Why Do Marijuana and Synthetic Cannabimimetics Induce Acute Myocardial Infarction in Healthy Young People?
Abstract
:1. Introduction
2. Endocannabinoid System in Humans and in Experimental Animals—Components and Anatomical Distribution
3. Tachycardia—General
- administration of an oromucosal spray containing THC and cannabidiol (Sativex®; [127]).
| Number (Characterization, Age in Years) | Agonist | Dose (mg) * | Application | Cardiac Effects | Comments/Suggested Mechanisms and Involvement of CB1-Rs | References |
|---|---|---|---|---|---|---|
| 10 healthy volunteers (21–33) | THC | 1–40 | cigarette | dose-dependent ↑HR; ↑BP | changes in BP better correlated to HR than to doses; antagonists not studied | [104] |
| 16 healthy volunteers (18–42) | THC | 25 | cigarette | ↑HR; ↓BP: normotensive < hypertensive persons | n.a. | [105] |
| 6 healthy volunteers (18–30) | THC | 10 | cigarette | ↑HR; ↑BP | tachycardia resulting from β-AR activation (diminished by propranolol 120 mg p.o.) | [107] |
| 10 healthy volunteers(30–40) | THC | 10 | cigarette | ↑HR; ECG: ↑amplitude and ↓width of P wave in Lead 2 and inversion of T wave in Lead 3 | tachycardia is mediated via β-ARs, since it was prevented by propranolol (40 mg/kg p.o.) but not by atropine (0.6 mg/kg s.c.) | [108] |
| 14 healthy volunteers (20–31) | THC | 6 | cigarette | ↑HR and ↑left ventricular performance (mean rate of internal diameter shortening) | tachycardia not accompanied by ↑plasma NA levels, since the respective maximal increases took place at 10 and 30 min, respectively | [110] |
| 21 experienced users of cannabis (21–45) | THC | 20–60 | 1 to 3 cigarettes | ↑HR; ↑cardiac output ↔stroke volume ↔ejection fraction | marijuana has no significant effect on myocardial contractility independent of its effect on HR | [113] |
| 91 cannabis users (19–25); double-blind, placebo-controlled, parallel-group randomized clinical trial | THC | 94 | cigarette | ↑HR; peak HR (~40 beats/min) and plasma THC concentration (~55 ng/mL) at 5 min; ↑HR different until +4 h | n.a. | [112] |
| 42 volunteers (mean age of 29); randomized, double blind, parallel group design | cannabis | 2.8% THC | cigarette | ↑HR | acute tachycardia depends on CB1-Rs since it was diminished by acute rimonabant (90 mg/kg p.o.) or its chronic application (40 mg/kg for 8 and 15 days) | [109] |
| 16 healthy volunteers (mean age of 28) | THC UR-144 | 1, 1.5 10, 20 | joints (smoking) | ↑HR, ↑BP ↑HR, ↑BP | (THC and the preferential CB2-R agonist UR-144 were administered in joints containing tobacco.) | [114] |
| 16 healthy volunteers (mean age of 30) | JWH-122 JWH-210 | 1 1.25 | joints (smoking) | ↑HR, ↑BP ↔HR, ↔BP | (compounds with high potency at CB1-/CB2-Rs, respectively) | [128] |
| 17 healthy volunteers (mean age of 27) | THC | 10 25 | smoked or vaporized | ↑HR ↑HR | THC-induced tachycardia slightly higher in the case of vaporization | [106] |
| 36 healthy volunteers (18–31) | THC | 2, 4 and 6 | inhalation by vaporizer | ↑HR in a dose-dependent manner | THC-induced tachycardia inhibited by the CB1-R antagonist AVE1625 (20, 60, 120 mg p.o.) | [116] |
| 30 healthy volunteers (18–45); double-blind, placebo-controlled, randomized, four-period six-sequence crossover study | THC | 2, 4 and 6 | inhalation by vaporizer | ↑HR | THC-induced tachycardia was inhibited by the CB1-R antagonist surinabant 20 and 60 mg p.o. | [118] |
| 12 healthy volunteers (21–27) | THC | 2, 4, 6 and 8 | vaporized | sharp ↑HR and rapid decline | THC-induced tachycardia is dose-dependent | [115] |
| 12 healthy volunteers | THC | 2, 4, 6 and 8 | vaporized | ↑HR | different sites of action for cardiac and CNS responses suggested: average population equilibration half-life for HR 8 min and for CNS 39–85 min | [117] |
| 11 frequent and 9 occasional cannabis smokers (mean age of 27 and 29, respectively) | THC | ~54 | smoked vaporized oral | ↑HR; ↑carbon monoxide ↑HR ↑HR | smoking produced higher increase in carbon monoxide compared to vaporization | [129] |
| 84 healthy volunteers (mean age of 32), naturalistic, ad libitum use | THC | average 51 average 16 | smoked or vaporized (flower cannabis), oral (edible cannabis) | ↑HR ↑HR | the flower group started with lower basal HR than the edible group at pre-use but had higher average HR at post-use | [111] |
| 16 healthy volunteers (mean age of 26) | THC CBD | 10 600 | capsules | ↑HR ↔HR | tachycardia induced by THC but not by CBD (has low affinity to CB1-Rs) | [119] |
| 14 healthy volunteers (21–45); randomized, double-blind design | nabilone Cesamet® dronabinol Marinol® | 4, 6, 8 10, 20 | capsules capsules | dose-dependent ↑HR, ↓systolic BP ↔HR, ↓systolic BP | nabilone has better bioavailability than dronabinol (THC) | [121] |
| 37 healthy volunteers (18–35) | THC | 7.5, 15 | capsules | ↑HR, ↓heart rate variability, ↔pre-ejection period, ↔BP | tachycardia results from parasympathetic inhibition; no changes in sympathetic tone | [120] |
| 9 healthy volunteers (mean age of 21.4) | THC (Namisol®) | 6.5 and 8.0 | tablets | slight ↑HR (by about 5 beats/min) | n.a. | [130] |
| 11 healthy volunteers (≥65) | THC (Namisol®) | 3, 5 or 6.5 | tablets | no clinically relevant changes in HR and ECG parameters | n.a. | [131] |
| 5 volunteers (22–29); regular marijuana smokers (at least once a day) | THC | 30 μg/kg | i.v. | ↑HR | tachycardia results from sympathetic stimulation and parasympathetic inhibition (diminished by i.v. propranolol and atropine 0.2 and 0.04 mg/kg, respectively) | [122] |
| 20 healthy male volunteers (22–30) | THC | 25 μg/kg ≈ 5 mg in one marijuana cigarette | i.v. | ↑HR, ↑total electromechanical systole; ↑left ventricular ejection time; ↓pre-ejection period | THC-induced changes in cardiac performance via the autonomic nervous system: partially diminished by propranolol 0.1 mg/kg i.v. and totally by propranolol 0.1 mg/kg plus atropine 2 mg/kg i.v. | [123,124] |
| 21 healthy volunteers | THC CBD | 0.2 mg/min 1.8 mg/min | i.v. | ↑HR ↑HR | CBD increases HR only at a much higher dose than THC | [126] |
| 6 patients undergoing diagnostic ECG evaluation | THC | 25 μg/kg | i.v. | ↓sinus length; ↓mean sinus node recovery, ↓maximal sinus node recovery times; ↓mean calculated sinoatrial conduction; ↓mean A-V nodal effective and functional refractory periods | (25 µg/kg i.v. correspond to ≈5 mg in one marijuana cigarette) enhancement of sinus automaticity and facilitation of sinoatrial and A-V nodal conduction | [125] |
| 9 cannabis users | THC Sativex®: THC: CBD | 5, 15 16.2:15.0 | oromucosal sprays | comparable ↑HR induced by THC and Sativex® | CBD fails to diminish the THC-induced tachycardia | [127] |
4. Tachycardia—Mechanisms
- i.
- the heart itself;
- ii.
- the autonomic nervous system;
- iii.
- the central nervous system;
- iv.
- the baroreceptor reflex.
4.1. Heart
| Species | Isolated Heart Preparation | CB-R Ligands, Concentrations 1 | Effects | Possible Mechanisms (CB1-Rs, CB2-Rs, Others) if Determined | References |
|---|---|---|---|---|---|
| humans | right atrial muscle | AEA, MethAEA and HU-210 AM251 | ↓contractility ↑contractility | CB1-R-mediated negative inotropic effect of AEA (antagonized by AM251 but not by AM630); endogenous tone at CB1-Rs | [70] |
| right atrial appendages | AEA and CP | ↓ electrically stimulated [3H]-NA release | presynaptic inhibitory CB1-Rs (effect antagonized by RIM and LY320135) | [168] | |
| Wistar rats | perfused heart 2 | THC | ↑HR, ↓CF, ↓ cardiac activity | cardiotoxicity, antagonists not used | [169] |
| SD rats | perfused heart 2 | THC ∆8-THC | ↑HR, ↓force of contraction ↔HR (arrhythmia), ↓force of contraction | antagonists not used | [170] |
| Wistar rats | perfused heart 2 | HU-210 | ↓HR | antagonists not used | [171] |
| Wistar rats | perfused heart 2 | HU-210, RIM and SR144528 | ↔HR, ↓LVDP, ↓dp/dt max, ↓dp/dt min | negative inotropism, mechanism unclear; partial agonism of RIM and SR144528? | [172] |
| Wistar rats | perfused heart 2 | HU-210 | ↓HR, ↓LVDP, ↓MRC, ↓MRR, ↓LVEDP alone and after ISO (100 nM) | negative chrono- and inotropism (mechanism?); ↓cardiostimulatory effect of ISO | [157,173] |
| SD rat | perfused heart 2 | AEA, MethAEA and ACEA PEA and JWH-015 | ↓LVDP, ↑CF ↔ LVDP, ↔CF | novel sites mediating AEA-induced negative inotropism (reduced by RIM and SR144528 but not AM251, AM630, CAPZ) and coronary vasodilation (reduced by RIM, SR144528 and AM251, but not AM630 and CAPZ) | [174] |
| Wistar rats | perfused heart 2 | AEA | ↔HR, ↔CF, ↓dp/dt max, ↓LVSP | antagonists not used | [175] |
| Wistar rats | perfused heart 2 | oleamide | ↑CF | CB1-R suggested but no proven | [176] |
| Wistar rats | perfused heart 2 with VP-induced coronary preconstriction | AEA or ACEA JWH-133 THC | ↔HR, ↑CF, ↑LVSP ↑CF, ↑LVSP ↓CF, ↓LVSP | coronary vasodilation and positive inotropism of AEA and ACEA (but not of THC and JWH-133) mediated via CB1-Rs (reduced by RIM and AM251 but not by SR144528 and O-1918); effects of THC and JWH-133 not modified by AM251 or AM630 | [175] |
| SD rats | perfused heart 2 | 2-AG WIN-2 | ↑CF, ↔LVDP ↑CF, ↓LVDP | coronary vasodilation is mediated via CB1-Rs; diminished by O-2050; negative inotropic effect by WIN-2 but not by 2-AG | [77] |
| SD rats | perfused heart 2 | O-2050 and orlistat | ↓effects induced by Ang II including ↓LVDP, ↓dp/dt max, ↓dp/dt min and ↓CF | 2-AG reduces negative inotropism + coronary constriction of Ang II via CB1-Rs | [77] |
| SD rats | perfused heart 2 | JWH-030 30 (but not 3) µM | ↓LVEDP, ↔LVSP, ↔LVDP, ↔HR | JWH-030 reduces cell viability via CB2-Rs (effect of AM630; no effect of RIM) | [156] |
| SD rats | perfused heart 2 or tachypaced | CB13 | ↔ dp/dt max, dp/dt min, HR, AV interval, LVDP, ↓tachypa- cing-induced shortening of the atrial effective refractory period | no effects on basal hemodynamic properties; beneficial effects against atrial fibrillation (antagonists not used) | [80] |
| Wistar rats | intramural coronary resistance artery | WIN-2 | relaxation | coronary vasodilation mediated by CB1-Rs (antagonized by O-2050 and AM251); CB1-R blockade enhanced myogenic tone | [90] |
| guinea pigs | atria | WIN-2 abn-CBD | ↓electrically stimulated [3H]-NA release ↔ electrically stimulated [3H]-NA release | presynaptic CB1-R (antagonized by RIM) but no GPR18 on sympathetic nerve endings | [177] |
| SD rats | atria | AEA, THC, PEA, JWH-015 | ↓stimulated [3H]-NA release | presynaptic CB1-Rs on sympathetic nerve endings (antagonism by RIM) | [178] |
| Wistar rats | right atria | WIN-2, MethAEA JWH-133 | ↔ HR; ↑chronotropic effect of NA ↔HR; ↔chronotropic effect of NA | slight enhancement of chronotropic effect of NA (antagonists not used) | [167] |
| Wistar rats | right atria | CP, CBD AM251 or AM630 | ↔chronotropic effect and ↑inotropic effect of ISO chronotropic effect of ISO ↑ 1 µM; ↓ 3 µM | mechanism of the effect of CP, AM251 and AM630 on the positive inotropism and/or chronotropism of ISO unclear | [179] |
| Wistar rats | left atria | AEA AEA ACEA JWH-015 | ↔dF/dt + AM251: ↑dF/dt + AM630: ↓dF/dt ↓dF/dt ↑dF/dt | negative inotropic effect via CB1-Rs and positive inotropic effect via CB2-Rs (AM251 and AM630 reduced the effects of ACEA and JWH-015, respectively) | [180] |
| rabbits | left ventricular myocytes | A-955840 | ↓FS, ↓dL/dt max, ↓L-type calcium current | CB2-R agonist A-955840 has negative inotropic effect independent of CB1-Rs and CB2-Rs (not modified by RIM and SR144528) | [38] |
| SD rats | ventricular myocytes | CB13 | ↔ contractile function | no inotropic effect | [18] |
| mice GPR55-/- | left ventricular cardiomyocyte | GPR55 deletion | ↑diastole sarcomere length ↔systole sarcomere length ↑transient Ca2+ amplitude ↓time from peak contraction to 50% and 90% relaxation | contractile changes dependent of GPR55 | [85] |
| SD rats | homogenized ventricles | THC ∆8-THC | ↓adenylate cyclase activity ↔adenylate cyclase activity | ↓adenylate cyclase activity may lead to cardiac depressant action of THC | [181] |
| humans | platelets | THC | ↑expression of glycoprotein IIb-IIIa and P-selectin involved in platelet activation | antagonists not used | [91] |
| platelets | THC | ↓adrenaline- or ADP-induced aggregation | antagonists not used | [182] | |
| platelets | AEA | ↑platelet activation ↑intracellular Ca2+ concentration | AA metabolites not involved (lack of effect of ASA and an FAAH inhibitor) | [183] | |
| platelets | AEA | ↔aggregation ↓aggregation induced by collagen, ADP, AA and TXA2 analogue but not by thrombin | antagonists not used | [184] | |
