Metabolic Profiling of CHO Cells during the Production of Biotherapeutics
Abstract
:1. Introduction
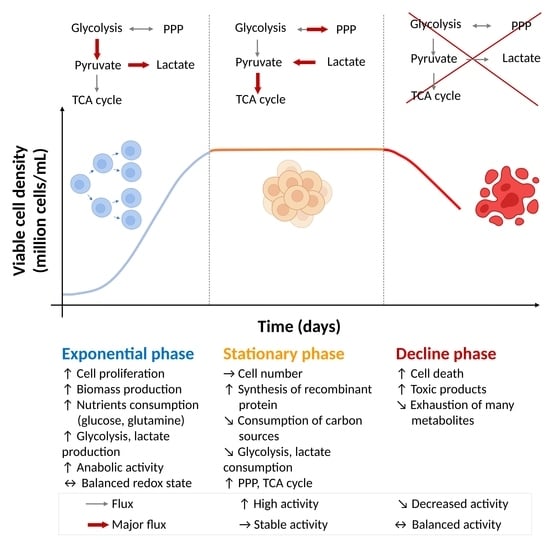
2. The Exponential Growth Phase: High Nutrient Uptake Enabling Cell Growth
2.1. Carbon Source Catabolism Generates Energy for the Cells as Nutrients Are Largely Available (Step 1)
2.2. The Use of Energy Enables Biomass Production (Step 2)
3. The Stationary Phase: Stabilized Growth and High Recombinant Protein Production
3.1. Alternative Carbon Source Catabolism Enables Energy Production and Cell Maintenance
3.2. The Use of Energy Enables Recombinant Protein Production
4. The Decline Phase: Media Exhaustion Resulting in Cell Death
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, S.; Kumar, N.K.; Dwiwedi, P.; Charan, J.; Kaur, R.; Sidhu, P.; Chugh, V.K. Monoclonal Antibodies: A Review. Curr. Clin. Pharmacol. 2018, 13, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Walsh, G. Biopharmaceutical benchmarks 2018. Nat. Biotechnol. 2018, 36, 1136–1145. [Google Scholar] [CrossRef] [PubMed]
- Kunert, R.; Reinhart, D. Advances in recombinant antibody manufacturing. Appl. Microbiol. Biotechnol. 2016, 100, 3451–3461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.; Vijayasankaran, N.; Shen, A.Y.; Kiss, R.; Amanullah, A. Cell culture processes for monoclonal antibody production. MAbs 2010, 2, 466–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ritacco, F.V.; Wu, Y.; Khetan, A. Cell culture media for recombinant protein expression in Chinese hamster ovary (CHO) cells: History, key components, and optimization strategies. Biotechnol. Prog. 2018, 34, 1407–1426. [Google Scholar] [CrossRef] [PubMed]
- Oliver, S.G.; Winson, M.K.; Kell, D.B.; Baganz, F. Systematic functional analysis of the yeast genome. Trends Biotechnol. 1998, 16, 373–378. [Google Scholar] [CrossRef]
- Zhang, A.; Sun, H.; Xu, H.; Qiu, S.; Wang, X. Cell metabolomics. Omics A J. Integr. Biol. 2013, 17, 495–501. [Google Scholar] [CrossRef] [Green Version]
- León, Z.; García-Cañaveras, J.C.; Donato, M.T.; Lahoz, A. Mammalian cell metabolomics: Experimental design and sample preparation. Electrophoresis 2013, 34, 2762–2775. [Google Scholar] [CrossRef]
- Cuperlović-Culf, M.; Barnett, D.A.; Culf, A.S.; Chute, I. Cell culture metabolomics: Applications and future directions. Drug Discov. Today 2010, 15, 610–621. [Google Scholar] [CrossRef]
- Roberts, L.D.; Souza, A.L.; Gerszten, R.E.; Clish, C.B. Targeted metabolomics. Curr. Protoc. Mol. Biol. 2012, 98, 30–32. [Google Scholar] [CrossRef]
- Ahn, W.S.; Antoniewicz, M.R. Metabolic flux analysis of CHO cells at growth and non-growth phases using isotopic tracers and mass spectrometry. Metab. Eng. 2011, 13, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Chong, W.P.; Goh, L.T.; Reddy, S.G.; Yusufi, F.N.; Lee, D.Y.; Wong, N.S.; Heng, C.K.; Yap, M.G.; Ho, Y.S. Metabolomics profiling of extracellular metabolites in recombinant Chinese Hamster Ovary fed-batch culture. Rapid Commun. Mass Spectrom. 2009, 23, 3763–3771. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, N.; Rose, S.T.; Morgan, J.A. Metabolic flux analysis of CHO cell metabolism in the late non-growth phase. Biotechnol. Bioeng. 2011, 108, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Sellick, C.A.; Croxford, A.S.; Maqsood, A.R.; Stephens, G.M.; Westerhoff, H.V.; Goodacre, R.; Dickson, A.J. Metabolite profiling of CHO cells: Molecular reflections of bioprocessing effectiveness. Biotechnol. J. 2015, 10, 1434–1445. [Google Scholar] [CrossRef]
- Karst, D.J.; Steinhoff, R.F.; Kopp, M.R.G.; Serra, E.; Soos, M.; Zenobi, R.; Morbidelli, M. Intracellular CHO Cell Metabolite Profiling Reveals Steady-State Dependent Metabolic Fingerprints in Perfusion Culture. Biotechnol. Prog. 2017, 33, 879–890. [Google Scholar] [CrossRef]
- Sellick, C.A.; Croxford, A.S.; Maqsood, A.R.; Stephens, G.; Westerhoff, H.V.; Goodacre, R.; Dickson, A.J. Metabolite profiling of recombinant CHO cells: Designing tailored feeding regimes that enhance recombinant antibody production. Biotechnol. Bioeng. 2011, 108, 3025–3031. [Google Scholar] [CrossRef]
