2008, 38, 1745–1755. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Fu, L.; Liang, Y.; Guo, Z.; Wang, L.; Ma, C.; Wang, H. Exosomes Originating from MSCs Stimulated with TGF-β and IFN-γ Promote Treg Differentiation. J. Cell. Physiol. 2018, 233, 6832–6840. [Google Scholar] [CrossRef]
- Lerrer, S.; Liubomirski, Y.; Bott, A.; Abnaof, K.; Oren, N.; Yousaf, A.; Körner, C.; Meshel, T.; Wiemann, S.; Ben-Baruch, A. Co-Inflammatory Roles of TGFβ1 in the Presence of TNFα Drive a Pro-Inflammatory Fate in Mesenchymal Stem Cells. Front. Immunol. 2017, 8, 479. [Google Scholar] [CrossRef] [Green Version]
- Waterman, R.S.; Tomchuck, S.L.; Henkle, S.L.; Betancourt, A.M. A New Mesenchymal Stem Cell (MSC) Paradigm: Polarization into a Pro-Inflammatory MSC1 or an Immunosuppressive MSC2 Phenotype. PLoS ONE 2010, 5, e10088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivera-Cruz, C.M.; Figueiredo, M.L. Evaluation of Human Adipose-Derived Mesenchymal Stromal Cell Toll-like Receptor Priming and Effects on Interaction with Prostate Cancer Cells. Cytotherapy 2022, 25, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Trayhurn, P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol. Rev. 2013, 93, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, K.; Kusminski, C.M.; Scherer, P.E. Adipose tissue remodeling and obesity. J. Clin. Investig. 2011, 121, 2094–2101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maumus, M.; Sengenès, C.; Decaunes, P.; Zakaroff-Girard, A.; Bourlier, V.; Lafontan, M.; Galitzky, J.; Bouloumié, A. Evidence of in Situ Proliferation of Adult Adipose Tissue-Derived Progenitor Cells: Influence of Fat Mass Microenvironment and Growth. J. Clin. Endocrinol. Metab. 2008, 93, 4098–4106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, P.; Nuotio-Antar, A.M.; Smith, C.W. Γδ T Cells Promote Inflammation and Insulin Resistance during High Fat Diet-Induced Obesity in Mice. J. Leukoc. Biol. 2015, 97, 121–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burke, S.J.; Karlstad, M.D.; Regal, K.M.; Sparer, T.E.; Lu, D.; Elks, C.M.; Grant, R.W.; Stephens, J.M.; Burk, D.H.; Collier, J.J. CCL20 Is Elevated during Obesity and Differentially Regulated by NF-ΚB Subunits in Pancreatic β-Cells. Biochim. Biophys. Acta 2015, 1849, 637–652. [Google Scholar] [CrossRef] [Green Version]
- Caër, C.; Rouault, C.; Le Roy, T.; Poitou, C.; Aron-Wisnewsky, J.; Torcivia, A.; Bichet, J.C.; Clément, K.; Guerre-Millo, M.; André, S. Immune Cell-Derived Cytokines Contribute to Obesity-Related Inflammation, Fibrogenesis and Metabolic Deregulation in Human Adipose Tissue. Sci. Rep. 2017, 7, 3000. [Google Scholar] [CrossRef] [Green Version]
- Toubal, A.; Kiaf, B.; Beaudoin, L.; Cagninacci, L.; Rhimi, M.; Fruchet, B.; Silva, J.; Corbett, A.J.; Simoni, Y.; Lantz, O.; et al. Mucosal-Associated Invariant T Cells Promote Inflammation and Intestinal Dysbiosis Leading to Metabolic Dysfunction during Obesity. Nat. Commun. 2020, 11, 3755. [Google Scholar] [CrossRef]
- Serena, C.; Keiran, N.; Ceperuelo-Mallafre, V.; Ejarque, M.; Fradera, R.; Roche, K.; Nuñez-Roa, C.; Vendrell, J.; Fernández-Veledo, S. Obesity and Type 2 Diabetes Alters the Immune Properties of Human Adipose Derived Stem Cells: Obesity Changes the Immune Properties of Stem Cells. Stem Cells 2016, 34, 2559–2573. [Google Scholar] [CrossRef]
- Aguirre, V.; Uchida, T.; Yenush, L.; Davis, R.; White, M.F. The C-Jun NH(2)-Terminal Kinase Promotes Insulin Resistance during Association with Insulin Receptor Substrate-1 and Phosphorylation of Ser(307). J. Biol. Chem. 2000, 275, 9047–9054. [Google Scholar] [CrossRef] [Green Version]
- Silva, K.R.; Baptista, L.S. Adipose-Derived Stromal/Stem Cells from Different Adipose Depots in Obesity Development. World J. Stem Cells 2019, 11, 147–166. [Google Scholar] [CrossRef]
- Silva, K.R.; Liechocki, S.; Carneiro, J.R.; Claudio-da-Silva, C.; Maya-Monteiro, C.M.; Borojevic, R.; Baptista, L.S. Stromal-Vascular Fraction Content and Adipose Stem Cell Behavior Are Altered in Morbid Obese and Post Bariatric Surgery Ex-Obese Women. Stem Cell Res. Ther. 2015, 6, 72. [Google Scholar] [CrossRef] [Green Version]