| platelets | 2-AG | ↑aggregation; ↑intracellular Ca2+ concentration; ↑AA and TXB2 release | CB1-Rs involved (inhibition by RIM but not by SR144528 and AA derivatives) | [185] | |
| platelets | THC 2-AG AM251 | ↔aggregation ↑aggregation ↔aggregation induced by ADP and thrombin | 2-AG induced aggregation was independent from CB1- and CB2-Rs (not antagonized by AM251 and AM630) but dependent on the conversion to AA (inhibited by MAGL inhibitor and ASA) | [186] | |
| platelets | 2-AG ACEA, JWH-015 | ↑aggregation, platelet activation ↔aggregation | platelet aggregation induced by 2-AG is independent from CB1- and CB2-Rs (not antagonized by RIM or SR144528 but connected with ↑TXA2) | [187] | |
| platelets | 2-AG, virodhamine AEA, ACEA, JWH015 | ↑aggregation, platelet activation ↔aggregation | platelet aggregation induced by 2-AG and virodhamine independent from CB1- and CB2-Rs but inhibited by MAGL inhibitor, ASA and TXA2-R antagonist | [188] | |
| platelets | AM251 or AM630 | ↔platelet count ↔aggregation induced by collagen, AA and ADP | platelet aggregation is independent of CB1- and CB2-Rs | [189] | |
| platelets | LPI | ↔aggregation ↓aggregation induced by ADP | GPR55 involved (effect of LPI reversed by the GPR55 antagonist CID16020046) | [26] | |
| rabbits | platelets | THC | ↓aggregation induced by ADP and PAF; ↓5-HT release from platelets | antagonists not used | [182] |
| rabbits | platelets | AEA HU-210 | ↑aggregation ↔aggregation | platelet aggregation induced by AEA independent from CB1-Rs (not antagonized by RIM but reduced by ASA) | [190] |
| mice | homogenised hearts | THC 100 µM | ↓oxygen consumption | antagonists not used | [191] |
| mice | cardiac mitochondria | THC 0.1 and 0.2 µM | ↓oxygen consumption ↓mitochondria coupled respiration | ↓oxygen consumption; not dependent on CB1-Rs (similar changes in CB1−/− mice) | [192] |
| beef | cardiacmitochondria | THC 120 µM | ↓respiration ↓ oxygen consumption | ↓mitochondrial oxygen consumption; antagonists not used | [193] |
| rats | cardiac mitochondria | THC, HU-210, AEA THC and HU-210 | ↓oxygen consumption and ↓mitochondrial membrane potential ↑mitochondrial hydrogen peroxide production | ↓mitochondrial oxygen consumption; antagonists not used | [194] |
| Wistar rats | cardiac mitochondria | THC up to 500 µM | ↔ROS production, no mitochondrial swelling ↔membrane potential, no oxidative stress, no lipid peroxidation | THC is not directly toxic in isolated cardiac mitochondria, and may even be helpful in reducing mitochondrial toxicity | [195] |
| SD rats | neonatal ventricular myocytes | CB13 | prevents ET1–induced ↓mitochondrial bioenergetics and mitochondrial membrane depolarization | improvement in cardiac mitochondrial function (precise mechanism unclear) | [196] |
| sheep | Purkinje fibers | THC | ↑APD90 | antagonists not used | [197] |
| rabbits | Purkinje fibers | JWH-030 JWH-210 | ↓APD90, ↔resting membrane potential ↔APD90, ↔resting membrane potential | only the highest concentration of JWH-030 reduces APD (mechanism unclear) | [156] |
| rabbits | sinoatrial node samples | AEA | ↓AP duration and ↓AP amplitude | ↓AP duration and ↓AP amplitude in SAN pacemaker cells via CB1-Rs (blocked by AM251 but not by AM630) | [198] |
| Wistar rats | papillary muscles; ventricular myocytes | AEA | ↓AP duration ↓AP amplitude ↓L-type Ca2+ current | antiarrhythmic properties; ↓AP and ↓L-type Ca2+ current through CB1-Rs (antagonized by AM251 but not AM630) | [199] |
| Wistar rats | ventricular myocytes | AEA | ↓Ito, ↑IKATP, ↔Iss, ↔IK1 | antiarrhythmic and cardioprotective properties: ↓Ito independent of CB1- and CB2-Rs; ↑IKATP mediated via CB2-Rs (antagonized by AM630 but not AM251) | [200] |
| Wistar rats | ventricular myocytes | AEA JWH-133 | ↔NCX1 [Ca2+]i: normal conditions; ↓NCX1 and [Ca2+]i: ischemia ↓NCX1 and [Ca2+]i: ischemia | ↓calcium overload through ↓NCX1 during ischemia via CB2-Rs (antagonized by AM630 but not by AM251; mimicked by JWH-133) | [201] |
| SD rats | neonatal ventricular myocytes | AEA, MethAEA, JWH-133 and CB13 | ↓ET1-induced induction of markers of hypertrophy | antihypertrophic properties via CB1- and CB2-Rs (antagonism by AM251/AM281 and AM630, respectively) | [18] |
| SD rats | neonatal ventricular myocytes | extracellular LPI administration intracellular LPI administration | ↑Ca2+ entry via L-type Ca2+ channels, long-lasting membrane depolarization ↑Ca2+ release from endolysosomal Ca2+ channels, short-lived membrane hyperpolarization | GPR55 Rs at the sarcolemma: ↑Ca2+ entry via L-type Ca2+ channels, leading to depolarization; GPR55 Rs at membranes of intracellular organelles: ↑intracellular Ca2+ release, leading to hyperpolarization (all effects blocked by ML193) | [86] |
| guinea pigs | ventricular cardiac nuclei | AEA | ↓IP3-mediated nuclear Ca2+ release | involves CB1- and CB2-Rs (effect reversed by AM251 and AM630, respectively) | [72] |
| rat cardiomyoblast cell line | H9c2 cells | THC-OH and THC-COOH THC | ↑cell migration and proliferation, ↑cell death and significant deterioration in cellular architecture ↔cell morphology or viability | the key metabolites of THC, as opposed to THC itself, elicit toxic cardiac effects (note that THC does not undergo metabolism in H9c2 cells) | [202] |
| rat cardiomyoblast cell line | H9c2 cells | JWH-030, JWH-210, JWH-250 or RCS-4 | all 0.1–100 µM: ↓cell viability, ↑cell apoptosis | synthetic cannabinoids induce cardiotoxicity via CB2-Rs (reduced by AM630 but not RIM) | [156] |
| mice | HL-1 atrial cardiomyocyte | THC 10 and 30 µM | ↑ER stress and apoptosis | cardiotoxicity independent of CB1-/CB2-Rs (not blocked by AM251 and AM630) | [203] |
4.2. Autonomic System
4.3. Central Nervous System
| Species | Conscious/ Anaesthetized with | Site of Injection | Drug under Study | Dose (nmol/rat), if Not Stated Otherwise | Effects | Possible/Suggested Mechanisms and Involvement of CB1-Rs/CB2-Rs/Others if Determined 1 | References |
|---|---|---|---|---|---|---|---|
| rabbits | conscious | intracisternal | CP or WIN-2 CP or WIN-2 WIN-3 | 0.1 and 1 µg/kg 10 µg/kg 0.1, 1 or 10 µg/kg | ↓HR, ↑RSNA, ↑plasma NA ↓HR, ↑RSNA, ↑plasma NA + ↑BP no effects | ↓HR and ↑BP, related to central CB1-Rs (diminished by i.v. RIM, not shared by inactive WIN-3) ↓HR related to muscarine receptors (also reduced by atropine) | [140,141] |
| mongrel dogs | pentobarbital | head circulation | THC | 2.5 mg/kg | ↓HR | THC-induced bradycardia has a central origin and involves an alteration of the central autonomic outflow regulating HR | [149] |
| cats | chloralose | lateral cerebral ventricle | THC | 2 mg/kg | ↓HR; ↔ BP | THC-induced bradycardia mediated centrally and not associated with a substantial reduction in BP | [151] |
| WKY | urethane | i.c.v. | ACEA | 1400 | ↔ HR, ↔ BP, ↔plasma NA and Adr | [210] | |
| Wistar rats | urethane | intracisternal | WIN-2 WIN-3 | both 1, 3, 10 and 30 µg/kg | WIN-2 unlike WIN-3: ↓HR, ↑BP and ↑plasma NA | CB1-Rs in the brain stem enhance cardiac vagal tone and sympathetic tone (all effects diminished by RIM) | [211] |
| Sprague Dawley rats | conscious | intracisternal | WIN-2 | 23 and 70 | immediate ↓HR but delayed ↑BP and ↑plasma NA (maximum at 10 min) | ↓HR, ↑BP and ↑plasma NA depend on CB1-Rs (reduced by AM251); ↑BP and ↑plasma NA but not ↓HR reduced by GABAA-R agonist muscimol | [212] |
| Sprague Dawley rats | conscious | RVLM | WIN-2 | 0.1, 0.2, 0.3 | ↑HR, ↑BP | difference in the HR response between Ibrahim and Abdel-Rahman [204] vs. [205] might be caused by the localized effect of WIN-2 within the RVLM compared to a more widespread effect after intracisternal administration | [213] |
| Wistar rats | conscious | RVLM | ACEA AM251 | 0.00005 0.00025 | ↑HR, ↑BP, ↑RSNA ↓HR, ↓BP, ↓RSNA | ↑HR and ↑BP mediated by CB1-Rs (reduced by AM251); CB1-Rs tonically active (AM had effects opposite in direction to those of ACEA) | [94] |
| Wistar rats | conscious | RVLM | AM251 | 0.00025 | ↓HR and ↓BP | CB1-Rs activated tonically by eCBs (cf. study by Wang et al. [94]) | [214] |
| Sprague Dawley rats | conscious | RVLM | abn-CBD NAGly O-1918 | 0.65, 1.3, 2.5 1.4, 2.8, 5.5, 11 0.7, 1.4, 2.8 | ↑HR, ↓BP ↔ HR, small ↓BP ↓HR, ↑BP | GPR18-Rs might mediate tachycardia and hypotension; probably activated by eCBs (O-1918 had effects opposite in direction to those of abn-CBD) | [215] |
| Sprague Dawley rats | urethane | RVLM | WIN-2 HU-210 | 0.00005, 0.0005 or 0.005 0.0005 | both agonists: ↔ HR, ↑ BP and ↑ sSNA | central sympathoexcitation mediated by CB1-Rs (reduced by AM281) | [216] |
| Wistar rats | urethane | RVLM | WIN-2 | 12 | ↔HR; slight ↓BP; ↔plasma NA | not examined | [159] |
| Sprague Dawley rats | pentobarbital | dPAG | AEA | 0.0018 | ↑HR, ↑BP, ↑RSNA; higher baseline HR connected with increased AEA content and decreased FAAH activity | eCBs can lead to sympathoexcitation via modulation of GABAergic inhibition by CB1-Rs at the level of the dPAG (responses reduced by AM281 and the GABAA-R antagonist gabazine) | [217,218,219] |
| Wistar rats | urethane | PVN | MethAEA CP MethAEA +AM251 or CP +AM251 or +AM6545 | 10 or 0.1 doses of agonists as above; antagonist doses (mg/kg): AM251 1.7 i.v. AM6545 8.3 i.p. | ↓HR, ↓BP ↑HR, ↑BP | the centrally induced ↑HR and ↑BP is mediated by CB1-Rs in the PVN (reduced by AM251 given into the PVN) and can be masked by peripheral CB1-Rs; the direction of the response (↑ or ↓ of HR and BP) probably depends on the sympathetic tone | [220,221] |
| Wistar rats | urethane | PVN | CP + AM251 1.7 mg/kg i.v. | 10 | ↑HR, ↑BP | pressor response of CP (after blockade of peripheral CB1-Rs by AM251) mediated via NMDA-, GABAA-, β2-, TP-, AT1-Rs and NO (antagonized by the respective inhibitors given i.v.) | [220,221] |
| Wistar rats | conscious | BNST | AM251 URB597 | 0.001, 0.03, 0.1 0.03 | ↑HR but not ↑BP induced by restraint stress increased by AM251 and decreased by URB597 | CB1-Rs and eCBs play a role in cardiac responses during stress via modulation of NMDA receptors in BNST and GABAA-Rs in the lateral hypothalamus; amplificatory effect of AM251 reduced by the respective antagonists LY235959 and SR95531 | [222,223,224] |
| dog | α-chloralose + urethane | NTS | WIN-2 RIM | 1.25–1.50 pmol 2.5–3.0 pmol | ↔BP, ↔BRS ↔BP, ↔BRS | [225] | |
| Sprague Dawley rats | urethane | NTS | WIN-2 CP AM281 | 10 10 14 | ↔ HR, ↓BP ↔ HR, ↓BP, ↔BRS ↔ HR, ↔ BP, ↔BRS | CB1-Rs in NTS do not modulate HR and baroreflex sensitivity | [226] |
| Sprague Dawley rats | pentobarbital | NTS | AEA AM404 (AEA transport inhibitor) | 0.0025 0.0035 | both drugs: ↔HR; ↔BP ↑BRS | CB1-Rs activated by eCBs in the NTS may have presynaptically attenuated GABA release, leading to enhanced BRS (effects of AEA inhibited by RIM and GABAA-R antagonist bicuculline) | [41,227] |
| Wistar rats | conscious | vMPFC | AM251 | 0.1 | ↔ HR and ↔ BP by itself; ↑BRS | CB1-Rs reduce the cardiac responses of the baroreflex | [228] |
4.4. Baroreceptor Reflex
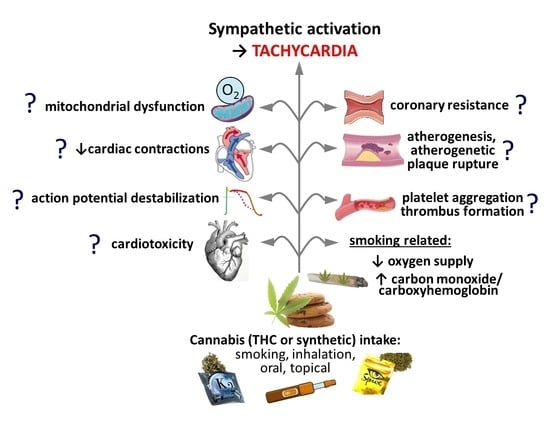
5. Thrombus Formation and Coronary Constriction?
6. Increased Energy Demand and Decreased Energy Supply?
7. Other Factors
8. General Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crocq, M.A. History of cannabis and the endocannabinoid system. Dialogues Clin. Neurosci. 2020, 22, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V. A brief history of cannabinoid and endocannabinoid pharmacology as inspired by the work of British scientists. Trends Pharmacol. Sci. 2006, 27, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Tang, Z.; Wu, X.; Spengler, R.; Jiang, H.; Yang, Y.; Boivin, N. The origins of cannabis smoking: Chemical residue evidence from the first millennium BCE in the Pamirs. Sci. Adv. 2019, 5, eaaw1391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dos Santos, R.G.; Hallak, J.E.C.; Crippa, J.A.S. Neuropharmacological effects of the main phytocannabinoids: A narrative review. Adv. Exp. Med. Biol. 2021, 1264, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Grubb, A.F.; Greene, S.J.; Fudim, M.; Dewald, T.; Mentz, R.J. Drugs of abuse and heart failure. J. Card. Fail. 2021, 27, 1260–1275. [Google Scholar] [CrossRef]
- Page, R.L., 2nd; Allen, L.A.; Kloner, R.A.; Carriker, C.R.; Martel, C.; Morris, A.A.; Piano, M.R.; Rana, J.S.; Saucedo, J.F.; American Heart Association Clinical Pharmacology Committee; et al. Medical marijuana, recreational cannabis, and cardiovascular health: A scientific statement from the American Heart Association. Circulation 2020, 142, e131–e152. [Google Scholar] [CrossRef] [PubMed]
- DrugBank Online. Available online: https://go.drugbank.com/ (accessed on 25 January 2022).