- Selvarasu, S.; Ho, Y.S.; Chong, W.P.; Wong, N.S.; Yusufi, F.N.; Lee, Y.Y.; Yap, M.G.; Lee, D.Y. Combined in silico modeling and metabolomics analysis to characterize fed-batch CHO cell culture. Biotechnol. Bioeng. 2012, 109, 1415–1429. [Google Scholar] [CrossRef]
- Templeton, N.; Dean, J.; Reddy, P.; Young, J.D. Peak antibody production is associated with increased oxidative metabolism in an industrially relevant fed-batch CHO cell culture. Biotechnol. Bioeng. 2013, 110, 2013–2024. [Google Scholar] [CrossRef]
- Dean, J.; Reddy, P. Metabolic analysis of antibody producing CHO cells in fed-batch production. Biotechnol. Bioeng. 2013, 110, 1735–1747. [Google Scholar] [CrossRef]
- Chong, W.P.; Reddy, S.G.; Yusufi, F.N.; Lee, D.Y.; Wong, N.S.; Heng, C.K.; Yap, M.G.; Ho, Y.S. Metabolomics-driven approach for the improvement of Chinese hamster ovary cell growth: Overexpression of malate dehydrogenase II. J. Biotechnol. 2010, 147, 116–121. [Google Scholar] [CrossRef]
- Mulukutla, B.C.; Kale, J.; Kalomeris, T.; Jacobs, M.; Hiller, G.W. Identification and control of novel growth inhibitors in fed-batch cultures of Chinese hamster ovary cells. Biotechnol. Bioeng. 2017, 114, 1779–1790. [Google Scholar] [CrossRef] [PubMed]
- Alden, N.; Raju, R.; McElearney, K.; Lambropoulos, J.; Kshirsagar, R.; Gilbert, A.; Lee, K. Using Metabolomics to Identify Cell Line-Independent Indicators of Growth Inhibition for Chinese Hamster Ovary Cell-based Bioprocesses. Metabolites 2020, 10, 199. [Google Scholar] [CrossRef] [PubMed]
- Nicolae, A.; Wahrheit, J.; Bahnemann, J.; Zeng, A.P.; Heinzle, E. Non-stationary 13C metabolic flux analysis of Chinese hamster ovary cells in batch culture using extracellular labeling highlights metabolic reversibility and compartmentation. BMC Syst. Biol. 2014, 8, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nargund, S.; Qiu, J.; Goudar, C.T. Elucidating the role of copper in CHO cell energy metabolism using 13C metabolic flux analysis. Biotechnol. Prog. 2015, 31, 1179–1186. [Google Scholar] [CrossRef]
- Kirsch, B.J.; Bennun, S.V.; Mendez, A.; Johnson, A.S.; Wang, H.; Qiu, H.; Li, N.; Lawrence, S.M.; Bak, H.; Betenbaugh, M.J. Metabolic Analysis of the Asparagine and Glutamine Dynamics in an Industrial CHO Fed-Batch Process. Biotechnol. Bioeng. 2021, 119, 807–819. [Google Scholar] [CrossRef]
- Torres, M.; Elvin, M.; Betts, Z.; Place, S.; Gaffney, C.; Dickson, A.J. Metabolic profiling of Chinese hamster ovary cell cultures at different working volumes and agitation speeds using spin tube reactors. Biotechnol. Prog. 2021, 37, e3099. [Google Scholar] [CrossRef]
- Miccheli, A.T.; Miccheli, A.; Di Clemente, R.; Valerio, M.; Coluccia, P.; Bizzarri, M.; Conti, F. NMR-based metabolic profiling of human hepatoma cells in relation to cell growth by culture media analysis. Biochim. Biophys. Acta 2006, 1760, 1723–1731. [Google Scholar] [CrossRef]
- Khoo, S.H.; Al-Rubeai, M. Metabolic characterization of a hyper-productive state in an antibody producing NS0 myeloma cell line. Metab. Eng. 2009, 11, 199–211. [Google Scholar] [CrossRef]
- Luo, J.; Vijayasankaran, N.; Autsen, J.; Santuray, R.; Hudson, T.; Amanullah, A.; Li, F. Comparative metabolite analysis to understand lactate metabolism shift in Chinese hamster ovary cell culture process. Biotechnol. Bioeng. 2012, 109, 146–156. [Google Scholar] [CrossRef]
- Matuszczyk, J.C.; Teleki, A.; Pfizenmaier, J.; Takors, R. Compartment-specific metabolomics for CHO reveals that ATP pools in mitochondria are much lower than in cytosol. Biotechnol. J. 2015, 10, 1639–1650. [Google Scholar] [CrossRef]
- Vodopivec, M.; Lah, L.; Narat, M.; Curk, T. Metabolomic profiling of CHO fed-batch growth phases at 10, 100, and 1,000 L. Biotechnol. Bioeng. 2019, 116, 2720–2729. [Google Scholar] [CrossRef] [PubMed]