- De Girolamo, L.; Stanco, D.; Salvatori, L.; Coroniti, G.; Arrigoni, E.; Silecchia, G.; Russo, M.A.; Niada, S.; Petrangeli, E.; Brini, A.T. Stemness and Osteogenic and Adipogenic Potential Are Differently Impaired in Subcutaneous and Visceral Adipose Derived Stem Cells (ASCs) Isolated from Obese Donors. Int. J. Immunopathol. Pharmacol. 2013, 26, 11–21. [Google Scholar] [CrossRef]
- Oñate, B.; Vilahur, G.; Camino-López, S.; Díez-Caballero, A.; Ballesta-López, C.; Ybarra, J.; Moscatiello, F.; Herrero, J.; Badimon, L. Stem Cells Isolated from Adipose Tissue of Obese Patients Show Changes in Their Transcriptomic Profile That Indicate Loss in Stemcellness and Increased Commitment to an Adipocyte-like Phenotype. BMC Genom. 2013, 14, 625. [Google Scholar] [CrossRef] [Green Version]
- Tokunaga, M.; Inoue, M.; Jiang, Y.; Barnes, R.H.; Buchner, D.A.; Chun, T.-H. Fat Depot-Specific Gene Signature and ECM Remodeling of Sca1(High) Adipose-Derived Stem Cells. Matrix Biol. 2014, 36, 28–38. [Google Scholar] [CrossRef] [Green Version]
- Harrison, M.A.A.; Wise, R.M.; Benjamin, B.P.; Hochreiner, E.M.; Mohiuddin, O.A.; Bunnell, B.A. Adipose-Derived Stem Cells from Obese Donors Polarize Macrophages and Microglia toward a Pro-Inflammatory Phenotype. Cells 2020, 10, 26. [Google Scholar] [CrossRef]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Morris, J.S.; Liu, J.; Hassan, M.M.; Day, R.S.; Bondy, M.L.; Abbruzzese, J.L. Body Mass Index and Risk, Age of Onset, and Survival in Patients with Pancreatic Cancer. JAMA 2009, 301, 2553–2562. [Google Scholar] [CrossRef] [Green Version]
- Levi, Z.; Kark, J.D.; Twig, G.; Katz, L.; Leiba, A.; Derazne, E.; Tzur, D.; Liphshitz, I.; Keinan-Boker, L.; Afek, A. Body Mass Index at Adolescence and Risk of Noncardia Gastric Cancer in a Cohort of 1.79 Million Men and Women. Cancer 2018, 124, 356–363. [Google Scholar] [CrossRef] [Green Version]
- Key, T.J.; Appleby, P.N.; Reeves, G.K.; Roddam, A.; Dorgan, J.F.; Longcope, C.; Stanczyk, F.Z.; Stephenson, H.E.; Falk, R.T.; Miller, R. Body Mass Index, Serum Sex Hormones, and Breast Cancer Risk in Postmenopausal Women. J. Natl. Cancer Inst. 2003, 95, 1218–1226. [Google Scholar] [PubMed]
- MacInnis, R.J.; English, D.R. Body Size and Composition and Prostate Cancer Risk: Systematic Review and Meta-Regression Analysis. Cancer Causes Control 2006, 17, 989–1003. [Google Scholar] [CrossRef] [PubMed]
- Renehan, A.G.; Tyson, M.; Egger, M.; Heller, R.F.; Zwahlen, M. Body-Mass Index and Incidence of Cancer: A Systematic Review and Meta-Analysis of Prospective Observational Studies. Lancet 2008, 371, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Daquinag, A.C.; Amaya-Manzanares, F.; Sirin, O.; Tseng, C.; Kolonin, M.G. Stromal Progenitor Cells from Endogenous Adipose Tissue Contribute to Pericytes and Adipocytes That Populate the Tumor Microenvironment. Cancer Res. 2012, 72, 5198–5208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, S.Q.; Cao, J.; Zhang, Q.Y.; Li, Y.Y.; Yan, Y.Q.; Yu, F.X. Adipose Tissue-Derived Stem Cells Promote Pancreatic Cancer Cell Proliferation and Invasion. Braz. J. Med. Biol. Res. 2013, 46, 758–764. [Google Scholar] [CrossRef]
- Zhao, B.-C.; Zhao, B.; Han, J.-G.; Ma, H.-C.; Wang, Z.-J. Adipose-Derived Stem Cells Promote Gastric Cancer Cell Growth, Migration and Invasion through SDF-1/CXCR4 Axis. Hepatogastroenterology 2010, 57, 1382–1389. [Google Scholar]
- Klopp, A.H.; Zhang, Y.; Solley, T.; Amaya-Manzanares, F.; Marini, F.; Andreeff, M.; Debeb, B.; Woodward, W.; Schmandt, R.; Broaddus, R. Omental Adipose Tissue–Derived Stromal Cells Promote Vascularization and Growth of Endometrial Tumors. Clin. Cancer Res. 2012, 18, 771–782. [Google Scholar] [CrossRef] [Green Version]
- Ritter, A.; Kreis, N.-N.; Hoock, S.C.; Solbach, C.; Louwen, F.; Yuan, J. Adipose Tissue-Derived Mesenchymal Stromal/Stem Cells, Obesity and the Tumor Microenvironment of Breast Cancer. Cancers 2022, 14, 3908. [Google Scholar] [CrossRef]
- Jotzu, C.; Alt, E.; Welte, G.; Li, J.; Hennessy, B.T.; Devarajan, E.; Krishnappa, S.; Pinilla, S.; Droll, L.; Song, Y.-H. Adipose Tissue-Derived Stem Cells Differentiate into Carcinoma-Associated Fibroblast-like Cells under the Influence of Tumor-Derived Factors. Anal. Cell. Pathol. 2010, 33, 61–79. [Google Scholar] [CrossRef]