- Zawilska, J.B. “Legal Highs”-An Emerging Epidemic of Novel Psychoactive Substances. Int. Rev. Neurobiol. 2015, 120, 273–300. [Google Scholar] [CrossRef]
- Chung, E.Y.; Cha, H.J.; Min, H.K.; Yun, J. Pharmacology and adverse effects of new psychoactive substances: Synthetic cannabinoid receptor agonists. Arch. Pharm. Res. 2021, 44, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Derakhshandeh, R.; Liu, J.; Narayan, S.; Nabavizadeh, P.; Le, S.; Danforth, O.M.; Pinnamaneni, K.; Rodriguez, H.J.; Luu, E.; et al. One Minute of Marijuana Secondhand Smoke Exposure Substantially Impairs Vascular Endothelial Function. J. Am. Heart Assoc. 2016, 5, e003858. [Google Scholar] [CrossRef] [Green Version]
- Pacher, P.; Steffens, S.; Haskó, G.; Schindler, T.H.; Kunos, G. Cardiovascular effects of marijuana and synthetic cannabinoids: The good, the bad, and the ugly. Nat. Rev. Cardiol. 2018, 15, 151–166. [Google Scholar] [CrossRef]
- Drummer, O.H.; Gerostamoulos, D.; Woodford, N.W. Cannabis as a cause of death: A review. Forensic Sci. Int. 2019, 298, 298–306. [Google Scholar] [CrossRef]
- Chetty, K.; Lavoie, A.; Deghani, P. A literature review of cannabis and myocardial infarction-What clinicians may not be aware of. CJC Open 2020, 3, 12–21. [Google Scholar] [CrossRef] [PubMed]
- DeFilippis, E.M.; Bajaj, N.S.; Singh, A.; Malloy, R.; Givertz, M.M.; Blankstein, R.; Bhatt, D.L.; Vaduganathan, M. Marijuana use in patients with cardiovascular disease: JACC review topic of the week. J. Am. Coll. Cardiol. 2020, 75, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Huffman, J.W.; Zengin, G.; Wu, M.J.; Lu, J.; Hynd, G.; Bushell, K.; Thompson, A.L.; Bushell, S.; Tartal, C.; Hurst, D.P.; et al. Structure-activity relationships for 1-alkyl-3-(1-naphthoyl)indoles at the cannabinoid CB(1) and CB(2) receptors: Steric and electronic effects of naphthoyl substituents. New highly selective CB(2) receptor agonists. Bioorg. Med. Chem. 2005, 13, 89–112. [Google Scholar] [CrossRef] [PubMed]
- Banister, S.D.; Stuart, J.; Kevin, R.C.; Edington, A.; Longworth, M.; Wilkinson, S.M.; Beinat, C.; Buchanan, A.S.; Hibbs, D.E.; Glass, M.; et al. Effects of bioisosteric fluorine in synthetic cannabinoid designer drugs JWH-018, AM-2201, UR-144, XLR-11, PB-22, 5F-PB-22, APICA, and STS-135. ACS Chem. Neurosci. 2015, 6, 1445–1458. [Google Scholar] [CrossRef] [Green Version]
- Marcinkowska, M.; Śniecikowska, J.; Fajkis, N.; Paśko, P.; Franczyk, W.; Kołaczkowski, M. Management of dementia-related psychosis, agitation and aggression: A review of the pharmacology and clinical effects of potential drug candidates. CNS Drugs 2020, 34, 243–268. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Akinwumi, B.C.; Shao, Z.; Anderson, H.D. Ligand activation of cannabinoid receptors attenuates hypertrophy of neonatal rat cardiomyocytes. J. Cardiovasc. Pharmacol. 2014, 64, 420–430. [Google Scholar] [CrossRef] [Green Version]
- Aung, M.M.; Griffin, G.; Huffman, J.W.; Wu, M.; Keel, C.; Yang, B.; Showalter, V.M.; Abood, M.E.; Martin, B.R. Influence of the N-1 alkyl chain length of cannabimimetic indoles upon CB(1) and CB(2) receptor binding. Drug Alcohol Depend. 2000, 60, 133–140. [Google Scholar] [CrossRef]
- Wiley, J.L.; Marusich, J.A.; Martin, B.R.; Huffman, J.W. 1-Pentyl-3-phenylacetylindoles and JWH-018 share in vivo cannabinoid profiles in mice. Drug Alcohol Depend. 2012, 123, 148–153. [Google Scholar] [CrossRef] [Green Version]
- Gu, S.M.; Lee, H.J.; Lee, T.H.; Song, Y.J.; Kim, Y.H.; Han, K.M.; Shin, J.; Park, H.K.; Kim, H.S.; Cha, H.J.; et al. A synthetic cannabinoid JWH-210 reduces lymphoid organ weights and T-cell activator levels in mice via CB2 receptors. Naunyn Schmiedebergs Arch. Pharmacol. 2017, 390, 1201–1209. [Google Scholar] [CrossRef]
- Beltramo, M.; Stella, N.; Calignano, A.; Lin, S.Y.; Makriyannis, A.; Piomelli, D. Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science 1997, 277, 1094–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frost, J.M.; Dart, M.J.; Tietje, K.R.; Garrison, T.R.; Grayson, G.K.; Daza, A.V.; El-Kouhen, O.F.; Yao, B.B.; Hsieh, G.C.; Pai, M.; et al. Indol-3-ylcycloalkyl ketones: Effects of N1 substituted indole side chain variations on CB(2) cannabinoid receptor activity. J. Med. Chem. 2010, 53, 295–315. [Google Scholar] [CrossRef]
- Banister, S.D.; Stuart, J.; Conroy, T.; Longworth, M.; Manohar, M.; Beinat, C.; Wilkinson, S.M.; Kevin, R.C.; Hibbs, D.E.; Glass, M.; et al. Structure–activity relationships of synthetic cannabinoid designer drug RCS-4 and its regioisomers and C4 homologues. Forensic Toxicol. 2015, 33, 355–366. [Google Scholar] [CrossRef] [Green Version]
- Banister, S.D.; Wilkinson, S.M.; Longworth, M.; Stuart, J.; Apetz, N.; English, K.; Brooker, L.; Goebel, C.; Hibbs, D.E.; Glass, M. The synthesis and pharmacological evaluation of adamantane-derived indoles: Cannabimimetic drugs of abuse. ACS Chem. Neurosci. 2013, 4, 1081–1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kargl, J.; Brown, A.J.; Andersen, L.; Dorn, G.; Schicho, R.; Waldhoer, M.; Heinemann, A. A selective antagonist reveals a potential role of G protein-coupled receptor 55 in platelet and endothelial cell function. J. Pharmacol. Exp. Ther. 2013, 346, 54–66. [Google Scholar] [CrossRef] [Green Version]
- Heynen-Genel, S.; Dahl, R.; Shi, S.; Milan, L.; Hariharan, S.; Sergienko, E.; Hedrick, M.; Dad, S.; Stonich, D.; Su, Y.; et al. Screening for Selective Ligands for GPR55—Antagonists. In Probe Reports from the NIH Molecular Libraries Program [Internet]; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2010; pp. 1–24. [Google Scholar]
- Ryberg, E.; Larsson, N.; Sjögren, S.; Hjorth, S.; Hermansson, N.O.; Leonova, J.; Elebring, T.; Nilsson, K.; Drmota, T.; Greasley, P.J. The orphan receptor GPR55 is a novel cannabinoid receptor. Br. J. Pharmacol. 2007, 152, 1092–1101. [Google Scholar] [CrossRef]
- McIntyre, P.; McLatchie, L.M.; Chambers, A.; Phillips, E.; Clarke, M.; Savidge, J.; Toms, C.; Peacock, M.; Shah, K.; Winter, J.; et al. Pharmacological differences between the human and rat vanilloid receptor 1 (VR1). Br. J. Pharmacol. 2001, 132, 1084–1094. [Google Scholar] [CrossRef] [Green Version]
- McHugh, D.; Page, J.; Dunn, E.; Bradshaw, H.B. Δ(9) -Tetrahydrocannabinol and N-arachidonyl glycine are full agonists at GPR18 receptors and induce migration in human endometrial HEC-1B cells. Br. J. Pharmacol. 2012, 165, 2414–2424. [Google Scholar] [CrossRef] [Green Version]
- Heblinski, M.; Santiago, M.; Fletcher, C.; Stuart, J.; Connor, M.; McGregor, I.S.; Arnold, J.C. Terpenoids commonly found in Cannabis sativa do not modulate the actions of phytocannabinoids or endocannabinoids on TRPA1 and TRPV1 channels. Cannabis Cannabinoid Res. 2020, 5, 305–317. [Google Scholar] [CrossRef] [Green Version]
- Zygmunt, P.M.; Chuang, H.; Movahed, P.; Julius, D.; Högestätt, E.D. The anandamide transport inhibitor AM404 activates vanilloid receptors. Eur. J. Pharmacol. 2000, 396, 39–42. [Google Scholar] [CrossRef]
- Bowles, N.P.; Karatsoreos, I.N.; Li, X.; Vemuri, V.K.; Wood, J.A.; Li, Z.; Tamashiro, K.L.; Schwartz, G.J.; Makriyannis, A.M.; Kunos, G.; et al. A peripheral endocannabinoid mechanism contributes to glucocorticoid-mediated metabolic syndrome. Proc. Natl. Acad. Sci. USA 2015, 112, 285–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinaldi-Carmona, M.; Barth, F.; Congy, C.; Martinez, S.; Oustric, D.; Pério, A.; Poncelet, M.; Maruani, J.; Arnone, M.; Finance, O.; et al. SR147778 [5-(4-bromophenyl)-1-(2,4-dichlorophenyl)-4-ethyl-N-(1-piperidinyl)1H-pyrazole-3-carb-oxamide], a new potent and selective antagonist of the CB1 cannabinoid receptor: Biochemical and pharmacological characterization. J. Pharmacol. Exp. Ther. 2004, 310, 905–914. [Google Scholar] [CrossRef]
- Black, M.D.; Stevens, R.J.; Rogacki, N.; Featherstone, R.E.; Senyah, Y.; Giardino, O.; Borowsky, B.; Stemmelin, J.; Cohen, C.; Pichat, P.; et al. AVE1625, a cannabinoid CB1 receptor antagonist, as a co-treatment with antipsychotics for schizophrenia: Improvement in cognitive function and reduction of antipsychotic-side effects in rodents. Psychopharmacology 2011, 215, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.Q.; Mikkelsen, T.S.; McKinney, M.K.; Lander, E.S.; Cravatt, B.F. A second fatty acid amide hydrolase with variable distribution among placental mammals. J. Biol. Chem. 2006, 281, 36569–36578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bisogno, T.; Howell, F.; Williams, G.; Minassi, A.; Cascio, M.G.; Ligresti, A.; Matias, I.; Schiano-Moriello, A.; Paul, P.; Williams, E.J.; et al. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J. Cell Biol. 2003, 163, 463–468. [Google Scholar] [CrossRef]
- Su, Z.; Preusser, L.; Diaz, G.; Green, J.; Liu, X.; Polakowski, J.; Dart, M. Negative inotropic effect of a CB2 agonist A-955840 in isolated rabbit ventricular myocytes is independent of CB1 and CB2 receptors. Curr. Drug Saf. 2011, 6, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G.; Howlett, A.C.; Abood, M.E.; Alexander, S.P.; Di Marzo, V.; Elphick, M.R.; Greasley, P.J.; Hansen, H.S.; Kunos, G.; Mackie, K.; et al. International union of basic and clinical pharmacology. LXXIX. Cannabinoid receptors and their ligands: Beyond CB1 and CB2. Pharmacol. Rev. 2010, 62, 588–631. [Google Scholar] [CrossRef] [Green Version]
- Malinowska, B.; Piszcz, J.; Koneczny, B.; Hryniewicz, A.; Schlicker, E. Modulation of the cardiac autonomic transmission of pithed rats by presynaptic opioid OP4 and cannabinoid CB1 receptors. Naunyn Schmiedebergs Arch. Pharmacol. 2001, 364, 233–241. [Google Scholar] [CrossRef]
- Brozoski, D.T.; Dean, C.; Hopp, F.A.; Seagard, J.L. Uptake blockade of endocannabinoids in the NTS modulates baroreflex-evoked sympathoinhibition. Brain Res. 2005, 1059, 197–202. [Google Scholar] [CrossRef]
- Legality of Cannabis. Available online: https://en.wikipedia.org/wiki/Legality_of_cannabis (accessed on 25 January 2022).