- Goudar, C.; Biener, R.; Boisart, C.; Heidemann, R.; Piret, J.; de Graaf, A.; Konstantinov, K. Metabolic flux analysis of CHO cells in perfusion culture by metabolite balancing and 2D [13C, 1H] COSY NMR spectroscopy. Metab. Eng. 2010, 12, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Ahn, W.S.; Antoniewicz, M.R. Parallel labeling experiments with [1,2-13C]glucose and [U-13C]glutamine provide new insights into CHO cell metabolism. Metab. Eng. 2013, 15, 34–47. [Google Scholar] [CrossRef]
- Templeton, N.; Lewis, A.; Dorai, H.; Qian, E.A.; Campbell, M.P.; Smith, K.D.; Lang, S.E.; Betenbaugh, M.J.; Young, J.D. The impact of anti-apoptotic gene Bcl-2∆ expression on CHO central metabolism. Metab. Eng. 2014, 25, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Slade, P.G.; Caspary, R.G.; Nargund, S.; Huang, C.J. Mannose metabolism in recombinant CHO cells and its effect on IgG glycosylation. Biotechnol. Bioeng. 2016, 113, 1468–1480. [Google Scholar] [CrossRef] [PubMed]
- Wijaya, A.W.; Verhagen, N.; Teleki, A.; Takors, R. Compartment-specific 13C metabolic flux analysis reveals boosted NADPH availability coinciding with increased cell-specific productivity for IgG1 producing CHO cells after MTA treatment. Eng. Life Sci. 2021, 21, 832–847. [Google Scholar] [CrossRef]
- Wijaya, A.W.; Ulmer, A.; Hundsdorfer, L.; Verhagen, N.; Teleki, A.; Takors, R. Compartment-specific metabolome labeling enables the identification of subcellular fluxes that may serve as promising metabolic engineering targets in CHO cells. Bioprocess Biosyst. Eng. 2021, 44, 2567–2578. [Google Scholar] [CrossRef]
- Pereira, S.; Kildegaard, H.F.; Andersen, M.R. Impact of CHO Metabolism on Cell Growth and Protein Production: An Overview of Toxic and Inhibiting Metabolites and Nutrients. Biotechnol. J. 2018, 13, e1700499. [Google Scholar] [CrossRef] [Green Version]
- Quek, L.E.; Dietmair, S.; Krömer, J.O.; Nielsen, L.K. Metabolic flux analysis in mammalian cell culture. Metab. Eng. 2010, 12, 161–171. [Google Scholar] [CrossRef]
- Junghans, L.; Teleki, A.; Wijaya, A.W.; Becker, M.; Schweikert, M.; Takors, R. From nutritional wealth to autophagy: In vivo metabolic dynamics in the cytosol, mitochondrion and shuttles of IgG producing CHO cells. Metab. Eng. 2019, 54, 145–159. [Google Scholar] [CrossRef]
- Sheikholeslami, Z.; Jolicoeur, M.; Henry, O. Probing the metabolism of an inducible mammalian expression system using extracellular isotopomer analysis. J. Biotechnol. 2013, 164, 469–478. [Google Scholar] [CrossRef]
- Sheikholeslami, Z.; Jolicoeur, M.; Henry, O. Elucidating the effects of postinduction glutamine feeding on the growth and productivity of CHO cells. Biotechnol. Prog. 2014, 30, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Chong, W.P.; Thng, S.H.; Hiu, A.P.; Lee, D.Y.; Chan, E.C.; Ho, Y.S. LC-MS-based metabolic characterization of high monoclonal antibody-producing Chinese hamster ovary cells. Biotechnol. Bioeng. 2012, 109, 3103–3111. [Google Scholar] [CrossRef] [PubMed]
- Carinhas, N.; Duarte, T.M.; Barreiro, L.C.; Carrondo, M.J.; Alves, P.M.; Teixeira, A.P. Metabolic signatures of GS-CHO cell clones associated with butyrate treatment and culture phase transition. Biotechnol. Bioeng. 2013, 110, 3244–3257. [Google Scholar] [CrossRef]
- Blondeel, E.J.M.; Ho, R.; Schulze, S.; Sokolenko, S.; Guillemette, S.R.; Slivac, I.; Durocher, Y.; Guillemette, J.G.; McConkey, B.J.; Chang, D.; et al. An omics approach to rational feed: Enhancing growth in CHO cultures with NMR metabolomics and 2D-DIGE proteomics. J. Biotechnol. 2016, 234, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Aranibar, N.; Borys, M.; Mackin, N.A.; Ly, V.; Abu-Absi, N.; Abu-Absi, S.; Niemitz, M.; Schilling, B.; Li, Z.J.; Brock, B.; et al. NMR-based metabolomics of mammalian cell and tissue cultures. J. Biomol. NMR 2011, 49, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Chong, W.P.; Yusufi, F.N.; Lee, D.Y.; Reddy, S.G.; Wong, N.S.; Heng, C.K.; Yap, M.G.; Ho, Y.S. Metabolomics-based identification of apoptosis-inducing metabolites in recombinant fed-batch CHO culture media. J. Biotechnol. 2011, 151, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Carvalhal, A.V.; Santos, S.S.; Calado, J.; Haury, M.; Carrondo, M.J. Cell growth arrest by nucleotides, nucleosides and bases as a tool for improved production of recombinant proteins. Biotechnol. Prog. 2003, 19, 69–83. [Google Scholar] [CrossRef]