- Cho, J.A.; Park, H.; Lim, E.H.; Kim, K.H.; Choi, J.S.; Lee, J.H.; Shin, J.W.; Lee, K.W. Exosomes from Ovarian Cancer Cells Induce Adipose Tissue-Derived Mesenchymal Stem Cells to Acquire the Physical and Functional Characteristics of Tumor-Supporting Myofibroblasts. Gynecol. Oncol. 2011, 123, 379–386. [Google Scholar] [CrossRef]
- Strong, A.L.; Pei, D.T.; Hurst, C.G.; Gimble, J.M.; Burow, M.E.; Bunnell, B.A. Obesity Enhances the Conversion of Adipose-Derived Stromal/Stem Cells into Carcinoma-Associated Fibroblast Leading to Cancer Cell Proliferation and Progression to an Invasive Phenotype. Stem Cells Int. 2017, 2017, 9216502. [Google Scholar] [CrossRef] [Green Version]
- Malvi, P.; Chaube, B.; Singh, S.V.; Mohammad, N.; Vijayakumar, M.V.; Singh, S.; Chouhan, S.; Bhat, M.K. Elevated Circulatory Levels of Leptin and Resistin Impair Therapeutic Efficacy of Dacarbazine in Melanoma under Obese State. Cancer Metab. 2018, 6, 2. [Google Scholar] [CrossRef] [Green Version]
- Ellerhorst, J.A.; Diwan, A.H.; Dang, S.M.; Uffort, D.G.; Johnson, M.K.; Cooke, C.P.; Grimm, E.A. Promotion of Melanoma Growth by the Metabolic Hormone Leptin. Oncol. Rep. 2010, 23, 901–907. [Google Scholar] [CrossRef]
- Olszańska, J.; Pietraszek-Gremplewicz, K.; Nowak, D. Melanoma Progression under Obesity: Focus on Adipokines. Cancers 2021, 13, 2281. [Google Scholar] [CrossRef]
- Amjadi, F.; Javanmard, S.H.; Zarkesh-Esfahani, H.; Khazaei, M.; Narimani, M. Leptin Promotes Melanoma Tumor Growth in Mice Related to Increasing Circulating Endothelial Progenitor Cells Numbers and Plasma NO Production. J. Exp. Clin. Cancer Res. 2011, 30, 21. [Google Scholar] [CrossRef] [Green Version]
- Horiguchi, A.; Oya, M.; Shimada, T.; Uchida, A.; Marumo, K.; Murai, M. Activation of Signal Transducer and Activator of Transcription 3 in Renal Cell Carcinoma: A Study of Incidence and Its Association with Pathological Features and Clinical Outcome. J. Urol. 2002, 168, 762–765. [Google Scholar] [CrossRef]
- Strong, A.L.; Ohlstein, J.F.; Biagas, B.A.; Rhodes, L.V.; Pei, D.T.; Tucker, H.A.; Llamas, C.; Bowles, A.C.; Dutreil, M.F.; Zhang, S.; et al. Leptin Produced by Obese Adipose Stromal/Stem Cells Enhances Proliferation and Metastasis of Estrogen Receptor Positive Breast Cancers. Breast Cancer Res. 2015, 17, 112. [Google Scholar] [CrossRef] [Green Version]
- Lo, J.C.; Ljubicic, S.; Leibiger, B.; Kern, M.; Leibiger, I.B.; Moede, T.; Kelly, M.E.; Bhowmick, D.C.; Murano, I.; Cohen, P.; et al. Adipsin Is an Adipokine That Improves β Cell Function in Diabetes. Cell 2014, 158, 41–53. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Banoy, N.; Guseh, J.S.; Li, G.; Rubio-Navarro, A.; Chen, T.; Poirier, B.; Putzel, G.; Rosselot, C.; Pabón, M.A.; Camporez, J.P.; et al. Adipsin Preserves Beta Cells in Diabetic Mice and Associates with Protection from Type 2 Diabetes in Humans. Nat. Med. 2019, 25, 1739–1747. [Google Scholar] [CrossRef]
- Goto, H.; Shimono, Y.; Funakoshi, Y.; Imamura, Y.; Toyoda, M.; Kiyota, N.; Kono, S.; Takao, S.; Mukohara, T.; Minami, H. Adipose-Derived Stem Cells Enhance Human Breast Cancer Growth and Cancer Stem Cell-like Properties through Adipsin. Oncogene 2019, 38, 767–779. [Google Scholar] [CrossRef]
- Benaiges, E.; Ceperuelo-Mallafré, V.; Madeira, A.; Bosch, R.; Núñez-Roa, C.; Ejarque, M.; Maymó-Masip, E.; Huber-Ruano, I.; Lejeune, M.; Vendrell, J.; et al. Survivin drives tumor-associated macrophage reprogramming: A novel mechanism with potential impact for obesity. Cell Oncol. 2021, 44, 777–792. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yi, T.; Kortylewski, M.; Pardoll, D.M.; Zeng, D.; Yu, H. IL-17 Can Promote Tumor Growth through an IL-6-Stat3 Signaling Pathway. J. Exp. Med. 2009, 206, 1457–1464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bank, I. Obesity and Cancer: The Gammadelta T Cell Link. Explor. Immunol. 2022, 2, 320–333. [Google Scholar] [CrossRef]
- Dalmas, E.; Venteclef, N.; Caer, C.; Poitou, C.; Cremer, I.; Aron-Wisnewsky, J.; Lacroix-Desmazes, S.; Bayry, J.; Kaveri, S.V.; Clément, K.; et al. T Cell–Derived IL-22 Amplifies IL-1b–Driven Inflammation in Human Adipose Tissue: Relevance to Obesity and Type 2 Diabetes. Diabetes 2014, 63, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Kolonin, M.G. Cytokine Signaling Regulating Adipose Stromal Cell Trafficking. Adipocyte 2016, 5, 369–374. [Google Scholar] [CrossRef] [Green Version]