- Latif, Z.; Garg, N. The Impact of Marijuana on the Cardiovascular System: A Review of the Most Common Cardiovascular Events Associated with Marijuana Use. J. Clin. Med. 2020, 9, 1925. [Google Scholar] [CrossRef] [PubMed]
- Motyka, M.A.; Al-Imam, A. Representations of psychoactive drugs’ use in mass culture and their impact on audiences. Int. J. Environ. Res. Public Health 2021, 18, 6000. [Google Scholar] [CrossRef] [PubMed]
- Borgonhi, E.M.; Volpatto, V.L.; Ornell, F.; Rabelo-da-Ponte, F.D.; Kessler, F.H.P. Multiple clinical risks for cannabis users during the COVID-19 pandemic. Addict. Sci. Clin. Pract. 2021, 16, 5. [Google Scholar] [CrossRef] [PubMed]
- Hollister, L.E. Health aspects of cannabis. Pharmacol Rev. 1986, 38, 1–20. [Google Scholar] [CrossRef]
- Tait, R.J.; Caldicott, D.; Mountain, D.; Hill, S.L.; Lenton, S. A systematic review of adverse events arising from the use of synthetic cannabinoids and their associated treatment. Clin. Toxicol. 2016, 54, 783–784. [Google Scholar] [CrossRef]
- Thomas, G.; Kloner, R.A.; Rezkalla, S. Adverse cardiovascular, cerebrovascular, and peripheral vascular effects of marijuana inhalation: What cardiologists need to know. Am. J. Cardiol. 2014, 113, 187–190. [Google Scholar] [CrossRef]
- Bachs, L.; Mørland, H. Acute cardiovascular fatalities following cannabis use. Forensic Sci. Int. 2001, 124, 200–203. [Google Scholar] [CrossRef]
- Mittleman, M.A.; Lewis, R.A.; Maclure, M.; Sherwood, J.B.; Muller, J.E. Triggering myocardial infarction by marijuana. Circulation 2001, 103, 2805–2809. [Google Scholar] [CrossRef] [Green Version]
- Jouanjus, E.; Leymarie, F.; Tubery, M.; Lapeyre-Mestre, M. Cannabis-related hospitalizations: Unexpected serious events identified through hospital databases. Br. J. Clin. Pharmacol. 2011, 71, 758–765. [Google Scholar] [CrossRef] [Green Version]
- Sagris, M.; Antonopoulos, A.S.; Theofilis, P.; Oikonomou, E.; Siasos, G.; Tsalamandris, S.; Antoniades, C.; Brilakis, E.S.; Kaski, J.C.; Tousoulis, D. Risk factors profile of young and older patients with Myocardial Infarction. Cardiovasc. Res. 2021, cvab264. [Google Scholar] [CrossRef]
- Chami, T.; Kim, C.H. Cannabis abuse and elevated risk of myocardial infarction in the young: A population-based study. Mayo Clin. Proc. 2019, 94, 1647–1649. [Google Scholar] [CrossRef]
- Jouanjus, E.; Raymond, V.; Lapeyre-Mestre, M.; Wolff, V. What is the current knowledge about the cardiovascular risk for users of cannabis-based products? A systematic review. Curr. Atheroscler. Rep. 2017, 19, 26. [Google Scholar] [CrossRef] [PubMed]
- Desai, R.; Patel, U.; Sharma, S.; Amin, P.; Bhuva, R.; Patel, M.S.; Sharma, N.; Shah, M.; Patel, S.; Savani, S.; et al. Recreational marijuana use and acute myocardial infarction: Insights from Nationwide Inpatient Sample in the United States. Cureus 2017, 9, e1816. [Google Scholar] [CrossRef] [Green Version]
- Kalla, A.; Krishnamoorthy, P.M.; Gopalakrishnan, A.; Figueredo, V.M. Cannabis use predicts risks of heart failure and cerebrovascular accidents: Results from the National Inpatient Sample. J. Cardiovasc. Med. 2018, 19, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Desai, R.; Fong, H.K.; Shah, K.; Kaur, V.P.; Savani, S.; Gangani, K.; Damarlapally, N.; Goyal, H. Rising trends in hospitalizations for cardiovascular events among young cannabis users (18-39 Years) without other substance abuse. Medicina 2019, 55, 438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fong, H.K.; Lodhi, M.U.; Kothapudi, V.N.; Singh, S.; Desai, R. Alarming trends in the frequency of malignant hypertension among admissions with a known cannabis use disorder. Int. J. Cardiol. Heart Vasc. 2021, 33, 100729. [Google Scholar] [CrossRef]
- Zongo, A.; Lee, C.; Dyck, J.R.B.; El-Mourad, J.; Hyshka, E.; Hanlon, J.G.; Eurich, D.T. Medical cannabis authorization and the risk of cardiovascular events: A longitudinal cohort study. BMC Cardiovasc. Disord. 2021, 21, 426. [Google Scholar] [CrossRef]
- Yang, P.K.; Odom, E.C.; Patel, R.; Loustalot, F.; Coleman King, S. Nonmedical marijuana use and cardiovascular events: A systematic review. Public Health Rep. 2022, 137, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Skipina, T.M.; Upadhya, B.; Soliman, E.Z. Cannabis use and electrocardiographic myocardial injury. Am. J. Cardiol. 2021, 151, 100–104. [Google Scholar] [CrossRef]
- Ladha, K.S.; Mistry, N.; Wijeysundera, D.N.; Clarke, H.; Verma, S.; Hare, G.M.T.; Mazer, C.D. Recent cannabis use and myocardial infarction in young adults: A cross-sectional study. CMAJ 2021, 193, E1377–E1384. [Google Scholar] [CrossRef]
- Pertwee, R.G. Endocannabinoids and their pharmacological actions. Handb. Exp. Pharmacol. 2015, 231, 1–37. [Google Scholar] [CrossRef]
- Toczek, M.; Malinowska, B. Enhanced endocannabinoid tone as a potential target of pharmacotherapy. Life Sci. 2018, 204, 20–45. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.; Lago-Fernandez, A.; Hurst, D.P.; Sotudeh, N.; Brailoiu, E.; Reggio, P.H.; Abood, M.E.; Jagerovic, N. Therapeutic exploitation of GPR18: Beyond the cannabinoids? J. Med. Chem. 2020, 63, 14216–14227. [Google Scholar] [CrossRef] [PubMed]
- Rabino, M.; Mallia, S.; Castiglioni, E.; Rovina, D.; Pompilio, G.; Gowran, A. The endocannabinoid system and cannabidiol: Past, present, and prospective for cardiovascular diseases. Pharmaceuticals 2021, 14, 936. [Google Scholar] [CrossRef] [PubMed]
- Valenta, I.; Varga, Z.V.; Valentine, H.; Cinar, R.; Horti, A.; Mathews, W.B.; Dannals, R.F.; Steele, K.; Kunos, G.; Wahl, R.L.; et al. Feasibility evaluation of myocardial cannabinoid type 1 receptor imaging in obesity: A translational approach. JACC Cardiovasc. Imaging 2018, 11, 320–332. [Google Scholar] [CrossRef]
- Van Esbroeck, A.; Varga, Z.V.; Di, X.; van Rooden, E.J.; Tóth, V.E.; Onódi, Z.; Kuśmierczyk, M.; Leszek, P.; Ferdinandy, P.; Hankemeier, T.; et al. Activity-based protein profiling of the human failing ischemic heart reveals alterations in hydrolase activities involving the endocannabinoid system. Pharmacol. Res. 2020, 151, 104578. [Google Scholar] [CrossRef]
- Weis, F.; Beiras-Fernandez, A.; Sodian, R.; Kaczmarek, I.; Reichart, B.; Beiras, A.; Schelling, G.; Kreth, S. Substantially altered expression pattern of cannabinoid receptor 2 and activated endocannabinoid system in patients with severe heart failure. J. Mol. Cell Cardiol. 2010, 48, 1187–1193. [Google Scholar] [CrossRef]
- Bonz, A.; Laser, M.; Küllmer, S.; Kniesch, S.; Babin-Ebell, J.; Popp, V.; Ertl, G.; Wagner, J.A. Cannabinoids acting on CB1 receptors decrease contractile performance in human atrial muscle. J. Cardiovasc. Pharmacol. 2003, 41, 657–664. [Google Scholar] [CrossRef]
- Cappellano, G.; Uberti, F.; Caimmi, P.P.; Pietronave, S.; Mary, D.A.; Dianzani, C.; Micalizzi, E.; Melensi, M.; Boldorini, R.; Nicosia, G.; et al. Different expression and function of the endocannabinoid system in human epicardial adipose tissue in relation to heart disease. Can. J. Cardiol. 2013, 29, 499–509. [Google Scholar] [CrossRef]
- Currie, S.; Rainbow, R.D.; Ewart, M.A.; Kitson, S.; Pliego, E.H.; Kane, K.A.; McCarron, J.G. IP(3)R-mediated Ca2+ release is modulated by anandamide in isolated cardiac nuclei. J. Mol. Cell Cardiol. 2008, 45, 804–811. [Google Scholar] [CrossRef]
- Wagner, J.A.; Hu, K.; Karcher, J.; Bauersachs, J.; Schäfer, A.; Laser, M.; Han, H.; Ertl, G. CB(1) cannabinoid receptor antagonism promotes remodeling and cannabinoid treatment prevents endothelial dysfunction and hypotension in rats with myocardial infarction. Br. J. Pharmacol. 2003, 138, 1251–1258. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.J.; Lu, Y.; Wang, H.W.; Zhang, H.; Wang, S.R.; Xu, W.Y.; Fu, H.L.; Yao, X.Y.; Yang, F.; Yuan, H.B. Activation of endocannabinoid receptor 2 as a mechanism of propofol pretreatment-induced Cardioprotection against ischemia-reperfusion injury in rats. Oxid. Med. Cell. Longev. 2017, 2017, 2186383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biernacki, M.; Łuczaj, W.; Jarocka-Karpowicz, I.; Ambrożewicz, E.; Toczek, M.; Skrzydlewska, E. The effect of long-term administration of fatty acid amide hydrolase inhibitor URB597 on oxidative metabolism in the heart of rats with primary and secondary hypertension. Molecules 2018, 23, 2350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukhopadhyay, P.; Bátkai, S.; Rajesh, M.; Czifra, N.; Harvey-White, J.; Haskó, G.; Zsengeller, Z.; Gerard, N.P.; Liaudet, L.; Kunos, G.; et al. Pharmacological inhibition of CB1 cannabinoid receptor protects against doxorubicin-induced cardiotoxicity. J. Am. Coll. Cardiol. 2007, 50, 528–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miklós, Z.; Wafa, D.; Nádasy, G.L.; Tóth, Z.E.; Besztercei, B.; Dörnyei, G.; Laska, Z.; Benyó, Z.; Ivanics, T.; Hunyady, L.; et al. Angiotensin II-induced cardiac effects are modulated by endocannabinoid-mediated CB1 receptor activation. Cells 2021, 10, 724. [Google Scholar] [CrossRef] [PubMed]
- Pędzińska-Betiuk, A.; Weresa, J.; Toczek, M.; Baranowska-Kuczko, M.; Kasacka, I.; Harasim-Symbor, E.; Malinowska, B. Chronic inhibition of fatty acid amide hydrolase by URB597 produces differential effects on cardiac performance in normotensive and hypertensive rats. Br. J. Pharmacol. 2017, 174, 2114–2129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Remiszewski, P.; Jarocka-Karpowicz, I.; Biernacki, M.; Jastrząb, A.; Schlicker, E.; Toczek, M.; Harasim-Symbor, E.; Pędzińska-Betiuk, A.; Malinowska, B. Chronic cannabidiol administration fails to diminish blood pressure in rats with primary and secondary hypertension despite its effects on cardiac and plasma endocannabinoid system, oxidative stress and lipid metabolism. Int. J. Mol. Sci. 2020, 21, 1295. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.I.; Murninkas, M.; Elyagon, S.; Etzion, Y.; Anderson, H.D. Cannabinoid receptor agonist inhibits atrial electrical remodeling in a tachypaced Ex Vivo rat model. Front. Pharmacol. 2021, 12, 642398. [Google Scholar] [CrossRef]
- Tomlinson, L.; Tirmenstein, M.A.; Janovitz, E.B.; Aranibar, N.; Ott, K.H.; Kozlosky, J.C.; Patrone, L.M.; Achanzar, W.E.; Augustine, K.A.; Brannen, K.C.; et al. Cannabinoid receptor antagonist-induced striated muscle toxicity and ethylmalonic-adipic aciduria in beagle dogs. Toxicol. Sci. 2012, 29, 268–279. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.F.; Jiang, L.S.; Bu, J.; Huang, X.J.; Song, W.; Du, Y.P.; He, B. Cannabinoid-2 receptor activation protects against infarct and ischemia-reperfusion heart injury. J. Cardiovasc. Pharmacol. 2012, 59, 301–307. [Google Scholar] [CrossRef]
- Rajesh, M.; Bátkai, S.; Kechrid, M.; Mukhopadhyay, P.; Lee, W.S.; Horváth, B.; Holovac, E.; Cinar, R.; Liaudet, L.; Mackie, K.; et al. Cannabinoid 1 receptor promotes cardiac dysfunction, oxidative stress, inflammation, and fibrosis in diabetic cardiomyopathy. Diabetes 2012, 61, 716–727. [Google Scholar] [CrossRef] [Green Version]
- Henstridge, C.M.; Balenga, N.A.; Kargl, J.; Andradas, C.; Brown, A.J.; Irving, A.; Sanchez, C.; Waldhoer, M. Minireview: Recent developments in the physiology and pathology of the lysophosphatidylinositol-sensitive receptor GPR55. Mol. Endocrinol. 2011, 25, 1835–1848. [Google Scholar] [CrossRef] [Green Version]
- Puhl, S.L.; Hilby, M.; Kohlhaas, M.; Keidel, L.M.; Jansen, Y.; Hristov, M.; Schindler, J.; Maack, C.; Steffens, S. Haematopoietic and cardiac GPR55 synchronize post-myocardial infarction remodelling. Sci. Rep. 2021, 11, 14385. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Deliu, E.; Zhang, X.Q.; Hoffman, N.E.; Carter, R.L.; Grisanti, L.A.; Brailoiu, G.C.; Madesh, M.; Cheung, J.Y.; Force, T.; et al. Differential activation of cultured neonatal cardiomyocytes by plasmalemmal versus intracellular G protein-coupled receptor 55. J. Biol. Chem. 2013, 288, 22481–22492. [Google Scholar] [CrossRef] [Green Version]
- Walsh, S.K.; Hector, E.E.; Andréasson, A.C.; Jönsson-Rylander, A.C.; Wainwright, C.L. GPR55 deletion in mice leads to age-related ventricular dysfunction and impaired adrenoceptor-mediated inotropic responses. PLoS ONE 2014, 9, e108999. [Google Scholar] [CrossRef] [Green Version]
- Sleep, S.L.; Shrestha, N.; Cuffe, J.; Holland, O.J.; Headrick, J.P.; McAinch, A.J.; Hryciw, D.H. The effect of high maternal linoleic acid on endocannabinoid signalling in rodent hearts. J. Dev. Orig. Health Dis. 2020, 11, 617–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajesh, M.; Mukhopadhyay, P.; Bátkai, S.; Haskó, G.; Liaudet, L.; Huffman, J.W.; Csiszar, A.; Ungvari, Z.; Mackie, K.; Chatterjee, S.; et al. CB2-receptor stimulation attenuates TNF-alpha-induced human endothelial cell activation, transendothelial migration of monocytes, and monocyte-endothelial adhesion. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H2210–H2218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szekeres, M.; Nádasy, G.L.; Soltész-Katona, E.; Hunyady, L. Control of myogenic tone and agonist induced contraction of intramural coronary resistance arterioles by cannabinoid type 1 receptors and endocannabinoids. Prostaglandins Other Lipid Mediat. 2018, 134, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Deusch, E.; Kress, H.G.; Kraft, B.; Kozek-Langenecker, S.A. The procoagulatory effects of delta-9-tetrahydrocannabinol in human platelets. Anesth. Analg. 2004, 99, 1127–1130. [Google Scholar] [CrossRef] [PubMed]