- Chen, X.G.; Wang, R.S.; Deng, M.X.; Ran, X.Z. Effects of exogenerous nucleotides on the apoptosis of intestinal epithelial cells IEC-6. J. Hyg. Res. 2005, 34, 701–704. [Google Scholar]
- Zhang, L.-X.; Zhang, W.-Y.; Wang, C.; Liu, J.-T.; Deng, X.-C.; Liu, X.-P.; Fan, L.; Tan, W.-S. Responses of CHO-DHFR cells to ratio of asparagine to glutamine in feed media: Cell growth, antibody production, metabolic waste, glutamate, and energy metabolism. Bioresour. Bioprocess. 2016, 3, 5. [Google Scholar] [CrossRef] [Green Version]
- Duarte, T.M.; Carinhas, N.; Barreiro, L.C.; Carrondo, M.J.; Alves, P.M.; Teixeira, A.P. Metabolic responses of CHO cells to limitation of key amino acids. Biotechnol. Bioeng. 2014, 111, 2095–2106. [Google Scholar] [CrossRef] [PubMed]
- Mohmad-Saberi, S.E.; Hashim, Y.Z.; Mel, M.; Amid, A.; Ahmad-Raus, R.; Packeer-Mohamed, V. Metabolomics profiling of extracellular metabolites in CHO-K1 cells cultured in different types of growth media. Cytotechnology 2013, 65, 577–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, D.J.; Jia, S.J.; Dai, Z.; Li, Y.J. Asymmetric dimethylarginine induces apoptosis via p38 MAPK/caspase-3-dependent signaling pathway in endothelial cells. J. Mol. Cell. Cardiol. 2006, 40, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Zamani, L.; Lundqvist, M.; Zhang, Y.; Aberg, M.; Edfors, F.; Bidkhori, G.; Lindahl, A.; Mie, A.; Mardinoglu, A.; Field, R.; et al. High Cell Density Perfusion Culture has a Maintained Exoproteome and Metabolome. Biotechnol. J. 2018, 13, e1800036. [Google Scholar] [CrossRef] [Green Version]
- Taschwer, M.; Hackl, M.; Hernández Bort, J.A.; Leitner, C.; Kumar, N.; Puc, U.; Grass, J.; Papst, M.; Kunert, R.; Altmann, F.; et al. Growth, productivity and protein glycosylation in a CHO EpoFc producer cell line adapted to glutamine-free growth. J. Biotechnol. 2012, 157, 295–303. [Google Scholar] [CrossRef]
- Sumit, M.; Dolatshahi, S.; Chu, A.A.; Cote, K.; Scarcelli, J.J.; Marshall, J.K.; Cornell, R.J.; Weiss, R.; Lauffenburger, D.A.; Mulukutla, B.C.; et al. Dissecting N-Glycosylation Dynamics in Chinese Hamster Ovary Cells Fed-batch Cultures using Time Course Omics Analyses. iScience 2019, 12, 102–120. [Google Scholar] [CrossRef] [Green Version]
- Chang, D.E.; Smalley, D.J.; Conway, T. Gene expression profiling of Escherichia coli growth transitions: An expanded stringent response model. Mol. Microbiol. 2002, 45, 289–306. [Google Scholar] [CrossRef]
- Chrysanthopoulos, P.K.; Goudar, C.T.; Klapa, M.I. Metabolomics for high-resolution monitoring of the cellular physiological state in cell culture engineering. Metab. Eng. 2010, 12, 212–222. [Google Scholar] [CrossRef]
- Altamirano, C.; Illanes, A.; Becerra, S.; Cairó, J.J.; Gòdia, F. Considerations on the lactate consumption by CHO cells in the presence of galactose. J. Biotechnol. 2006, 125, 547–556. [Google Scholar] [CrossRef]
- Vcelar, S.; Jadhav, V.; Melcher, M.; Auer, N.; Hrdina, A.; Sagmeister, R.; Heffner, K.; Puklowski, A.; Betenbaugh, M.; Wenger, T.; et al. Karyotype variation of CHO host cell lines over time in culture characterized by chromosome counting and chromosome painting. Biotechnol. Bioeng. 2018, 115, 165–173. [Google Scholar] [CrossRef]
- Huhn, S.; Chang, M.; Kumar, A.; Liu, R.; Jiang, B.; Betenbaugh, M.; Lin, H.; Nyberg, G.; Du, Z. Chromosomal instability drives convergent and divergent evolution toward advantageous inherited traits in mammalian CHO bioproduction lineages. iScience 2022, 25, 104074. [Google Scholar] [CrossRef] [PubMed]