- Mani, S.A.; Guo, W.; Liao, M.-J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The Epithelial-Mesenchymal Transition Generates Cells with Properties of Stem Cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef] [Green Version]
- Su, F.; Daquinag, A.C.; Ahn, S.; Saha, A.; Dai, Y.; Zhao, Z.; DiGiovanni, J.; Kolonin, M.G. Progression of Prostate Carcinoma Is Promoted by Adipose Stromal Cell-Secreted CXCL12 Signaling in Prostate Epithelium. Npj Precis. Oncol. 2021, 5, 26. [Google Scholar] [CrossRef]
- Su, F.; Ahn, S.; Saha, A.; DiGiovanni, J.; Kolonin, M.G. Adipose Stromal Cell Targeting Suppresses Prostate Cancer Epithelial-Mesenchymal Transition and Chemoresistance. Oncogene 2019, 38, 1979–1988. [Google Scholar] [CrossRef]
- Komiyama, Y.; Nakae, S.; Matsuki, T.; Nambu, A.; Ishigame, H.; Kakuta, S.; Sudo, K.; Iwakura, Y. IL-17 Plays an Important Role in the Development of Experimental Autoimmune Encephalomyelitis. J. Immunol. 2006, 177, 566–573. [Google Scholar] [CrossRef] [Green Version]
- Langrish, C.L.; Chen, Y.; Blumenschein, W.M.; Mattson, J.; Basham, B.; Sedgwick, J.D.; McClanahan, T.; Kastelein, R.A.; Cua, D.J. IL-23 Drives a Pathogenic T Cell Population That Induces Autoimmune Inflammation. J. Exp. Med. 2005, 201, 233–240. [Google Scholar] [CrossRef] [Green Version]
- McGinley, A.M.; Sutton, C.E.; Edwards, S.C.; Leane, C.M.; DeCourcey, J.; Teijeiro, A.; Hamilton, J.A.; Boon, L.; Djouder, N.; Mills, K.H.G. Interleukin-17A Serves a Priming Role in Autoimmunity by Recruiting IL-1β-Producing Myeloid Cells That Promote Pathogenic T Cells. Immunity 2020, 52, 342–356.e6. [Google Scholar] [CrossRef]
- Gianfrancesco, M.A.; Barcellos, L.F. Obesity and Multiple Sclerosis Susceptibility: A Review. J. Neurol. Neuromed. 2016, 1, 1–5. [Google Scholar] [CrossRef]
- Huppke, B.; Ellenberger, D.; Hummel, H.; Stark, W.; Röbl, M.; Gärtner, J.; Huppke, P. Association of Obesity With Multiple Sclerosis Risk and Response to First-Line Disease Modifying Drugs in Children. JAMA Neurol. 2019, 76, 1157–1165. [Google Scholar] [CrossRef]
- Versini, M.; Jeandel, P.-Y.; Rosenthal, E.; Shoenfeld, Y. Obesity in Autoimmune Diseases: Not a Passive Bystander. Autoimmun. Rev. 2014, 13, 981–1000. [Google Scholar] [CrossRef]
- Parikh, S.J.; Edelman, M.; Uwaifo, G.I.; Freedman, R.J.; Semega-Janneh, M.; Reynolds, J.; Yanovski, J.A. The Relationship between Obesity and Serum 1,25-Dihydroxy Vitamin D Concentrations in Healthy Adults. J. Clin. Endocrinol. Metab. 2004, 89, 1196–1199. [Google Scholar] [CrossRef] [Green Version]
- Smotkin-Tangorra, M.; Purushothaman, R.; Gupta, A.; Nejati, G.; Anhalt, H.; Ten, S. Prevalence of vitamin D insufficiency in obese children and adolescents. J. Pediatr. Endocrinol. Metab. 2007, 20, 817–823. [Google Scholar] [CrossRef]
- Munger, K.L.; Levin, L.I.; Hollis, B.W.; Howard, N.S.; Ascherio, A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA 2006, 296, 2832–2838. [Google Scholar] [CrossRef] [Green Version]
- Redondo, J.; Sarkar, P.; Kemp, K.; Virgo, P.F.; Pawade, J.; Norton, A.; Emery, D.C.; Guttridge, M.G.; Marks, D.I.; Wilkins, A.; et al. Reduced Cellularity of Bone Marrow in Multiple Sclerosis with Decreased MSC Expansion Potential and Premature Ageing in Vitro. Mult. Scler 2018, 24, 919–931. [Google Scholar] [CrossRef] [Green Version]
- Redondo, J.; Sarkar, P.; Kemp, K.; Heesom, K.J.; Wilkins, A.; Scolding, N.J.; Rice, C.M. Dysregulation of Mesenchymal Stromal Cell Antioxidant Responses in Progressive Multiple Sclerosis. Stem Cells Transl. Med. 2018, 7, 748–758. [Google Scholar] [CrossRef] [Green Version]
- Strong, A.L.; Bowles, A.C.; Wise, R.M.; Morand, J.P.; Dutreil, M.F.; Gimble, J.M.; Bunnell, B.A. Human Adipose Stromal/Stem Cells from Obese Donors Show Reduced Efficacy in Halting Disease Progression in the Experimental Autoimmune Encephalomyelitis Model of Multiple Sclerosis: Obesity Reduces the Therapeutic Efficacy of ASCs. Stem Cells 2016, 34, 614–626. [Google Scholar] [CrossRef] [Green Version]
- Yousefi, M.; Nabipour, A.; Ganjalikhani Hakemi, M.; Ashja-Arvan, M.; Amirpour, N.; Salehi, H. Transplantation of Human Adipose-Derived Stem Cells Overexpressing LIF/IFN-β Promotes Recovery in Experimental Autoimmune Encephalomyelitis (EAE). Sci. Rep. 2022, 12, 17835. [Google Scholar] [CrossRef] [PubMed]