- Mackie, K. Distribution of cannabinoid receptors in the central and peripheral nervous system. Handb. Exp. Pharmacol. 2005, 168, 299–325. [Google Scholar] [CrossRef]
- Ibrahim, B.M.; Abdel-Rahman, A.A. A pivotal role for enhanced brainstem Orexin receptor 1 signaling in the central cannabinoid receptor 1-mediated pressor response in conscious rats. Brain Res. 2015, 1622, 51–63. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Li, G.Q.; Zhang, H.P.; Zhang, Y.; Li, Q. Overactivation of cannabinoid receptor type 1 in rostral ventrolateral medulla promotes cardiovascular responses in spontaneously hypertensive rats. J. Hypertens. 2017, 35, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Puente, N.; Elezgarai, I.; Lafourcade, M.; Reguero, L.; Marsicano, G.; Georges, F.; Manzoni, O.J.; Grandes, P. Localization and function of the cannabinoid CB1 receptor in the anterolateral bed nucleus of the stria terminalis. PLoS ONE 2010, 5, e8869. [Google Scholar] [CrossRef] [PubMed]
- Lange, M.D.; Daldrup, T.; Remmers, F.; Szkudlarek, H.J.; Lesting, J.; Guggenhuber, S.; Ruehle, S.; Jüngling, K.; Seidenbecher, T.; Lutz, B.; et al. Cannabinoid CB1 receptors in distinct circuits of the extended amygdala determine fear responsiveness to unpredictable threat. Mol. Psychiatry 2017, 22, 1422–1430. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Park, J.Y.; Oh, J.Y.; Bae, S.J.; Jang, H.; Jeon, S.; Kim, J.; Park, H.J. Novel analgesic effects of melanin-concentrating hormone on persistent neuropathic and inflammatory pain in mice. Sci. Rep. 2018, 8, 707. [Google Scholar] [CrossRef]
- Ruginsk, S.G.; Vechiato, F.M.; Uchoa, E.T.; Elias, L.L.; Antunes-Rodrigues, J. Type 1 cannabinoid receptor modulates water deprivation-induced homeostatic responses. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R1358–R1368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mostafeezur, R.M.; Zakir, H.M.; Takatsuji, H.; Yamada, Y.; Yamamura, K.; Kitagawa, J. Cannabinoids facilitate the swallowing reflex elicited by the superior laryngeal nerve stimulation in rats. PLoS ONE 2012, 7, e50703. [Google Scholar] [CrossRef]
- Casarotto, P.C.; Terzian, A.L.; Aguiar, D.C.; Zangrossi, H.; Guimarães, F.S.; Wotjak, C.T.; Moreira, F.A. Opposing roles for cannabinoid receptor type-1 (CB1) and transient receptor potential vanilloid type-1 channel (TRPV1) on the modulation of panic-like responses in rats. Neuropsychopharmacology 2012, 37, 478–486. [Google Scholar] [CrossRef]
- Zuurman, L.; Ippel, A.E.; Moin, E.; van Gerven, J.M. Biomarkers for the effects of cannabis and THC in healthy volunteers. Br. J. Clin. Pharmacol. 2009, 67, 5–21. [Google Scholar] [CrossRef] [Green Version]
- Gopinathannair, R.; Etheridge, S.P.; Marchlinski, F.E.; Spinale, F.G.; Lakkireddy, D.; Olshansky, B. Arrhythmia-induced cardiomyopathies: Mechanisms, recognition, and management. J. Am. Coll. Cardiol. 2015, 66, 1714–1728. [Google Scholar] [CrossRef] [Green Version]
- Palatini, P.; Julius, S. Elevated heart rate: A major risk factor for cardiovascular disease. Clin. Exp. Hypertens. 2004, 26, 637–644. [Google Scholar] [CrossRef]
- Johnson, S.; Domino, E.F. Some cardiovascular effects of marihuana smoking in normal volunteers. Clin. Pharmacol. Ther. 1971, 12, 762–768. [Google Scholar] [CrossRef]
- Crawford, W.J.; Merritt, J.C. Effects of tetrahydrocannabinol on arterial and intraocular hypertension. Int. J. Clin. Pharmacol. Biopharm. 1979, 17, 191–196. [Google Scholar]
- Spindle, T.R.; Cone, E.J.; Schlienz, N.J.; Mitchell, J.M.; Bigelow, G.E.; Flegel, R.; Hayes, E.; Vandrey, R. Acute effects of smoked and vaporized cannabis in healthy adults who infrequently use cannabis: A crossover trial. JAMA Netw. Open 2018, 1, e184841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sulkowski, A.; Vachon, L.; Rich, E.S., Jr. Propranolol effects on acute marihuana intoxication in man. Psychopharmacology 1977, 52, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Beaconsfield, P.; Ginsburg, J.; Rainsbury, R. Marihuana smoking: Cardiovascular effects in man and possible mechanisms. N. Engl. J. Med. 1972, 287, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Huestis, M.A.; Boyd, S.J.; Heishman, S.J.; Preston, K.L.; Bonnet, D.; Le Fur, G.; Gorelick, D.A. Single and multipmillmle doses of rimonabant antagonize acute effects of smoked cannabis in male cannabis users. Psychopharmacology 2007, 194, 505–515. [Google Scholar] [CrossRef] [Green Version]
- Gash, A.; Karliner, J.S.; Janowsky, D.; Lake, C.R. Effects of smoking marihuana on left ventricular performance and plasma norepinephrine: Studies in normal men. Ann. Intern. Med. 1978, 89, 448–452. [Google Scholar] [CrossRef]
- Bidwell, L.C.; Karoly, H.C.; Torres, M.O.; Master, A.; Bryan, A.D.; Hutchison, K.E. A naturalistic study of orally administered vs. inhaled legal market cannabis: Cannabinoids exposure, intoxication, and impairment. Psychopharmacology 2021, 239, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Brands, B.; Mann, R.E.; Wickens, C.M.; Sproule, B.; Stoduto, G.; Sayer, G.S.; Burston, J.; Pan, J.F.; Matheson, J.; Stefan, C. Acute and residual effects of smoked cannabis: Impact on driving speed and lateral control, heart rate, and self-reported drug effects. Drug Alcohol Depend. 2019, 205, 107641. [Google Scholar] [CrossRef] [PubMed]
- Tashkin, D.P.; Levisman, J.A.; Abbasi, A.S.; Shapiro, B.J.; Ellis, N.M. Short-term effects of smoked marihuana on left ventricular function in man. Chest 1977, 72, 20–26. [Google Scholar] [CrossRef]
- Maida, N.; Papaseit, E.; Martínez, L.; Pérez-Mañá, C.; Poyatos, L.; Pellegrini, M.; Pichini, S.; Pacifici, R.; Ventura, M.; Galindo, L.; et al. Acute pharmacological effects and oral fluid biomarkers of the synthetic cannabinoid UR-144 and THC in recreational users. Biology 2021, 10, 257. [Google Scholar] [CrossRef] [PubMed]
- Zuurman, L.; Roy, C.; Schoemaker, R.C.; Hazekamp, A.; den Hartigh, J.; Bender, J.C.; Verpoorte, R.; Pinquier, J.L.; Cohen, A.F.; van Gerven, J.M. Effect of intrapulmonary tetrahydrocannabinol administration in humans. J. Psychopharmacol. 2008, 22, 707–716. [Google Scholar] [CrossRef]
- Zuurman, L.; Roy, C.; Schoemaker, R.C.; Amatsaleh, A.; Guimaeres, L.; Pinquier, J.L.; Cohen, A.F.; van Gerven, J.M. Inhibition of THC-induced effects on the central nervous system and heart rate by a novel CB1 receptor antagonist AVE1625. J. Psychopharmacol. 2010, 24, 363–371. [Google Scholar] [CrossRef]
- Strougo, A.; Zuurman, L.; Roy, C.; Pinquier, J.L.; van Gerven, J.M.; Cohen, A.F.; Schoemaker, R.C. Modelling of the concentration—Effect relationship of THC on central nervous system parameters and heart rate—Insight into its mechanisms of action and a tool for clinical research and development of cannabinoids. J. Psychopharmacol. 2008, 22, 717–726. [Google Scholar] [CrossRef]
- Klumpers, L.E.; Roy, C.; Ferron, G.; Turpault, S.; Poitiers, F.; Pinquier, J.L.; van Hasselt, J.G.; Zuurman, L.; Erwich, F.A.; van Gerven, J.M. Surinabant, a selective cannabinoid receptor type 1 antagonist, inhibits Δ9-tetrahydrocannabinol-induced central nervous system and heart rate effects in humans. Br. J. Clin. Pharmacol. 2013, 76, 65–77. [Google Scholar] [CrossRef] [Green Version]
- Martin-Santos, R.; Crippa, J.A.; Batalla, A.; Bhattacharyya, S.; Atakan, Z.; Borgwardt, S.; Allen, P.; Seal, M.; Langohr, K.; Farré, M.; et al. Acute effects of a single, oral dose of Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD) administration in healthy volunteers. Curr. Pharm. Des. 2012, 18, 4966–4979. [Google Scholar] [CrossRef]
- Pabon, E.; Rockwood, F.; Norman, G.J.; de Wit, H. Acute effects of oral delta-9-tetrahydrocannabinol (THC) on autonomic cardiac activity and their relation to subjective and anxiogenic effects. Psychophysiology 2022, 59, e13955. [Google Scholar] [CrossRef]
- Bedi, G.; Cooper, Z.D.; Haney, M. Subjective, cognitive and cardiovascular dose-effect profile of nabilone and dronabinol in marijuana smokers. Addict. Biol. 2013, 18, 872–881. [Google Scholar] [CrossRef]
- Benowitz, N.L.; Rosenberg, J.; Rogers, W.; Bachman, J.; Jones, R.T. Cardiovascular effects of intravenous delta-9-tetrahydrocannabinol: Autonomic nervous mechanisms. Clin. Pharmacol. Ther. 1979, 25, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Kanakis, C., Jr.; Pouget, J.M.; Rosen, K.M. The effects of delta-9-tetrahydrocannabinol (cannabis) on cardiac performance with and without beta blockade. Circulation 1976, 53, 703–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanakis, C.; Pouget, M.; Rosen, K.M. Lack of cardiovascular effects of delta-9-tetrahydrocannabinol in chemically denervated men. Ann. Intern. Med. 1979, 91, 571–574. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.H.; Dhingra, R.C.; Kanakis, C., Jr.; Amat-y-Leon, F.; Rosen, K.M. The electrophysiological effects of delta-9-tetrahydrocannabinol (cannabis) on cardiac conduction in man. Am. Heart J. 1977, 94, 740–747. [Google Scholar] [CrossRef]
- Perez-Reyes, M.; Timmons, M.C.; Davis, K.H.; Wall, E.M. A comparison of the pharmacological activity in man of intravenously administered delta9-tetrahydrocannabinol, cannabinol, and cannabidiol. Experientia 1973, 29, 1368–1369. [Google Scholar] [CrossRef] [PubMed]
- Karschner, E.L.; Darwin, W.D.; McMahon, R.P.; Liu, F.; Wright, S.; Goodwin, R.S.; Huestis, M.A. Subjective and physiological effects after controlled Sativex and oral THC administration. Clin. Pharmacol. Ther. 2011, 89, 400–407. [Google Scholar] [CrossRef] [Green Version]
- Martínez, L.; La Maida, N.; Papaseit, E.; Pérez-Mañá, C.; Poyatos, L.; Pellegrini, M.; Pichini, S.; Ventura, M.; Galindo, L.; Busardò, F.P.; et al. Acute pharmacological effects and oral fluid concentrations of the synthetic cannabinoids JWH-122 and JWH-210 in humans after self-administration: An observational study. Front. Pharmacol. 2021, 12, 705643. [Google Scholar] [CrossRef]
- Newmeyer, M.N.; Swortwood, M.J.; Abulseoud, O.A.; Huestis, M.A. Subjective and physiological effects, and expired carbon monoxide concentrations in frequent and occasional cannabis smokers following smoked, vaporized, and oral cannabis administration. Drug Alcohol Depend. 2017, 175, 67–76. [Google Scholar] [CrossRef]
- Klumpers, L.E.; Beumer, T.L.; van Hasselt, J.G.; Lipplaa, A.; Karger, L.B.; Kleinloog, H.D.; Freijer, J.I.; de Kam, M.L.; van Gerven, J.M. Novel Δ(9) -tetrahydrocannabinol formulation Namisol® has beneficial pharmacokinetics and promising pharmacodynamic effects. Br. J. Clin. Pharmacol. 2012, 74, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.I.; van den Elsen, G.A.; Colbers, A.; van der Marck, M.A.; Burger, D.M.; Feuth, T.B.; Rikkert, M.; Kramers, C. Safety and pharmacokinetics of oral delta-9-tetrahydrocannabinol in healthy older subjects: A randomized controlled trial. Eur. Neuropsychopharmacol. 2014, 24, 1475–1482. [Google Scholar] [CrossRef]
- Russo, E.B. Cannabinoids in the management of difficult to treat pain. Ther. Clin. Risk Manag. 2008, 4, 245–259. [Google Scholar] [CrossRef] [Green Version]
- Fredericks, A.B.; Benowitz, N.L.; Savanapridi, C.Y. The cardiovascular and autonomic effects of repeated administration of delta-9-tetrahydrocannabinol to rhesus monkeys. J. Pharmacol. Exp. Ther. 1981, 216, 247–253. [Google Scholar]
- Matsuzaki, M.; Casella, G.A.; Ratner, M. Δ9-Tetrahydrocannabinol: EEG changes, bradycardia and hypothermia in the rhesus monkey. Brain Res. Bull. 1987, 19, 223–229. [Google Scholar] [CrossRef]
- Vivian, J.A.; Kishioka, S.; Butelman, E.R.; Broadbear, J.; Lee, K.O.; Woods, J.H. Analgesic, respiratory and heart rate effects of cannabinoid and opioid agonists in rhesus monkeys: Antagonist effects of SR141716A. J. Pharmacol. Exp. Ther. 1998, 286, 697–703. [Google Scholar] [PubMed]
- Stewart, J.L.; McMahon, L.R. Rimonabant-induced Delta9-tetrahydrocannabinol withdrawal in rhesus monkeys: Discriminative stimulus effects and other withdrawal signs. J. Pharmacol. Exp. Ther. 2010, 334, 347–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jandhyala, B.S.; Hamed, A.T. Pulmonary and systemic hemodynamic effects of delta9-tetrahydrocannabinol in conscious and morphine-chloralose-anesthetized dogs: Anesthetic influence on drug action. Eur. J. Pharmacol. 1978, 53, 63–68. [Google Scholar] [CrossRef]
- Fernández-Trapero, M.; Pérez-Díaz, C.; Espejo-Porras, F.; de Lago, E.; Fernández-Ruiz, J. Pharmacokinetics of Sativex® in dogs: Towards a potential cannabinoid-based therapy for canine disorders. Biomolecules 2020, 10, 279. [Google Scholar] [CrossRef] [Green Version]
- Szabo, B.; Nordheim, U.; Niederhoffer, N. Effects of cannabinoids on sympathetic and parasympathetic neuroeffector transmission in the rabbit heart. J. Pharmacol. Exp. Ther. 2001, 297, 819–826. [Google Scholar]
- Niederhoffer, N.; Szabo, B. Effect of the cannabinoid receptor agonist WIN55212-2 on sympathetic cardiovascular regulation. Br. J. Pharmacol. 1999, 126, 457–466. [Google Scholar] [CrossRef] [Green Version]