- Weinguny, M.; Klanert, G.; Eisenhut, P.; Lee, I.; Timp, W.; Borth, N. Subcloning induces changes in the DNA-methylation pattern of outgrowing Chinese hamster ovary cell colonies. Biotechnol. J. 2021, 16, e2000350. [Google Scholar] [CrossRef] [PubMed]
- Templeton, N.; Smith, K.D.; McAtee-Pereira, A.G.; Dorai, H.; Betenbaugh, M.J.; Lang, S.E.; Young, J.D. Application of 13C flux analysis to identify high-productivity CHO metabolic phenotypes. Metab. Eng. 2017, 43, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Ellet, J.; Okediadi, C.; Hermes, P.; McCormick, E.; Casnocha, S. A single nutrient feed supports both chemically defined NS0 and CHO fed-batch processes: Improved productivity and lactate metabolism. Biotechnol. Prog. 2009, 25, 1353–1363. [Google Scholar] [CrossRef]
- Huang, Z.; Lee, D.Y.; Yoon, S. Quantitative intracellular flux modeling and applications in biotherapeutic development and production using CHO cell cultures. Biotechnol. Bioeng. 2017, 114, 2717–2728. [Google Scholar] [CrossRef]

| Amino Acid Derivative | Behavior | Reference | Comments |
|---|---|---|---|
| Alanine | Intracellular production and accumulation in media | [11,14,16,17,18,32,36,37,41,42,44] | |
| Production mainly from cytosolic pyruvate | [23] | ||
| Arginine | Intracellular consumption leading to concentration decrease in media | [12,20,32,41] | Different conclusion might be due to feeding method and medium composition that is specific to [17] |
| Accumulation from late-exponential phase onwards, indicating over-supply in the fed-batch | [17] | ||
| Asparagine | Intracellular consumption leading to concentration decrease in media | [17,23,25,32,36,37,41] | |
| The most consumed amino acid from media. Intracellular deamination to aspartic acid generates ammonia | [44] | ||
| Consumption (measures performed on cell substrate). Represents 5% of incoming carbon source. Linked to aspartic acid production | [18] | ||
| Intracellular concentration increase in early exponential phase In terms of uptake from the media, the highest compared to other growth phases. In high producer clone, consumption beyond stoichiometric requirements together with glutamine to replenish the TCA intermediates | [19,31] | ||
| Aspartic acid | Production linked to asparagine uptake (measures performed on cell substrate) | [18,31,41,42] | |
| Consumption (measures performed on cell culture supernatant) | [32] | ||
| Consumption (measures performed on cell substrate) | [23,44] | ||
| Cysteine | Consumption (measures performed on cell culture supernatant) | [41] | |
| Glutamine | Cells use more glutamine when cultivated in a media containing more glutamine | [25,42,52] | |
| Consumption during all culture phases, with the highest during the exponential phase; source of lactate formation | [11,19,32,36,37] | ||
| Main amino acid consumed | [23,41] | ||
| Consumption linked to glutamic acid production | [18] | ||
| Uptake from the media, the highest compared to other growth phases. In high producer clone, consumption beyond stoichiometric requirements together with asparagine to replenish the TCA intermediates | [19] | ||
| Glutamic acid | Intracellular production leading to increased concentration in media | [11,23,31,32,36,41,42] | |
| Production associated with glutamine uptake | [18,40] | ||
| Glycine | Accumulation in media during culture | [14,16,17,23,41,42] | The low uptake identified in [32] might be due to the analysis method (NMR) used, which is different from that used in other studies focused on glycine metabolism |
| Accumulation in media during culture; produced by serine catabolism | [44] | ||
| Low uptake from media | [32] | ||
| Its accumulation in media together with the other identified metabolites leads to growth inhibition in HIPDOG culture | [21] | ||
| Histidine | Uptake from media | [32,41] | |
| Isoleucine | Uptake from media | [23,32,41] | |
| Leucine | Uptake from media | [23,32,41] | The particularity of study [21] is the use of the specific HiDPOG culture process that might explain the difference observed |