- Ben-Sasson, S.Z.; Hu-Li, J.; Quiel, J.; Cauchetaux, S.; Ratner, M.; Shapira, I.; Dinarello, C.A.; Paul, W.E. IL-1 Acts Directly on CD4 T Cells to Enhance Their Antigen-Driven Expansion and Differentiation. Proc. Natl. Acad. Sci. USA 2009, 106, 7119–7124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michalak-Stoma, A.; Pietrzak, A.; Szepietowski, J.C.; Zalewska-Janowska, A.; Paszkowski, T.; Chodorowska, G. Cytokine Network in Psoriasis Revisited. Eur. Cytokine Netw. 2011, 22, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, J.; Zhao, Y.; Geng, L.; Song, F.; Chen, H.D. Psoriasis Is Associated with Increased Levels of Serum Leptin. Br. J. Dermatol. 2008, 158, 1134–1135. [Google Scholar] [CrossRef] [PubMed]
- Johnston, A.; Arnadottir, S.; Gudjonsson, J.E.; Aphale, A.; Sigmarsdottir, A.A.; Gunnarsson, S.I.; Steinsson, J.T.; Elder, J.T.; Valdimarsson, H. Obesity in Psoriasis: Leptin and Resistin as Mediators of Cutaneous Inflammation. Br. J. Dermatol. 2008, 159, 342–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, K.; Liu, H.; Jian, Q.; Liu, B.; Zhu, D.; Zhang, M.; Gao, L.; Li, C. Leptin Induces Secretion of Pro-Inflammatory Cytokines by Human Keratinocytes in Vitro–a Possible Reason for Increased Severity of Psoriasis in Patients with a High Body Mass Index. Exp. Dermatol. 2013, 22, 406–410. [Google Scholar] [CrossRef]
- Jensen, P.; Skov, L. Psoriasis and Obesity. Dermatology 2017, 232, 633–639. [Google Scholar] [CrossRef]
- Setty, A.R.; Curhan, G.; Choi, H.K. Obesity, Waist Circumference, Weight Change, and the Risk of Psoriasis in Women: Nurses’ Health Study II. Arch. Intern. Med. 2007, 167, 1670–1675. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, A.W.; Harskamp, C.T.; Armstrong, E.J. The Association between Psoriasis and Obesity: A Systematic Review and Meta-Analysis of Observational Studies. Nutr. Diabetes 2012, 2, e54. [Google Scholar] [CrossRef] [Green Version]
- Bryld, L.E.; Sørensen, T.I.; Andersen, K.K.; Jemec, G.B.; Baker, J.L. High body mass index in adolescent girls precedes psoriasis hospitalization. Acta Derm. Venereol. 2010, 90, 488–493. [Google Scholar] [CrossRef] [Green Version]
- Zhu, K.-J.; Zhang, C.; Li, M.; Zhu, C.-Y.; Shi, G.; Fan, Y.-M. Leptin Levels in Patients with Psoriasis: A Meta-Analysis. Clin. Exp. Dermatol. 2013, 38, 478–483. [Google Scholar] [CrossRef]
- Henno, A.; Blacher, S.; Lambert, C.; Colige, A.; Seidel, L.; Noël, A.; Lapière, C.; de la Brassinne, M.; Nusgens, B.V. Altered Expression of Angiogenesis and Lymphangiogenesis Markers in the Uninvolved Skin of Plaque-Type Psoriasis. Br. J. Dermatol. 2009, 160, 581–590. [Google Scholar] [CrossRef]
- Orciani, M.; Campanati, A.; Salvolini, E.; Lucarini, G.; Di Benedetto, G.; Offidani, A.; Di Primio, R. The Mesenchymal Stem Cell Profile in Psoriasis. Br. J. Dermatol. 2011, 165, 585–592. [Google Scholar] [CrossRef]
- Liu, R.; Wang, Y.; Zhao, X.; Yang, Y.; Zhang, K. Lymphocyte Inhibition Is Compromised in Mesenchymal Stem Cells from Psoriatic Skin. Eur. J. Dermatol. 2014, 24, 560–567. [Google Scholar] [CrossRef]
- Campanati, A.; Orciani, M.; Consales, V.; Lazzarini, R.; Ganzetti, G.; Di Benedetto, G.; Di Primio, R.; Offidani, A. Characterization and Profiling of Immunomodulatory Genes in Resident Mesenchymal Stem Cells Reflect the Th1-Th17/Th2 Imbalance of Psoriasis. Arch. Dermatol. Res. 2014, 306, 915–920. [Google Scholar] [CrossRef]
- Yao, D.; Ye, S.; He, Z.; Huang, Y.; Deng, J.; Wen, Z.; Chen, X.; Li, H.; Han, Q.; Deng, H.; et al. Adipose-Derived Mesenchymal Stem Cells (AD-MSCs) in the Treatment for Psoriasis: Results of a Single-Arm Pilot Trial. Ann. Transl. Med. 2021, 10, 1653. [Google Scholar] [CrossRef]
- Owczarczyk-Saczonek, A.; Krajewska-Włodarczyk, M.; Kruszewska, A.; Placek, W.; Maksymowicz, W.; Wojtkiewicz, J. Stem Cells as Potential Candidates for Psoriasis Cell-Replacement Therapy. Int. J. Mol. Sci. 2017, 18, 2182. [Google Scholar] [CrossRef] [Green Version]
- Cerhan, J.R.; Saag, K.G.; Criswell, L.A.; Merlino, L.A.; Mikuls, T.R. Blood Transfusion, Alcohol Use, and Anthropometric Risk Factors for Rheumatoid Arthritis in Older Women. J. Rheumatol. 2002, 29, 246–254. [Google Scholar]