- Niederhoffer, N.; Szabo, B. Cannabinoids cause central sympathoexcitation and bradycardia in rabbits. J. Pharmacol. Exp. Ther. 2000, 294, 707–713. [Google Scholar]
- Kawasaki, H.; Watanabe, S.; Oishi, R.; Ueki, S. Effects of delta-9-tetrahydrocannabinol on the cardiovascular system, and pressor and behavioral responses to brain stimulation in rats. Jpn. J. Pharmacol. 1980, 30, 493–502. [Google Scholar] [CrossRef]
- Gardiner, S.M.; March, J.E.; Kemp, P.A.; Bennett, T. Influence of the CB(1) receptor antagonist, AM 251, on the regional haemodynamic effects of WIN-55212-2 or HU 210 in conscious rats. Br. J. Pharmacol. 2002, 136, 581–587. [Google Scholar] [CrossRef] [Green Version]
- Schindler, C.W.; Gramling, B.R.; Justinova, Z.; Thorndike, E.B.; Baumann, M.H. Synthetic cannabinoids found in “spice” products alter body temperature and cardiovascular parameters in conscious male rats. Drug Alcohol Depend. 2017, 179, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Wróbel, A.; Szopa, A.; Serefko, A.; Poleszak, E. A Novel alternative in the treatment of detrusor overactivity? In vivo activity of O-1602, the newly synthesized agonist of GPR55 and GPR18 cannabinoid receptors. Molecules 2020, 25, 1384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ledent, C.; Valverde, O.; Cossu, G.; Petitet, F.; Aubert, J.F.; Beslot, F.; Böhme, G.A.; Imperato, A.; Pedrazzini, T.; Roques, B.P.; et al. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science 1999, 283, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Silvani, A.; Berteotti, C.; Bastianini, S.; Cohen, G.; Lo Martire, V.; Mazza, R.; Pagotto, U.; Quarta, C.; Zoccoli, G. Cardiorespiratory anomalies in mice lacking CB1 cannabinoid receptors. PLoS ONE 2014, 9, e100536. [Google Scholar] [CrossRef]
- Fantauzzi, M.F.; Cass, S.P.; McGrath, J.J.C.; Thayaparan, D.; Wang, P.; Stampfli, M.R.; Hirota, J.A. Development and validation of a mouse model of contemporary cannabis smoke exposure. ERJ Open Res. 2021, 7, 00107–02021. [Google Scholar] [CrossRef] [PubMed]
- Cavero, I.; Buckley, J.P.; Jandhyala, B.S. Hemodynamic and myocardial effects of (-)-delta9-trans-tetrahydrocannabinol in anesthetized dogs. Eur. J. Pharmacol. 1973, 24, 243–251. [Google Scholar] [CrossRef]
- Cavero, I.; Lokhandwala, M.F.; Buckley, J.P.; Jandhyala, B.S. The effect of (minus)-delta 9-trans-tetrahydrocannibinol on myocardial contractility and venous return in anesthetized dogs. Eur. J. Pharmacol. 1974, 29, 74–82. [Google Scholar] [CrossRef]
- Vollmer, R.R.; Cavero, I.; Ertel, R.J.; Solomon, T.A.; Buckley, J.P. Role of the central autonomic nervous system in the hypotension and bradycardia induced by (-)-delta 9-trans-tetrahydrocannabinol. J. Pharm. Pharmacol. 1974, 26, 186–192. [Google Scholar] [CrossRef]
- Graham, J.D.; Li, D.M. Cardiovascular and respiratory effects of cannabis in cat and rat. Br. J. Pharmacol. 1973, 49, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Waldman, M.; Hochhauser, E.; Fishbein, M.; Aravot, D.; Shainberg, A.; Sarne, Y. An ultra-low dose of tetrahydrocannabinol provides cardioprotection. Biochem. Pharmacol. 2013, 85, 1626–1633. [Google Scholar] [CrossRef]
- Bátkai, S.; Pacher, P.; Járai, Z.; Wagner, J.A.; Kunos, G. Cannabinoid antagonist SR-141716 inhibits endotoxic hypotension by a cardiac mechanism not involving CB1 or CB2 receptors. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H595–H600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lake, K.D.; Compton, D.R.; Varga, K.; Martin, B.R.; Kunos, G. Cannabinoid-induced hypotension and bradycardia in rats mediated by CB1-like cannabinoid receptors. J. Pharmacol. Exp. Ther. 1997, 281, 1030–1037. [Google Scholar] [PubMed]
- Yun, J.; Yoon, K.S.; Lee, T.H.; Lee, H.; Gu, S.M.; Song, Y.J.; Cha, H.J.; Han, K.M.; Seo, H.; Shin, J.; et al. Synthetic cannabinoid, JWH-030, induces QT prolongation through hERG channel inhibition. Toxicol. Res. 2016, 5, 1663–1671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krylatov, A.V.; Maslov, L.N.; Ermakov, S.; Lasukova, O.V.; Barzakh, E.I.; Crawford, D.; Pertwee, R.G. Significance of cardiac cannabinoid receptors in regulation of cardiac rhythm, myocardial contractility, and electrophysiologic processes in heart. Izv. Akad. Nauk Ser. Biol. 2007, 34, 28–35. [Google Scholar] [CrossRef]
- Pacher, P.; Bátkai, S.; Kunos, G. Haemodynamic profile and responsiveness to anandamide of TRPV1 receptor knock-out mice. J. Physiol. 2004, 558, 647–657. [Google Scholar] [CrossRef]
- Niederhoffer, N.; Schmid, K.; Szabo, B. The peripheral sympathetic nervous system is the major target of cannabinoids in eliciting cardiovascular depression. Naunyn Schmiedebergs Arch. Pharmacol. 2003, 367, 434–443. [Google Scholar] [CrossRef]
- Baranowska, U.; Göthert, M.; Rudz, R.; Malinowska, B. Methanandamide allosterically inhibits in vivo the function of peripheral nicotinic acetylcholine receptors containing the alpha 7-subunit. J. Pharmacol. Exp. Ther. 2008, 326, 912–919. [Google Scholar] [CrossRef]
- Kurz, C.; Baranowska, U.; Lupiński, S.; Göthert, M.; Malinowska, B.; Schlicker, E. Urethane, but not pentobarbitone, attenuates presynaptic receptor function in rats: A contribution to the choice of anaesthetic. Br. J. Pharmacol. 2009, 157, 1474–1482. [Google Scholar] [CrossRef]
- Zakrzeska, A.; Schlicker, E.; Baranowska, M.; Kozłowska, H.; Kwolek, G.; Malinowska, B. A cannabinoid receptor, sensitive to O-1918, is involved in the delayed hypotension induced by anandamide in anaesthetized rats. Br. J. Pharmacol. 2010, 160, 574–584. [Google Scholar] [CrossRef] [Green Version]
- Li, D.M. The lack of β-adrenoceptor involvement in the cardiac action of Δ1-tetrahydrocannabinol in rats. Clin. Exp. Pharmacol. Physiol. 1980, 7, 23–29. [Google Scholar] [CrossRef]
- Grambow, E.; Strüder, D.; Klar, E.; Hinz, B.; Vollmar, B. Differential effects of endogenous, phyto and synthetic cannabinoids on thrombogenesis and platelet activity. Biofactors 2016, 42, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Zandstra, T.E.; Notenboom, R.G.E.; Wink, J.; Kiès, P.; Vliegen, H.W.; Egorova, A.D.; Schalij, M.J.; De Ruiter, M.C.; Jongbloed, M.R.M. Asymmetry and heterogeneity: Part and parcel in cardiac autonomic innervation and function. Front. Physiol. 2021, 12, 665298. [Google Scholar] [CrossRef]
- Malinowska, B.; Baranowska-Kuczko, M.; Schlicker, E. Triphasic blood pressure responses to cannabinoids: Do we understand the mechanism? Br. J. Pharmacol. 2012, 165, 2073–2088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maggo, S.; Ashton, J.C. Effect of cannabinoid receptor agonists on isolated rat atria. J. Cardiovasc. Pharmacol. 2018, 72, 191–194. [Google Scholar] [CrossRef]
- Molderings, G.J.; Likungu, J.; Göthert, M. Presynaptic cannabinoid and imidazoline receptors in the human heart and their potential relationship. Naunyn Schmiedebergs Arch. Pharmacol. 1999, 360, 157–164. [Google Scholar] [CrossRef]
- Nahas, G.; Trouve, R. Effects and interactions of natural cannabinoids on the isolated heart. Proc. Soc. Exp. Biol. Med. 1985, 180, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Smiley, K.A.; Karler, R.; Turkanis, S.A. Effects of cannabinoids on the perfused rat heart. Res. Commun. Chem. Pathol. Pharmacol. 1976, 14, 659–675. [Google Scholar] [PubMed]
- Krylatov, A.V.; Maslov, L.N.; Ermakov, S.Y.; Barzakh, E.I.; Lasukova, O.V.; Crawford, D.; Ghadessy, R.; Serebrov, V.Y. Negative chronotropic effect of cannabinoids and their water-soluble emulsion is related to activation of cardiac CB1 receptors. Bull. Exp. Biol. Med. 2006, 142, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Krylatov, A.V.; Maslov, L.N.; Lasukova, O.V.; Pertwee, R.G. Cannabinoid receptor antagonists SR141716 and SR144528 exhibit properties of partial agonists in experiments on isolated perfused rat heart. Bull. Exp. Biol. Med. 2005, 139, 558–561. [Google Scholar] [CrossRef]
- Maslov, L.N.; Lasukova, O.V.; Krylatov, A.V.; Uzhachenko, R.V.; Pertwee, R. Selective cannabinoid receptor agonist HU-210 decreases pump function of isolated perfused heart: Role of cAMP and cGMP. Bull. Exp. Biol. Med. 2004, 138, 550–553. [Google Scholar] [CrossRef]
- Ford, W.R.; Honan, S.A.; White, R.; Hiley, C.R. Evidence of a novel site mediating anandamide-induced negative inotropic and coronary vasodilatator responses in rat isolated hearts. Br. J. Pharmacol. 2002, 135, 1191–1198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, J.A.; Abesser, M.; Karcher, J.; Laser, M.; Kunos, G. Coronary vasodilator effects of endogenous cannabinoids in vasopressin-preconstricted unpaced rat isolated hearts. J. Cardiovasc. Pharmacol. 2005, 46, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Díaz, C.; Juárez-Oropeza, M.A.; Mascher, D.; Pavón, N.; Regla, I.; Paredes-Carbajal, M.C. Effects of oleamide on the vasomotor responses in the rat. Cannabis Cannabinoid Res. 2020, 5, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Kurz, C.M.; Gottschalk, C.; Schlicker, E.; Kathmann, M. Identification of a presynaptic cannabinoid CB1 receptor in the guinea-pig atrium and sequencing of the guinea-pig CB1 receptor. J. Physiol. Pharmacol. 2008, 59, 3–15. [Google Scholar]
- Ishac, E.J.; Jiang, L.; Lake, K.D.; Varga, K.; Abood, M.E.; Kunos, G. Inhibition of exocytotic noradrenaline release by presynaptic cannabinoid CB1 receptors on peripheral sympathetic nerves. Br. J. Pharmacol. 1996, 118, 2023–2028. [Google Scholar] [CrossRef] [Green Version]
- Weresa, J.; Pędzińska-Betiuk, A.; Kossakowski, R.; Malinowska, B. Cannabinoid CB1 and CB2 receptors antagonists AM251 and AM630 differentially modulate the chronotropic and inotropic effects of isoprenaline in isolated rat atria. Pharmacol. Rep. 2019, 71, 82–89. [Google Scholar] [CrossRef]
- Sterin-Borda, L.; Del Zar, C.F.; Borda, E. Differential CB1 and CB2 cannabinoid receptor-inotropic response of rat isolated atria: Endogenous signal transduction pathways. Biochem. Pharmacol. 2005, 69, 1705–1713. [Google Scholar] [CrossRef]
- Li, D.M.; Ng, C.K. Effects of delta 1- and delta 6-tetrahydrocannabinol on the adenylate cyclase activity in ventricular tissue of the rat heart. Clin. Exp. Pharmacol. Physiol. 1984, 11, 81–85. [Google Scholar] [CrossRef]
- Formukong, E.A.; Evans, A.T.; Evans, F.J. The inhibitory effects of cannabinoids, the active constituents of Cannabis sativa L. on human and rabbit platelet aggregation. J. Pharm. Pharmacol. 1989, 41, 705–709. [Google Scholar] [CrossRef]
- Maccarrone, M.; Bari, M.; Menichelli, A.; Del Principe, D.; Agrò, A.F. Anandamide activates human platelets through a pathway independent of the arachidonate cascade. FEBS Lett. 1999, 447, 277–282. [Google Scholar] [CrossRef] [Green Version]
- De Angelis, V.; Koekman, A.C.; Weeterings, C.; Roest, M.; de Groot, P.G.; Herczenik, E.; Maas, C. Endocannabinoids control platelet activation and limit aggregate formation under flow. PLoS ONE 2014, 9, e108282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Signorello, M.G.; Giacobbe, E.; Leoncini, G. Activation by 2-arachidonoylglycerol of platelet p38MAPK/cPLA2 pathway. J. Cell Biochem. 2011, 112, 2794–2802. [Google Scholar] [CrossRef] [PubMed]
- Keown, O.P.; Winterburn, T.J.; Wainwright, C.L.; Macrury, S.M.; Neilson, I.; Barrett, F.; Leslie, S.J.; Megson, I.L. 2-arachidonyl glycerol activates platelets via conversion to arachidonic acid and not by direct activation of cannabinoid receptors. Br. J. Clin. Pharmacol. 2010, 70, 180–188. [Google Scholar] [CrossRef] [Green Version]
- Baldassarri, S.; Bertoni, A.; Bagarotti, A.; Sarasso, C.; Zanfa, M.; Catani, M.V.; Avigliano, L.; Maccarrone, M.; Torti, M.; Sinigaglia, F. The endocannabinoid 2-arachidonoylglycerol activates human platelets through non-CB1/CB2 receptors. J. Thromb. Haemost. 2008, 6, 1772–1779. [Google Scholar] [CrossRef] [PubMed]
- Brantl, S.A.; Khandoga, A.L.; Siess, W. Mechanism of platelet activation induced by endocannabinoids in blood and plasma. Platelets 2014, 25, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Almaghrabi, S.; Geraghty, D.; Ahuja, K.; Adams, M. Inhibition of platelet aggregation by vanilloid-like agents is not mediated by transient receptor potential vanilloid-1 channels or cannabinoid receptors. Clin. Exp. Pharmacol. Physiol. 2016, 43, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Braud, S.; Bon, C.; Touqui, L.; Mounier, C. Activation of rabbit blood platelets by anandamide through its cleavage into arachidonic acid. FEBS Lett. 2000, 471, 12–16. [Google Scholar] [CrossRef] [Green Version]
- Chiu, P.; Karler, R.; Craven, C.; Olsen, D.M.; Turkanis, S.A. The influence of delta9-tetrahydrocannabinol, cannabinol and cannabidiol on tissue oxygen consumption. Res. Commun. Chem. Pathol. Pharmacol. 1975, 12, 267–286. [Google Scholar]
- Mendizabal-Zubiaga, J.; Melser, S.; Bénard, G.; Ramos, A.; Reguero, L.; Arrabal, S.; Elezgarai, I.; Gerrikagoitia, I.; Suarez, J.; Rodríguez De Fonseca, F.; et al. Cannabinoid CB1 receptors are localized in striated muscle mitochondria and regulate mitochondrial respiration. Front. Physiol. 2016, 7, 476. [Google Scholar] [CrossRef] [Green Version]
- Whyte, D.A.; Al-Hammadi, S.; Balhaj, G.; Brown, O.M.; Penefsky, H.S.; Souid, A.K. Cannabinoids inhibit cellular respiration of human oral cancer cells. Pharmacology 2010, 85, 328–335. [Google Scholar] [CrossRef]