| Its accumulation in media together with the other identified metabolites leads to growth inhibition in HIPDOG culture | [21] | ||
| Lysine | Uptake from media | [23,32,41] | |
| Methionine | Uptake from media | [23,32,41] | The particularity of study [21] is the use of a specific HiDPOG culture process that might explain the difference observed |
| Its accumulation together with the other identified metabolites leads to growth inhibition in HIPDOG culture | [21] | ||
| Phenylalanine | Uptake from media | [12,20,23,32,41] | The particularity of study [21] is the use of a specific HiDPOG culture process that might explain the difference observed |
| Its accumulation in media together with the other identified metabolites leads to growth inhibition in HIPDOG culture | [21] | ||
| Proline | Accumulation in media | [32] | In study [41], a medium different from that of the two other studies was used, potentially explaining this discrepancy |
| Slight increase in concentration in media with time | [11] | ||
| Uptake from media | [41] | ||
| Serine | An increase in the intracellular concentration during the early exponential phase | [31] | The particularity of study [21] is the use of a specific HiDPOG culture process that might explain the difference observed |
| Uptake from media | [23,32,41,44] | ||
| Its accumulation together with the other identified metabolites leads to growth inhibition in HIPDOG culture | [21] | ||
| Threonine | Uptake from media | [23,32,41] | |
| Tryptophan | Uptake from media | [32] | The particularity of study [21] is the use of a specific HiDPOG culture process that might explain the difference observed |
| Depleted in media despite constant addition of feed | [12,20] | ||
| Its accumulation together with the other identified metabolites leads to growth inhibition in HIPDOG culture | [21] | ||
| Tyrosine | Uptake from media | [23,32,41] | The particularity of study [21] is the use of a specific HiDPOG culture process that might explain the difference observed |
| Its accumulation together with the other identified metabolites leads to growth inhibition in HIPDOG culture | [21] | ||
| Valine | Uptake from media | [23,32,41] | |
| Amino acid derivative | Behavior | Reference | Comments |
| Aspartylphenylalanine | Accumulation in media; known to be toxic | [12,20] | |
| Glutamylalanine | Accumulation in media | [12,20] | |
| Glutamylphenylalanine | Accumulation in media; known to be detrimental to cell growth | [12,20] | |
| Formylmethionine | Accumulating in media | [12,20] | |
| 5-L-glutamyl-L-alanine | Accumulation in media | [17] | |
| Dimethyl-L-arginine | Accumulation in media; associated with apoptosis | [17,53] | |
| N-acetyl-L-Leucine | Accumulation in media | [17] | |
| N-acetyl-L-phenylalanine | Accumulation in media; associated with apoptosis | [17] | |
| N-acetylmethionine | Accumulation in media | [17] | |
| N-formyl-L-methionine | Accumulation in media | [17] | |
| L-homocysteine | Accumulation in media. Metabolic source is methionine. Its accumulation together with the other identified metabolites leads to growth inhibition in HIPDOG culture | [21] | |
| 3-(4-Hydroxyphenyl) lactate | Accumulation in media. Metabolic sources are phenylalanine and tyrosine. Its accumulation together with the other identified metabolites leads to growth inhibition in HiPDOG culture | [21] | |
| Phenyllactate | Accumulation in media. Metabolic source is phenylalanine. Its accumulation together with the other identified metabolites leads to growth inhibition in HiPDOG culture | [21] | |
| Indole 3-lactate, indole-3-carboxylate, 2-hydroxyburtyrate, and 4-hydroxyphenylpyruvate | Accumulation in media. Metabolic source is tryptophan. Its accumulation together with the other identified metabolites leads to growth inhibition in HiPDOG culture | [21] | |