- Pedersen, M.; Jacobsen, S.; Klarlund, M.; Pedersen, B.V.; Wiik, A.; Wohlfahrt, J.; Frisch, M. Environmental Risk Factors Differ between Rheumatoid Arthritis with and without Auto-Antibodies against Cyclic Citrullinated Peptides. Arthritis Res. Ther. 2006, 8, R133. [Google Scholar] [CrossRef] [Green Version]
- Crowson, C.S.; Matteson, E.L.; Davis, J.M.; Gabriel, S.E. Contribution of Obesity to the Rise in Incidence of Rheumatoid Arthritis. Arthritis Care Res. 2013, 65, 71–77. [Google Scholar] [CrossRef] [Green Version]
- Benedetti, G.; Miossec, P. Interleukin 17 Contributes to the Chronicity of Inflammatory Diseases Such as Rheumatoid Arthritis. Eur. J. Immunol. 2014, 44, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Eljaafari, A.; Tartelin, M.-L.; Aissaoui, H.; Chevrel, G.; Osta, B.; Lavocat, F.; Miossec, P. Bone Marrow-Derived and Synovium-Derived Mesenchymal Cells Promote Th17 Cell Expansion and Activation through Caspase 1 Activation: Contribution to the Chronicity of Rheumatoid Arthritis. Arthritis Rheum. 2012, 64, 2147–2157. [Google Scholar] [CrossRef] [PubMed]
- Kongdang, P.; Chokchaitaweesuk, C.; Tangyuenyong, S.; Ongchai, S. Proinflammatory Effects of IL-1β Combined with IL-17A Promoted Cartilage Degradation and Suppressed Genes Associated with Cartilage Matrix Synthesis In Vitro. Molecules 2019, 24, 3682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skalska, U.; Kuca-Warnawin, E.; Kornatka, A.; Janicka, I.; Musiałowicz, U.; Burakowski, T.; Kontny, E. Articular and Subcutaneous Adipose Tissues of Rheumatoid Arthritis Patients Represent Equal Sources of Immunoregulatory Mesenchymal Stem Cells. Autoimmunity 2017, 50, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Greuter, T.; Porchet, F.; Braga-Neto, M.B.; Rossel, J.B.; Biedermann, L.; Schreiner, P.; Scharl, M.; Schoepfer, A.M.; Safroneeva, E.; Straumann, A.; et al. Impact of Obesity on Disease Activity and Disease Outcome in Inflammatory Bowel Disease: Results from the Swiss Inflammatory Bowel Disease Cohort. United Eur. Gastroenterol. J. 2020, 8, 1196–1207. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Gonzalez, F.; Dubuquoy, L.; Rousseaux, C.; Dubuquoy, C.; Decourcelle, C.; Saudemont, A.; Tachon, M.; Béclin, E.; Odou, M.F.; et al. Mesenteric Fat as a Source of C Reactive Protein and as a Target for Bacterial Translocation in Crohn’s Disease. Gut 2012, 61, 78–85. [Google Scholar] [CrossRef]
- Mendall, M.A.; Gunasekera, A.V.; John, B.J.; Kumar, D. Is Obesity a Risk Factor for Crohn’s Disease? Dig. Dis. Sci. 2011, 56, 837–844. [Google Scholar] [CrossRef]
- Khalili, H.; Ananthakrishnan, A.N.; Konijeti, G.G.; Higuchi, L.M.; Fuchs, C.S.; Richter, J.M.; Chan, A.T. Measures of Obesity and Risk of Crohn’s Disease and Ulcerative Colitis. Inflamm. Bowel. Dis. 2015, 21, 361–368. [Google Scholar] [CrossRef] [Green Version]
- Chan, S.S.; Luben, R.; Olsen, A.; Tjonneland, A.; Kaaks, R.; Teucher, B.; Lindgren, S.; Grip, O.; Key, T.; Crowe, F.L.; et al. Body Mass Index and the Risk for Crohn’s Disease and Ulcerative Colitis: Data from a European Prospective Cohort Study (The IBD in EPIC Study. Am. J. Gastroenterol. 2013, 108, 575–582. [Google Scholar] [CrossRef]
- Ungar, B.; Kopylov, U.; Goitein, D.; Lahat, A.; Bardan, E.; Avidan, B.; Lang, A.; Maor, Y.; Eliakim, R.; Ben-Horin, S. Severe and Morbid Obesity in Crohn’s Disease Patients: Prevalence and Disease Associations. Digestion 2013, 88, 26–32. [Google Scholar] [CrossRef]
- Blain, A.; Cattan, S.; Beaugerie, L.; Carbonnel, F.; Gendre, J.P.; Cosnes, J. Crohn’s Disease Clinical Course and Severity in Obese Patients. Clin. Nutr. 2002, 21, 51–57. [Google Scholar] [CrossRef]
- Serena, C.; Keiran, N.; Madeira, A.; Maymó-Masip, E.; Ejarque, M.; Terrón-Puig, M.; Espin, E.; Martí, M.; Borruel, N.; Guarner, F.; et al. Crohn’s Disease Disturbs the Immune Properties of Human Adipose-Derived Stem Cells Related to Inflammasome Activation. Stem Cell Rep. 2017, 9, 1109–1123. [Google Scholar] [CrossRef] [Green Version]
- Frazier, T.P.; Gimble, J.M.; Devay, J.W.; Tucker, H.A.; Chiu, E.S.; Rowan, B.G. Body Mass Index Affects Proliferation and Osteogenic Differentiation of Human Subcutaneous Adipose Tissue-Derived Stem Cells. BMC Cell Biol. 2013, 14, 34. [Google Scholar] [CrossRef] [Green Version]