- Athanasiou, A.; Clarke, A.B.; Turner, A.E.; Kumaran, N.M.; Vakilpour, S.; Smith, P.A.; Bagiokou, D.; Bradshaw, T.D.; Westwell, A.D.; Fang, L.; et al. Cannabinoid receptor agonists are mitochondrial inhibitors: A unified hypothesis of how cannabinoids modulate mitochondrial function and induce cell death. Biochem. Biophys. Res. Commun. 2007, 364, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Salimi, A.; Niknejad, M.; Minouei., M.; Mojarad Aylar, E. Analysis of toxicity effects of delta-9-tetrahydrocannabinol on isolated rat heart mitochondria. Toxicol. Mech. Methods 2022, 32, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Lee, D.I.; Roy Chowdhury, S.; Lu, P.; Kamboj, A.; Anderson, C.M.; Fernyhough, P.; Anderson, H.D. Activation of cannabinoid receptors attenuates endothelin-1-induced mitochondrial dysfunction in rat ventricular myocytes. J. Cardiovasc. Pharmacol. 2020, 75, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, R.D.; Turner, S.R.; Cooper, G.J.; Tattersall, J.E. Effects of seven drugs of abuse on action potential repolarisation in sheep cardiac Purkinje fibres. Eur. J. Pharmacol. 2005, 511, 99–107. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.Y.; Zhou, J.J.; Wei, Y.; Li, Q.; Yang, J.; Zhang, Y. Inhibitory effects of endocannabinoid on the action potential of pacemaker cells in sinoatrial nodes of rabbits. Sheng Li Xue Bao 2013, 651, 29–34. [Google Scholar]
- Li, Q.; Ma, H.J.; Zhang, H.; Qi, Z.; Guan, Y.; Zhang, Y. Electrophysiological effects of anandamide on rat myocardium. Br. J. Pharmacol. 2009, 158, 2022–2029. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Ma, H.J.; Song, S.L.; Shi, M.; Ma, H.; Li, D.P.; Zhang, Y. Effects of anandamide on potassium channels in rat ventricular myocytes: A suppression of I(to) and augmentation of K(ATP) channels. Am. J. Physiol. Cell Physiol. 2012, 302, C924–C930. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Cui, N.; Du, Y.; Ma, H.; Zhang, Y. Anandamide reduces intracellular Ca2+ concentration through suppression of Na+/Ca2+ exchanger current in rat cardiac myocytes. PLoS ONE 2013, 8, e63386. [Google Scholar] [CrossRef] [Green Version]
- Merve, A.O.; Sobiecka, P.; Remeškevičius, V.; Taylor, L.; Saskoy, L.; Lawton, S.; Jones, B.P.; Elwakeel, A.; Mackenzie, F.E.; Polycarpou, E.; et al. Metabolites of cannabis induce cardiac toxicity and morphological alterations in cardiac myocytes. Int. J. Mol. Sci. 2022, 23, 1401. [Google Scholar] [CrossRef]
- Murata, T.; Noritake, K.; Aki, T.; Uemura, K. Possible roles of AMPK and macropinocytosis in the defense responses against Δ9-THC toxicity on HL-1 cardiomyocytes. Toxicol. Rep. 2021, 8, 980–987. [Google Scholar] [CrossRef]
- Perper, J.A.; Van Thiel, D.H. Cardiovascular complications of cocaine abuse. Recent Dev. Alcohol. 1992, 10, 343–361. [Google Scholar] [CrossRef] [PubMed]
- Isoardi, K.Z.; Ayles, S.F.; Harris, K.; Finch, C.J.; Page, C.B. Methamphetamine presentations to an emergency department: Management and complications. Emerg. Med. Australas. 2019, 31, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.P.; Snyder, S.H.; Mechoulam, R. Cannabinoids: Influence on neurotransmitter uptake in rat brain synaptosomes. J. Pharmacol. Exp. Ther. 1975, 194, 74–81. [Google Scholar]
- Ilayan, E.; Feliszek, M.; Malinowska, B.; Schlicker, E. Do cannabinoids exhibit a tyramine-like effect? Naunyn Schmiedebergs Arch. Pharmacol. 2013, 386, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- Bönisch, H.; Fink, K.B.; Malinowska, B.; Molderings, G.J.; Schlicker, E. Serotonin and beyond—A tribute to Manfred Göthert (1939-2019). Naunyn Schmiedebergs Arch. Pharmacol. 2021, 394, 1829–1867. [Google Scholar] [CrossRef] [PubMed]
- Clyburn, C.; Sepe, J.J.; Habecker, B.A. What gets on the nerves of cardiac patients? Pathophysiological changes in cardiac innervation. J. Physiol. 2022, 600, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Yamamoto, M.; Zou, S.; Shimizu, S.; Higashi, Y.; Saito, M. Stimulation of brain cannabinoid CB1 receptors can ameliorate hypertension in spontaneously hypertensive rats. Clin. Exp. Pharmacol. Physiol. 2020, 47, 1254–1262. [Google Scholar] [CrossRef]
- Pfitzer, T.; Niederhoffer, N.; Szabo, B. Central effects of the cannabinoid receptor agonist WIN55212-2 on respiratory and cardiovascular regulation in anaesthetised rats. Br. J. Pharmacol. 2004, 142, 943–952. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, B.M.; Abdel-Rahman, A.A. Role of brainstem GABAergic signaling in central cannabinoid receptor evoked sympathoexcitation and pressor responses in conscious rats. Brain Res. 2011, 1414, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, B.M.; Abdel-Rahman, A.A. Enhancement of rostral ventrolateral medulla neuronal nitric-oxide synthase-nitric-oxide signaling mediates the central cannabinoid receptor 1-evoked pressor response in conscious rats. J. Pharmacol. Exp. Ther. 2012, 341, 579–586. [Google Scholar] [CrossRef] [Green Version]
- Li, G.Q.; Wang, Z.; Zhao, T.; Dai, S.X.; Liu, J.M.; Jia, B.Z.; Zhang, Y.; Li, Q. Role of cannabinoid receptor type 1 in rostral ventrolateral medulla in high-fat diet-induced hypertension in rats. J. Hypertens. 2018, 36, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Penumarti, A.; Abdel-Rahman, A.A. The novel endocannabinoid receptor GPR18 is expressed in the rostral ventrolateral medulla and exerts tonic restraining influence on blood pressure. J. Pharmacol. Exp. Ther. 2014, 349, 29–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padley, J.R.; Li, Q.; Pilowsky, P.M.; Goodchild, A.K. Cannabinoid receptor activation in the rostral ventrolateral medulla oblongata evokes cardiorespiratory effects in anaesthetised rats. Br. J. Pharmacol. 2003, 140, 384–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dean, C. Cannabinoid and GABA modulation of sympathetic nerve activity and blood pressure in the dorsal periaqueductal gray of the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R1765–R1772. [Google Scholar] [CrossRef] [Green Version]
- Dean, C. Endocannabinoid modulation of sympathetic and cardiovascular responses to acute stress in the periaqueductal gray of the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R771–R779. [Google Scholar] [CrossRef] [Green Version]
- Dean, C.; Hillard, C.J.; Seagard, J.L.; Hopp, F.A.; Hogan, Q.H. Components of the cannabinoid system in the dorsal periaqueductal gray are related to resting heart rate. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R254–R262. [Google Scholar] [CrossRef] [Green Version]
- Grzęda, E.; Schlicker, E.; Luczaj, W.; Harasim, E.; Baranowska-Kuczko, M.; Malinowska, B. Bi-directional CB1 receptor-mediated cardiovascular effects of cannabinoids in anaesthetized rats: Role of the paraventricular nucleus. J. Physiol. Pharmacol. 2015, 66, 343–353. [Google Scholar]
- Grzęda, E.; Schlicker, E.; Toczek, M.; Zalewska, I.; Baranowska-Kuczko, M.; Malinowska, B. CB1 receptor activation in the rat paraventricular nucleus induces bi-directional cardiovascular effects via modification of glutamatergic and GABAergic neurotransmission. Naunyn Schmiedebergs Arch. Pharmacol. 2017, 390, 25–35. [Google Scholar] [CrossRef] [Green Version]
- Gomes-de-Souza, L.; Oliveira, L.A.; Benini, R.; Rodella, P.; Costa-Ferreira, W.; Crestani, C.C. Involvement of endocannabinoid neurotransmission in the bed nucleus of stria terminalis in cardiovascular responses to acute restraint stress in rats. Br. J. Pharmacol. 2016, 173, 2833–2844. [Google Scholar] [CrossRef] [Green Version]
- Gomes-de-Souza, L.; Costa-Ferreira, W.; Oliveira, L.A.; Benini, R.; Crestani, C.C. Cannabinoid receptor type 1 in the bed nucleus of the stria terminalis modulates cardiovascular responses to stress via local N-methyl-D-aspartate receptor/neuronal nitric oxide synthase/soluble guanylate cyclase/protein kinase G signaling. J. Psychopharmacol. 2020, 34, 429–440. [Google Scholar] [CrossRef]
- Gomes-de-Souza, L.; Costa-Ferreira, W.; Mendonça, M.M.; Xavier, C.H.; Crestani, C.C. Lateral hypothalamus involvement in control of stress response by bed nucleus of the stria terminalis endocannabinoid neurotransmission in male rats. Sci. Rep. 2021, 11, 16133. [Google Scholar] [CrossRef] [PubMed]
- Rademacher, D.J.; Patel, S.; Hopp, F.A.; Dean, C.; Hillard, C.J.; Seagard, J.L. Microinjection of a cannabinoid receptor antagonist into the NTS increases baroreflex duration in dogs. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H1570–H1576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durakoglugil, M.S.; Orer, H.S. Cannabinoid receptor activation in the nucleus tractus solitaries produces baroreflex-like responses in the rat. Int. J. Biomed. Sci. 2008, 4, 229–237. [Google Scholar] [PubMed]
- Seagard, J.L.; Dean, C.; Patel, S.; Rademacher, D.J.; Hopp, F.A.; Schmeling, W.T.; Hillard, C.J. Anandamide content and interaction of endocannabinoid/GABA modulatory effects in the NTS on baroreflex-evoked sympathoinhibition. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H992–H1000. [Google Scholar] [CrossRef] [Green Version]
- Lagatta, D.C.; Kuntze, L.B.; Ferreira-Junior, N.C.; Resstel, L.B.M. Medial prefrontal cortex TRPV1 and CB1 receptors modulate cardiac baroreflex activity by regulating the NMDA receptor/nitric oxide pathway. Pflugers Arch. 2018, 470, 1521–1542. [Google Scholar] [CrossRef]
- D’Souza, D.C.; Ranganathan, M.; Braley, G.; Gueorguieva, R.; Zimolo, Z.; Cooper, T.; Perry, E.; Krystal, J. Blunted psychotomimetic and amnestic effects of delta-9-tetrahydrocannabinol in frequent users of cannabis. Neuropsychopharmacology 2008, 33, 2505–2516. [Google Scholar] [CrossRef] [Green Version]
- El-Dahan, K.S.; Machtoub, D.; Massoud, G.; Nasser, S.A.; Hamam, B.; Kobeissy, F.; Zouein, F.A.; Eid, A.H. Cannabinoids and myocardial ischemia: Novel insights, updated mechanisms, and implications for myocardial infarction. Curr. Med. Chem. 2021. [Google Scholar] [CrossRef]
- Furberg, C.D.; Psaty, B.M.; Meyer, J.V. Nifedipine. Dose-related increase in mortality in patients with coronary heart disease. Circulation 1995, 92, 1326–1331. [Google Scholar] [CrossRef]
- Dorey, A.; Scheerlinck, P.; Nguyen, H.; Albertson, T. Acute and chronic carbon monoxide toxicity from tobacco smoking. Mil. Med. 2020, 185, 61–67. [Google Scholar] [CrossRef]
- Ramachandra, C.J.A.; Hernandez-Resendiz, S.; Crespo-Avilan, G.E.; Lin, Y.H.; Hausenloy, D.J. Mitochondria in acute myocardial infarction and cardioprotection. EBioMedicine 2020, 57, 102884. [Google Scholar] [CrossRef]
- Zhou, H.; Ren, J.; Toan, S.; Mui, D. Role of mitochondrial quality surveillance in myocardial infarction: From bench to bedside. Ageing Res. Rev. 2021, 66, 101250. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, H. Mitochondrial quality control mechanisms as molecular targets in cardiac ischemia-reperfusion injury. Acta Pharm. Sin. B 2020, 10, 1866–1879. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Tan, Y.; Du, W.; Li, Y.; Toan, S.; Mui, D.; Tian, F.; Zhou, H. Phosphoglycerate mutase 5 exacerbates cardiac ischemia-reperfusion injury through disrupting mitochondrial quality control. Redox Biol. 2021, 38, 101777. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Mui, D.; Toan, S.; Zhu, P.; Li, R.; Zhou, H. SERCA overexpression improves mitochondrial quality control and attenuates cardiac microvascular ischemia-reperfusion injury. Mol. Ther. Nucleic Acids 2020, 22, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Lochner, A.; Wang, H.H.; Wang, S.; Zhu, H.; Ren, J.; Zhou, H. Coronary microvascular injury in myocardial infarction: Perception and knowledge for mitochondrial quality control. Theranostics 2021, 11, 6766–6785. [Google Scholar] [CrossRef] [PubMed]


| Type of study Number of Patients/ Sample/Evaluation Period | Results | Final Conclusion | References |
|---|---|---|---|
| Systematic review 115 articles (81 case reports, 29 observational studies, 3 clinical trials and 2 experimental studies). 116 individuals in case reports, mean age 31 (2011–2016) |
| association between exposure to cannabis-based products and cardiovascular disease; evidence stronger for ischemic strokes than for any other cardiovascular diseases | [54] |
| Epidemiologic study from NIS 2,451,933 acute MI patients, mean age 49 (2010-2014) | cannabis use raised the risk of acute MI by 3–8% | importance of patient history including recreational drug use in identifying the etiology of an unexplained MI | [55] |
| Epidemiologic study from NIS 316,397 patients, aged 18–55 (2009–2010) | heart failure (1.4 vs. 1.2%), cerebrovascular accidents (1.03 vs. 0.62%), CAD (5.0 vs. 4.6%) and sudden cardiac death (0.21 vs. 0.17%) significantly higher in cannabis users | cannabis use is an independent predictor of heart failure and cerebrovascular accidents | [56] |
| Retrospective cohort analysis 292,770 patients with a history of cannabis abuse and 10,542,348 age- and sex-matched controls, mean age 37 (2011–2016) | 3-year cumulative incidence of MI significantly higher in the cannabis-abuse group than in controls (1.37% vs. 0.54%); most pronounced risk in the young and middle-aged | cannabis abuse may be associated with an elevated risk of MI independent of other cardiovascular risk factors, with higher relative risk in women and younger age groups | [53] |