| Isovalerate | Accumulation in media. Metabolic source is leucine. Its accumulation together with the other identified metabolites leads to growth inhibition in HiPDOG culture | [21,44] | |
| Formate | Accumulation in media. Metabolic sources are serine, threonine and glycine. Its accumulation together with the other identified metabolites leads to growth inhibition in HiPDOG culture | [21,44] | |
| Isobutyrate | Accumulation in media | [44] | |
| Ammonia | Secreted in media during exponential and transition phase, with this being correlated with glutamine and asparagine consumption. Accumulation is known to affect productivity and inhibit cell growth | [17,44] | |
| Ammonium | Intracellular and media accumulation mainly due to breakdown of glutamine and several amino acids; detrimental effects on growth presumably due to apoptosis induction | [11,31,38] |
| Amino Acid | Behavior | Reference | Comments |
|---|---|---|---|
| Alanine | Accumulation in media | [16] | The switch observed in [44] might be due to the analysis method (NMR), which is different to those used in other studies |
| Constant increase in concentration with time | [11] | ||
| Switch from alanine secretion to uptake in media during stationary phase at the same time as aspartate and asparagine exhaustion | [44] | ||
| Arginine | Intracellular level decreases during the stationary phase | [31] | |
| Asparagine | Depleted at entry into stationary phase. Exponential growth and antibody production continue if it is added again in the media | [14,16,36,40,44] | The asparagine concentrations used in these studies might be different, leading to its exhaustion at different times of the culture |
| Intracellular consumption. Represents 8% of incoming carbon source | [18] | ||
| Uptake from the media, with this being lower than at exponential phase | [19] | ||
| Aspartic acid | Below detection level once cells enter stationary phase. Exponential growth and antibody production resumes when added to media | [14,16] | The aspartic acid concentrations used in these studies might be different, leading to its exhaustion at different times of the culture |
| Depleted in media during stationary phase | [44] | ||
| Cysteine | Depletion at entry into stationary phase | [14] | |
| Glutamine | Consumption | [11,31] | The glutamine concentrations used in these studies might be different, leading to its exhaustion at different times of the culture |
| Depleted in media at entry in stationary phase | [23,36,40] | ||
| Uptake from the media, with this being lower than at exponential phase | [19] | ||
| Glutamic acid | Below detection level once cells enter stationary phase. Exponential growth and antibody production resumes when added to media | [16] | The glutamic acid concentrations used in these studies might be different, leading to its exhaustion at different times of the culture |
| Constant detection | [11] | ||
| Glycine | NA | ||
| Histidine | NA | ||
| Isoleucine | NA | ||
| Leucine | NA | ||
| Lysine | NA | ||
| Methionine | NA | ||
| Phenylalanine | Intracellular level dropped during stationary phase | [31] | |
| Proline | Slight increase in concentration with time | [11] | |
| Serine | Consumption from media | [44] | |
| Threonine | Intracellular level decreases during stationary phase | [31] | The particularity of study [21] is the use of a specific HiDPOG culture process that might explain the difference observed |
| Accumulation in media identified as growth inhibitor in HiPDOG culture | [21] | ||
| Tryptophan | Intracellular level decreases during stationary phase | [31] | |
| Increased availability in media correlates with diminished viable cell density and accumulation of an intermediate during tryptophan metabolism, 5-hydroxyindolacetaldehyde (5-HIAAld), which is suspected to be an inhibitor of cell growth | [22] | ||
| Tyrosine | Depletion at entry into stationary phase | [14] | |
| Valine | Intracellular level decreases in the stationary phase | [31] | |
| Amino acid derivative | Behavior | Reference | Comments |