- Pachón-Peña, G.; Serena, C.; Ejarque, M.; Petriz, J.; Duran, X.; Oliva-Olivera, W.; Simó, R.; Tinahones, F.J.; Fernández-Veledo, S.; Vendrell, J. Obesity Determines the Immunophenotypic Profile and Functional Characteristics of Human Mesenchymal Stem Cells From Adipose Tissue. Stem Cells Transl. Med. 2016, 5, 464–475. [Google Scholar] [CrossRef]
- Patel, R.S.; Carter, G.; El Bassit, G.; Patel, A.A.; Cooper, D.R.; Murr, M.; Patel, N.A. Adipose-Derived Stem Cells from Lean and Obese Humans Show Depot Specific Differences in Their Stem Cell Markers, Exosome Contents and Senescence: Role of Protein Kinase C Delta (PKCδ) in Adipose Stem Cell Niche. Stem Cell Investig. 2016, 3, 2. [Google Scholar] [CrossRef]
- Roldan, M.; Macias-Gonzalez, M.; Garcia, R.; Tinahones, F.J.; Martin, M. Obesity Short-Circuits Stemness Gene Network in Human Adipose Multipotent Stem Cells. FASEB J. 2011, 25, 4111–4126. [Google Scholar] [CrossRef]
- Strong, A.L.; Hunter, R.S.; Jones, R.B.; Bowles, A.C.; Dutreil, M.F.; Gaupp, D.; Hayes, D.J.; Gimble, J.M.; Levi, B.; McNulty, M.A.; et al. Obesity Inhibits the Osteogenic Differentiation of Human Adipose-Derived Stem Cells. J. Transl. Med. 2016, 14, 27. [Google Scholar] [CrossRef] [Green Version]
- van Harmelen, V.; Skurk, T.; Röhrig, K.; Lee, Y.-M.; Halbleib, M.; Aprath-Husmann, I.; Hauner, H. Effect of BMI and Age on Adipose Tissue Cellularity and Differentiation Capacity in Women. Int. J. Obes. 2003, 27, 889–895. [Google Scholar] [CrossRef] [Green Version]
- Louwen, F.; Ritter, A.; Kreis, N.N.; Yuan, J. Insight into the Development of Obesity: Functional Alterations of Adipose-Derived Mesenchymal Stem Cells. Obes. Rev. 2018, 19, 888–904. [Google Scholar] [CrossRef]
- Strong, A.L.; Burow, M.E.; Gimble, J.M.; Bunnell, B.A. Concise Review: The Obesity Cancer Paradigm: Exploration of the Interactions and Crosstalk with Adipose Stem Cells. Stem Cells 2015, 33, 318–326. [Google Scholar] [CrossRef] [Green Version]
- Klomjit, N.; Conley, S.M.; Zhu, X.Y.; Sadiq, I.M.; Libai, Y.; Krier, J.D.; Ferguson, C.M.; Jordan, K.L.; Tang, H.; Lerman, A.; et al. Effects of Obesity on Reparative Function of Human Adipose Tissue-Derived Mesenchymal Stem Cells on Ischemic Murine Kidneys. Int. J. Obes. 2022, 46, 1222–1233. [Google Scholar] [CrossRef] [PubMed]
- Ritter, A.; Kreis, N.-N.; Roth, S.; Friemel, A.; Jennewein, L.; Eichbaum, C.; Solbach, C.; Louwen, F.; Yuan, J. Restoration of Primary Cilia in Obese Adipose-Derived Mesenchymal Stem Cells by Inhibiting Aurora A or Extracellular Signal-Regulated Kinase. Stem Cell Res. Ther. 2019, 10, 255. [Google Scholar] [CrossRef] [PubMed]
- Strong, A.L.; Strong, T.A.; Rhodes, L.V.; Semon, J.A.; Zhang, X.; Shi, Z.; Zhang, S.; Gimble, J.M.; Burow, M.E.; Bunnell, B.A. Obesity Associated Alterations in the Biology of Adipose Stem Cells Mediate Enhanced Tumorigenesis by Estrogen Dependent Pathways. Breast Cancer Res. 2013, 15, R102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chehimi, M.; Ward, R.; Pestel, J.; Robert, M.; Pesenti, S.; Bendridi, N.; Michalski, M.-C.; Laville, M.; Vidal, H.; Eljaafari, A. Omega-3 Polyunsaturated Fatty Acids Inhibit IL-17A Secretion through Decreased ICAM-1 Expression in T Cells Co-Cultured with Adipose-Derived Stem Cells Harvested from Adipose Tissues of Obese Subjects. Mol. Nutr. Food Res. 2019, 63, e1801148. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.-Y.; Liu, J.; Wang, Y.-L.; Liu, Y.; Shao, Y.; Han, Y.; Qin, Y.-R.; Xiao, F.-J.; Li, P.-F.; Zhao, L.-J.; et al. Adipose-Derived Mesenchymal Stem Cells Ameliorate Lipid Metabolic Disturbance in Mice. Stem Cells Transl. Med. 2016, 5, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Pan, Q.; Dong, H.; Yuan, X.; Li, Y.; Sun, Z.; Dong, X.; Wang, H. Adipose-Derived Mesenchymal Stem Cells Improve Glucose Homeostasis in High-Fat Diet-Induced Obese Mice. Stem Cell Res. Ther. 2015, 6, 208. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Santos, M.E.; Garcia-Arranz, M.; Andreu, E.J.; García-Hernández, A.M.; López-Parra, M.; Villarón, E.; Sepúlveda, P.; Fernández-Avilés, F.; García-Olmo, D.; Prosper, F.; et al. Optimization of Mesenchymal Stromal Cell (MSC) Manufacturing Processes for a Better Therapeutic Outcome. Front. Immunol. 2022, 13, 918565. [Google Scholar] [CrossRef]