| Epidemiologic study from NIS 52,290,927 hospitalized patients, aged 18–39 years (2007–2014) | hospitalizations among young cannabis users, compared to non-users increased by 50% for MI, 79% for arrhythmias, 300% for stroke and 75% for venous thromboembolic events | young cannabis users are more at risk in hospitalizations for acute MI, arrhythmia, and stroke | [57] |
| Scoping review 92 articles (1 randomized control trial, 4 systematic reviews, 19 literature reviews, 11 large database reviews, and 42 case reports/series) (2003–2018) |
|
| [13] |
| Epidemiologic study from NHANES 89.6 million adults who reported marijuana use (2005–2016) | 2015–2016: 2 million (2.3%) cannabis users have a cardiovascular disease | screening for marijuana use should be conducted in young patients with cardiovascular disease | [14] |
| Epidemiologic study from NIS 3,307,310 hospitalizations were noted with cannabis use disorder, mean age 36 (2007–2014) | among cannabis users 0.7% (n = 24,148) had malignant hypertension | it is necessary to develop an intervention to raise awareness regarding the deleterious effect of cannabis use and to curtail additional healthcare costs | [58] |
| Retrospective longitudinal cohort study 18,653 adult cannabis patients were matched to 51,243 controls, aged 31–60 years (2014–2017) | incidence rates for ACS or stroke were 7.19/1000 and 5.67/1000 person/year in the cannabis and control group | medical cannabis authorization associated with increased risk of ED visits or hospitalization for cardiovascular events including stroke and ACS | [59] |
| Systematic review 16 studies: 4 cohort, 8 case-control studies, 1 case-crossover study, 2 randomized controlled trials and 1 descriptive study with 10 to 118,659,619 participants (1970–2018) | marijuana use was related to an increased risk of MI in 2 studies and of stroke in 6 studies (within 24 h) | marijuana users may be at increased risk of cardiovascular events | [60] |
| Epidemiologic study from NHANES 3634 participants (1988–1994) | about 26.0% (n = 900) of participants (mean age 48) were ever cannabis users and 15.5% (n = 538) had myocardial injury | cannabis use increased risk of myocardial injury among people without cardiovascular disease; effect increased by coexistent hypertension | [61] |
| Epidemiologic study from BRFSS 33,173 young adult par-ticipants, 4610 (17.5%) respondents reported recent cannabis use (2017–2018) | MI more frequent among recent cannabis users (61 of 4610, 1.3%) relative to non-users (240 of 28,563, 0.8%) history of MI associated with cannabis use of more than 4 times per month and with smoking as the primary method of consumption |
| [62] |
| Species | Anesthesia | Agonist | Dose (mg/kg) and Route of Administration 1 | Effects | Mechanisms and Involvement of CB1-Rs/CB2-Rs/Others if Determined | References |
|---|---|---|---|---|---|---|
| rhesus monkeys | conscious | THC | 0.5 i.v. | ↑HR | antagonists not used | [133] |
| rhesus monkeys | conscious | THC | 0.75–4 i.p. | ↓HR | antagonists not used | [134] |
| rhesus monkeys | conscious | THC WIN-2 | 0.1–10 i.m. 0.1–10 i.m. | ↓HR | bradycardia is mediated viaCB1-Rs (prevented by RIM) | [135] |
| rhesus monkeys | conscious | RIM | 3.2 i.v. | ↑HR | CB1-Rs responsible for bradycardia are activated via endocannabinoids | [136] |
| mongrel dogs | conscious | THC | 1 and 2.5 i.v. | ↓HR and CO dose-dependent; ↑RVSW | antagonists not used | [137] |
| beagle dogs | conscious | Sativex® (CBD: THC) | spray; max. plasma levels 10.5:18.5 ng/mL | ↔HR | antagonists not used | [138] |
| rabbits | conscious | WIN-2 | 0.005 and 0.05 i.v. 0.5 i.v. | ↓HR ↑HR | both bradycardia and tachycardia are mediated by CB1-Rs, since RIM reduced bradycardia and reversed tachycardia | [139] |
| rabbits | conscious | CP WIN-2 WIN-3 | each 0.0001, 0.001, 0.01 i.c. | ↓HR; dose-dependent ↓HR; dose-dependent ↔HR | bradycardia related to an increase in cardiac vagal activity and CB1-Rs (prevented/diminished by i.v. atropine and RIM) | [140,141] |
| Wistar rats | conscious | THC | 4, 6, 8 i.p. | ↓HR | antagonists not used | [142] |
| Sprague Dawley rats | conscious | WIN-2 | 0.005, 0.05 0.25 i.v. | ↔HR ↓HR | since WIN-2 increased BP authors suggested that the clear dissociation between the effects of WIN-2 on BP and HR are consistent with the bradycardia being mediated centrally | [143] |
| Sprague Dawley rats | conscious | THC JWH-018 AM2201 XLR-11 CP | 0.3–3.0 s.c. 0.18–0.56 0.1–0.3 0.1–3.0 0.01–0.1 | ↔HR ↔HR ↔HR ↔HR ↔HR | THC, CP and the synthetic cannabinoids JWH-018, AM2201 and XLR-11 increase BP only | [144] |
| Wistar rats | conscious | O-1602 | 0.25 i.a. | ↔HR | antagonists not used | [145] |
| CB1+/+ and CB1−/− mice | conscious | AEA WIN-2 | 0.25 2 i.v. | ↓HR but only in CB1+/+ but not in CB1−/− | bradycardia is mediated via CB1-Rs | [146] |
| CB1+/+ and CB1−/− mice | conscious | deletion of CB1-R | - | basal HR was higher in CB1−/− than in CB1+/+ but only during active period | eCBs induce bradycardia via the activation of CB1-Rs but only during active period | [147] |
| mice GPR55+/+ GPR55−/− | conscious or isoflurane | deletion of GPR55 | - | in GPR55−/−: ↓basal HR ↑LVEDV, LV mass, heart weight, ↔LVID, CO, EF | GPR55 affects preload and chronotropy | [85] |
| mice | conscious | THC SDB-001 JWH-018 | 1–10 i.p. 0.3–3 1–10 | ↓HR ↓HR ↓HR | the effect of THC on HR is shared by another two CB-R agonists (antagonists not used) | [25] |
| mice | conscious | cannabis THC (10–14%) and CBD (0–2%) | cigarettes via Smoke Inhalation System | ↑serum COHb modulation of the proportionality of innate immune-cell populations in the lungs | increase in serum COHb level and modification of immunological system | [148] |
| mongrel dogs | morphine, chloralose | THC | 1 and 2.5 i.v. | ↑HR (dose-dependent) ↓RVSW | antagonists not used | [137] |
| mongrel dogs | pentobarbital | THC | 2.5 i.v | ↓HR | the maximal THC-induced bradycardia occurred only when both sympathetic and parasympathetic innervation to the heart was intact | [149] |
| mongrel dogs | pentobarbital | THC | 2.5 i.v | HR was constant by electrical pacing; ↓CO, ↓SV, ↓LVP ↓LVEDP, ↓+dp/dt | ↓CO mainly due to diminished venous return to the heart and not to impaired contractile force of the myocardium (experiments in which CO was constant by a right heart-bypass procedure) | [150] |
| cats | chloralose | THC THC | 2 i.v. 2 i.c.v. | ↓HR ↓HR | bradycardia induced by a central mechanism was diminished by cervical cardiac denervation but not by vagotomy | [151] |
| cats | pentobarbital | THC | 0.2 i.v | ↓HR | bradycardia antagonists not used | [152] |
| mice | isoflurane | THC | 0.002 i.p. | ↔HR, LVESD, LVEDD, FS | antagonists not used; lack of effect not surprising since a very low dose was chosen | [153] |
| Wistar rats | urethane | THC | 1, 2, 5 i.v. | ↓HR | bradycardia due to alteration of efferent vagal activity (blocked by vagotomy and atropine i.v.) | [142] |
| Sprague Dawley rats | urethane | THC ∆8-THC | 0.5 i.v. 0.5 i.v. | ↓HR ↓HR | bradycardia | [152] |
| Sprague Dawley rats | pentobarbital | RIM AM251 | 3 i.v. 3 i.v. | prevention (by RIM but not AM251) of the LPS-induced ↓cardiac contractility (+dp/dt and LVSP) but not of ↑HR | a cardiac receptor distinct from CB1-R or CB2-R mediates negative inotropy | [154] |
| Sprague Dawley rats | diethyl ether + urethane | THC THC HU-210 CP WIN-2 JWH-015 AEA | 0.03–10 i.v. 30 i.v. 0.003–0.3 i.v. 0.001–0.3 i.v. 0.01–10 i.v. 3–30 i.v. 4 i.v. | ↓HR dose-dependent ↑HR ↓HR dose-dependent ↓HR dose-dependent ↓HR dose-dependent ↓HR dose-dependent initial and delayed ↓HR | bradycardia induced by all compounds mediated by CB1-Rs (blocked by RIM); in the case of AEA, only the delayed ↓HR was diminished by RIM | [155] |
| Sprague Dawley rats | pentobarbital + isoflurane | JWH-030 | 0.1 i.v. 0.5 i.v. | ↔ QT interval ↑ QT interval, ↔RR interval | prolongation of the QT interval may be associated with adverse cardiovascular effects in abusers of synthetic cannabinoids | [156] |
| Wistar rats | chloralose | HU-210 AEA MethAEA ACPA | 0.1 i.v. 2.5 i.v. 2.5 i.v. 0.125 i.v. | ↓HR, ↔ECG ↓HR, ↑duration QRS ↓HR ↔ ECG ↓HR ↔ ECG | bradycardia mediated by CB1-Rs (inhibited by RIM but not by SR144528) | [157] |
| mice TRPV1+/+ TRPV1−/− | pentobarbital | AEA | 20 i.v. | brief (Phase I) and profound (Phase II) ↓HR, LVSP, LVEDP, +dp/dt, −dp/dt | brief (Phase I) and profound (Phase II) bradycardia and ↓cardiac contractility due to AEA mediated via TRPV1- and CB1-Rs, respectively (absent/present in TRPV1−/− and not modified/blocked by RIM, respectively); basal LVSP, LVEDP, +dp/dt, −dp/dt and HR did not differ between TRPV1+/+ and TRPV1−/− | [158] |
| Wistar rats | urethane | WIN-2 CP | 0.03–1 i.v. 0.03–1 i.v. | ↓HR and plasma [NA] less pronounced in ventilated than in spontaneously breathing rats | depressive action of CBs depends on the respiratory state of the animals and CB1-Rs inhibiting sympathetic and intensifying cardiac vagal tone, since ↓HR and plasma [NA] were diminished by RIM and methylatropine | [159] |
| urethane plus pancuronium | WIN-2 | 0.03–1 i.v. | ||||
| mice GPR55+/+ GPR55−/− | ketamine/ xylazine | deletion of GPR55 | - | GPR55−/−: young: ↑HR, ↔ most other cardiac functions; mature: cardiac dysfunction (↑LVESV, ↑LVEDV, ↓EF) and ventricular remodeling (↓LV free wall thickness, ↓heart weight/body weight ratio); young and mature: ↓cardiostimulatory responses to α1/β1-AR agonist dobutamine | GPR55 involved in the control of adrenergic signalling | [87] |
| mongrel dogs (spinal) | pentobarbital | THC | 2.5 i.v | ↔ HR; ↔ increases in HR induced by ES or by ISO | THC is devoid of any ganglionic or β-adrenergic blocking properties | [149] |
| Wistar rats (pithed) 2 | Pentobarbital 3 | WIN-2 CP MethAEA WIN-3 | 0.0005–0.5 i.v. 0.0004–0.4 i.v. 1.1, 3.6 i.v. 0.0005–0.5 i.v. | ↔HR, ↓increases in HR induced by ES 5 and NIC ↔increases in HR induced by ISO ↔ ES 5 increases in HR | ↓neurogenic sympathetic neuroeffector transmission in the heart via CB1-Rs located prejunctionally on the postganglionic rather than on the preganglionic sympathetic nerve fibers innervating the heart (blocked by RIM and/or AM251) and not on the chromaffin cells of the adrenal medulla (inhibitory effect of MethAEA on NIC-induced increase in HR not modified by AM251) | [40,160] |
| Wistar rats (pithed, adrenalectomized) 2 | Pentobarbital 3 | WIN-2 CP | 0.0005–0.5 i.v. 0.0004–0.4 i.v. | ↔HR, ↓increases in HR induced by ES 5 and NIC | [40] | |
| Wistar rats (pithed) 2 | pentobarbital or urethane 3 | CP | 0.4 i.v. | ↔HR, ↓increases in HR induced by ES 5 | stronger inhibitory effect of CP on the neurogenic tachycardic response in pentobarbitone- than in urethane-anaesthetized rats | [161] |
| Wistar rats (pithed) 3 | Pentobarbital 2 | WIN-2 CP WIN-3 | 0.0005–0.5 i.v. 0.0004–0.4 i.v. 0.0005-0.5 i.v. | ↔ HR, ↓decreases in HR induced by ES of n. vagus ↔decreases in HR induced by methacholine | presynaptic CB1-Rs located on the post- and/or preganglionic cardiac vagal nerve fibers did not modify the vagal bradycardia | [40] |
| rabbits (pithed) 4 | pentobarbital | WIN-2 CP | 0.005–1.5 i.v. 0.003–1 i.v. | both: ↓increase in HR induced by ES 5 WIN-2: ↔increase in HR induced by ISO | ↓neurogenic sympathetic and vagal neuroeffector transmission in the heart (via CB1-Rs on pre- or postganglionic neurons; blocked by RIM) | [139] |
| WIN-2 CP | 0.005–0.5 i.v. 0.003–0.3 i.v. | ↓ES decreases in HR elicited by ES of n. vagus | ||||
| Wistar rats (pithed) 2 | Urethane 3 | AEA CP | 1 i.v. 0.4 i.v. | ↔HR; ↓increases in HR induced by ES 5 | ↓neurogenic sympathetic tachycardia due to the presynaptic CB1-R but not GPR18 (blocked by AM251 but not O-1918, respectively) | [162] |
| Sprague Dawley rats (pithed) | ether | THC | 1 i.v. | ↔HR; ↔alterations in HR induced by ISO and propranolol | β-adrenoceptors not involved in the cardiac action of THC | [163] |
| hairless mice | ketamine and xylazine | AEA CBD WIN-2 | 5 i.p. 5 i.p. 5 i.p. | AEA (unlike CBD and WIN-2) ↓venular thrombus formation in ear venules | ↓thrombus formation evoked by AEA was dependent on cyclooxygenase metabolites (it was reduced by INDO i.p.) | [164] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weresa, J.; Pędzińska-Betiuk, A.; Mińczuk, K.; Malinowska, B.; Schlicker, E. Why Do Marijuana and Synthetic Cannabimimetics Induce Acute Myocardial Infarction in Healthy Young People? Cells 2022, 11, 1142. https://doi.org/10.3390/cells11071142
Weresa J, Pędzińska-Betiuk A, Mińczuk K, Malinowska B, Schlicker E. Why Do Marijuana and Synthetic Cannabimimetics Induce Acute Myocardial Infarction in Healthy Young People? Cells. 2022; 11(7):1142. https://doi.org/10.3390/cells11071142
Chicago/Turabian StyleWeresa, Jolanta, Anna Pędzińska-Betiuk, Krzysztof Mińczuk, Barbara Malinowska, and Eberhard Schlicker. 2022. "Why Do Marijuana and Synthetic Cannabimimetics Induce Acute Myocardial Infarction in Healthy Young People?" Cells 11, no. 7: 1142. https://doi.org/10.3390/cells11071142