| Acetylphenylalanine | Accumulation in media at the beginning of stationary phase. Known to be detrimental to cell growth | [12,20] | |
| Dimethylarginine | Accumulation in media at the beginning of stationary phase. By-product of protein degradation and can induce apoptosis | [12,20] | |
| N-formimino-L-glutamate | Accumulation in media as culture enters stationary phase. Metabolite of the degradation of histidine or glutamate | [22] | |
| Ammonia | Accumulation in media | [44] | |
| Ammonium | Intracellular and media accumulation mainly due to breakdown of glutamine and several amino acids; has detrimental effects on growth presumably due to apoptosis induction | [11,31] |
| Amino Acid | Behavior | Reference | Comments |
|---|---|---|---|
| Alanine | Accumulation in extracellular media after addition of anti-apoptotic agent | [46] | |
| Arginine | NA | ||
| Asparagine and aspartic acid | Depleted from extracellular media after addition of anti-apoptotic agent | [46] | The asparagine and aspartic acid concentrations used in these studies might be different, leading to their exhaustion at different times of the culture |
| Intracellular concentration decreases compared to other growth phases | [31] | ||
| Depletion during stationary phase | [44] | ||
| Cysteine | NA | ||
| Glutamine | NA | ||
| Glutamic acid | Accumulation in extracellular media after addition of anti-apoptotic agent | [46] | The glutamic acid concentration used in these studies might be different, leading to its exhaustion at different times of the culture |
| Decreased intracellular concentration compared to other growth phases | [31] | ||
| Glycine | NA | ||
| Histidine and isoleucine | Extracellular concentration decreases after addition of anti-apoptotic agent | [46] | |
| Leucine | Exhaustion at entry into decline phase | [16] | |
| Lysine | Extracellular concentration decreases after addition of anti-apoptotic agent. | [46] | The glutamic acid concentration used in these studies might be different, leading to its exhaustion at different times of the culture |
| Exhaustion at entry into decline phase | [16] | ||
| Detected; indicates over-supply | [52] | ||
| Methionine and phenylalanine | Extracellular concentration decreases after addition of anti-apoptotic agent | [46] | |
| Proline | NA | ||
| Serine | Extracellular concentration decreases after addition of anti-apoptotic agent | [46] | The glutamic acid concentration used in these studies might be different, leading to its exhaustion at different times of the culture |
| Exhaustion at entry into decline phase | [16] | ||
| Intracellular concentration decreases compared to other growth phases | [31] | ||
| Threonine, tryptophan, tyrosine, valine | Extracellular concentration decreases after addition of anti-apoptotic agent | [46] | |
| Ornithine | Detected. Known to have apoptotic properties | [52] | |
| Amino acid derivative | Behavior | Reference | Comments |
| Pyroglutamate | Extracellular concentration increases after addition of anti-apoptotic agent | [46] | |
| 4-hydroxyproline | Extracellular concentration decreases after addition of anti-apoptotic agent | [46] | |
| Dimethylarginine, flutamylphenylalanine, glycerophosphocholine, hexanoglycine | Caspase activity increases after exposition of cells to these metabolites | [47] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coulet, M.; Kepp, O.; Kroemer, G.; Basmaciogullari, S. Metabolic Profiling of CHO Cells during the Production of Biotherapeutics. Cells 2022, 11, 1929. https://doi.org/10.3390/cells11121929
Coulet M, Kepp O, Kroemer G, Basmaciogullari S. Metabolic Profiling of CHO Cells during the Production of Biotherapeutics. Cells. 2022; 11(12):1929. https://doi.org/10.3390/cells11121929
Chicago/Turabian StyleCoulet, Mathilde, Oliver Kepp, Guido Kroemer, and Stéphane Basmaciogullari. 2022. "Metabolic Profiling of CHO Cells during the Production of Biotherapeutics" Cells 11, no. 12: 1929. https://doi.org/10.3390/cells11121929