- Georgiev-Hristov, T.; García-Arranz, M.; Trébol-López, J.; Barba-Recreo, P.; García-Olmo, D. Searching for the Optimal Donor for Allogenic Adipose-Derived Stem Cells: A Comprehensive Review. Pharmaceutics 2022, 14, 2338. [Google Scholar] [CrossRef]



| General Indication | Clinical Indication | Cell Source | Injection | Phase | Results (Safety/Efficacy) | Trial Number | Ref |
|---|---|---|---|---|---|---|---|
| Autoimmune disease | Type 1 Diabetes Rheumatoid Arthritis Systemic Lupus | Auto BM Allo AT Allo UC Auto BM Allo AT Auto UC Allo UC | Systemic Systemic Systemic Local Local Systemic Systemic | N.A N.A N.A 2/3 1/2 1 1/2 | Safe and clinical efficacy Safe and clinical efficacy Safe and clinical efficacy Safe and clinical efficacy Safe and trend for efficacy Safe and clinical efficacy Safe and clinical efficacy | NCT01068951 NCT03920397 X NCT01873625 NCT01663116 NCT03171194 NCT01741857 | [42] [43] [44] [45] [46] [47] [48] |
| Cardiovascular disease | Type 2 Diabetes Myocardial infarction Heart failure Ischemic stroke | Allo UC Auto BM Auto AT Allo BM Auto BM Auto BM Auto BM Auto BM Allo BM Allo BM Allo BM Allo UC Auto BM | Systemic Local Local Systemic Local Local Local Local Syst/Local Local Systemic Systemic Systemic | 2 2 N.A 1/2 2 2/3 2/3 1/2 1/2 2 2 1/2 2 | Safe and clinical efficacy Safe and clinical efficacy Safe and clinical efficacy Safe and clinical efficacy Safe and clinical efficacy Safe and clinical efficacy Safe and no efficacy Safe and clinical efficacy Safe and no efficacy Safe and clinical efficacy Safe and clinical efficacy Safe and clinical efficacy Safe and clinical efficacy | NCT02302599 NCT02384018 NCT03276312 NCT01576328 NCT01759823 NCT01392105 NCT04421274 NCT01076920 NCT00883727 NCT02013674 NCT01436487 NCT01739777 NCT00875654 | [49] [50] [51] [52] [53] [54] [55] [56] [57] [58] [59] [60] [61] |
| GvHD | Acute GvHD | Allo BM Allo BM Allo BM | Systemic Systemic Systemic | 3 3 1 | Safe and differential efficacy Safe and clinical efficacy Safe and clinical efficacy | NCT00366145 NCT02336230 X | [62] [63] [64] |
| Intestinal bowel disease | Crohn’s disease | Allo BM Allo AT | Local Local | 1/2 3 | Safe and clinical efficacy Safe and clinical remission | NCT01144962 NCT01541579 | [65] [66] |
| Organ transplantation | Kidney transplantation | Auto BM Auto BM | Systemic Systemic | 1/2 N/A | Safe and clinical efficacy Safe and clinical efficacy | NCT00734396 NCT00658073 | [67] [68] |
| Neuro-degenerative disease | Amyotrophic lateral sclerosis Multiple sclerosis | Auto BM Auto BM Auto BM Auto BM Allo UC Auto NP Auto BM Auto BM Auto BM Auto BM | Systemic Syst/Local Syst/Local Systemic Systemic Local Syst/Local Systemic Systemic Syst/Local | 1/2 1 1/2 1 1/2 1 1/2 1/2 2 2 | Safe and clinical efficacy Safe and clinical efficacy Safe and clinical efficacy Safe and unknown efficacy Safe and clinical efficacy Safe and clinical efficacy Safe and clinical efficacy Safe and clinical efficacy Safe and trend for efficacy Safe and clinical efficacy | NCT04821479 NCT01759797 NCT04823000 NCT00813969 NCT02034188 NCT01933802 NCT00781872 NCT01745783 NCT10228266 NCT02166021 | [69] [70] [71] [72] [73] [74] [75] [76] [77] [78] |
| Viral infection | SARS-CoV-2 | Allo AT Allo X Allo BM | Systemic Systemic Systemic | 1 1 1/2 | Safe and clinical efficacy Safe and no efficacy Safe and clinical efficacy | NCT04276987 NCT04535856 NCT05019287 | [79] [80] [81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pestel, J.; Blangero, F.; Eljaafari, A. Pathogenic Role of Adipose Tissue-Derived Mesenchymal Stem Cells in Obesity and Obesity-Related Inflammatory Diseases. Cells 2023, 12, 348. https://doi.org/10.3390/cells12030348
Pestel J, Blangero F, Eljaafari A. Pathogenic Role of Adipose Tissue-Derived Mesenchymal Stem Cells in Obesity and Obesity-Related Inflammatory Diseases. Cells. 2023; 12(3):348. https://doi.org/10.3390/cells12030348
Chicago/Turabian StylePestel, Julien, Ferdinand Blangero, and Assia Eljaafari. 2023. "Pathogenic Role of Adipose Tissue-Derived Mesenchymal Stem Cells in Obesity and Obesity-Related Inflammatory Diseases" Cells 12, no. 3: 348. https://doi.org/10.3390/cells12030348