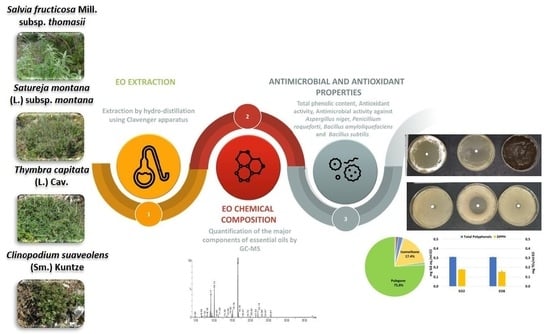
1. Introduction
Bread and bakery products represent fundamental foods in the daily diet. Although they are considered as staple foods, the microbiological spoilage occurs a few days after production if no preservative systems have been used, then causing economic losses for industries and consumers due to the wastes. The most common contaminants are molds, which grow on the product surface and originate from the bakery environment [
1,
2], but the presence of bacterial sporeformers belonging to the
Bacillus genus is also a relevant issue for the bakery industries, since they can cause the “rope” spoilage often confused as insufficient bake [
3,
4,
5]. While spores from fungi contaminate the product after baking [
2],
Bacillus spores originate from grains and flours but survive to the cooking process, germinating during storage into the bread crumb [
4,
6]. The most common solutions for shelf-life extension are those including the use of chemical preservatives (mainly propionate and sorbate salts), packaging (active or MAP), biopreservatives (including sourdough or essential oils from plants) [
7,
8,
9,
10]. The last trend in food manufacture is toward the application of biopreserving systems: the fermentation by lactic acid bacteria in sourdough is a well-known biotechnological strategy appreciated by consumers since the pleasant flavor given to the final products [
11,
12,
13]. The use of essential oils from medicinal plants in food manufacturing is an ancient technology mainly used to add specific flavor to foods. These substances contain a complex pattern of chemical compounds (essential oils, hereafter EOs) characterized by several biological activities as antioxidants, antimicrobial, anticancer, etc. [
14,
15,
16]. The EOs influence the multiplication and development of microorganisms interfering in physiological and biochemical processes. These activities are different for bacteria and fungi; in fact, some bacteria are more susceptible to essential oils because there are no hydrophilic lipopolysaccharides in the outer membrane that ensure protection to antimicrobial compounds [
17], whereas the antifungal activities of essential oils are manifold: they have an action on the disintegration of fungal hyphae operated by the terpene compounds or increase the membrane permeability and influence the enzymes involved in the synthesis of the cell wall [
18,
19].
Generally, EOs are synthesized in the aromatic vascular plants as a defense mechanism to exert antifungal, anti-parasitic, antiviral, and antibacterial activities [
20]. The use of medicinal and aromatic herbs in the treatment of infectious diseases dates back thousands of years. Many of the secondary metabolites of these plants have been shown to have important biological activities that are desperately needed even now [
16]. Plant-derived antimicrobial compounds capable of killing bacteria with a different mechanism than antibiotics may represent a valid approach especially for the treatment of infections caused by resistant microbe strains [
21].
The use of wild medicinal plants has long since been lost, but the process of speciation and extinction has continued. This has led to a lack of knowledge, as these “recent” medicinal plants have been described by taxonomists as new species, never used by man for medicinal or food purposes. Furthermore, it must be added that some ancient medicinal species have never been studied from a biochemical point of view, because they are very rare, with limited distribution or endemic.
The
Lamiaceae (formerly known as Labiatae) constitute the family that best represents officinal plants, including the Lamiales order with 258 genera and more 6000 species [
22]. It can inhabit different natural ecosystems and can be cultivated.
Lamiaceae are distributed globally with a particularly high concentration in the Mediterranean region, including Italy, which provides a great level of biodiversity [
23]. Most of the species belonging to the family are aromatic and possess essential oils (EOs) making them valuable in cosmetics, perfumery, food, agriculture, and medicine [
24,
25]. The use of
Lamiaceae species as antimicrobial agents for bread and bakery products preservation has also been reported [
7,
26,
27,
28,
29].
For their interesting properties, investigation into the mode of action, biological activities, and potential uses of essential oils has regained momentum. Moreover, as the public is becoming more informed about issues of food safety, nutrition, and health, a strong demand has appeared for reducing chemicals in agriculture and food commodities and to produce healthier and more natural alternatives. Therefore, it becomes worthy to develop a better understanding of the chemical complexity and the biological properties of these extracts in order to invent new and valuable applications in fields related to human health, agriculture, and environment without inducing the same secondary effects that chemicals may cause. The strong variation in the chemical composition confers to EOs different biological properties. However, the major constituents are not the sole agents responsible for these properties [
30]; the inactive and minor compounds can influence the rate of reactions and the bioactivity of active compounds. Therefore, the synergy between all phytochemicals can play a significant role in enhancing or reducing the power of the biological effects that EOs can induce [
30]. Hence, EOs are a possible alternative providing natural compounds with antimicrobial properties. These natural extracts are considered by the Food and Drug Administration as GRAS (Generally Recognized as Safe) (U.S code of Federal Regulations, 2016), making them a good option for food preservation and packaging.
Particularly, active food packaging can be applied on different food matrices, and the system has gained increasing attention mainly due to its ability to reduce fungal contaminations occurring on the food surface. In the case of bacterial contaminations occurring inside the product, it would be more convenient to add EOs as ingredients into the food formulation [
7,
8].
The objective of the present study was to evaluate the antimicrobial properties of EOs from two common officinal wild species
Thymbra capitata (L.) Cav.,
Satureja montana L. subsp.
montana, and two threatened taxa, which are both listed in the Regional Red List [
31], the rare
Clinopodium suaveolens (Sm.) Kuntze and the endemic
Salvia fruticosa Mill. subsp.
thomasii (Lacaita) Brullo, Guglielmo, Pavone & Terrasi, against common fungal and bacterial contaminants of bakery products, and to correlate the chemical composition of EOs with their biological activity (antimicrobial and antioxidant properties). In particular, the EOs were investigated for their ability to inhibit the growth of two fungal strains,
Aspergillus niger and
Penicillium roqueforti, and two bacterial strains causing the rope spoilage (
Bacillus amyloliquefaciens and
Bacillus subtilis) to have a preliminary understanding on the suitability of the plant species as sources of potential biopreservatives for bakery products.
4. Discussion
In the current study, the EOs from four
Lamiaceae species from Southern Italy have been chemically and biologically characterized. Results demonstrated the high variability and complexity of EOs composition and biological effect mainly in relation to the antimicrobial activity. The compound composition is characteristic for each plant depending on the species, specific site, temperature, rainfall, altitude, sun radiation, and soil features [
38,
39,
40]. In the current research, four wild plant species belonging to the
Lamiaceae family (
C. suaveolens,
S. fruticosa subsp.
thomasii,
S. montana subsp.
montana, and
T. capitata), recently characterized for the chemical composition of their EOs in Perrino et al. [
32], were further studied to obtain an exact quantification of the most abundant compounds occurring in the EOs and the relevant biological activity. In particular, the antimicrobial activity of EOs was tested against fungal and bacterial contaminants of bakery products to evaluate their potential as bio-preservatives in these food products.
The strong variability between the plant species and within the same species observed in our preliminary study [
32] reflects the antioxidant activity registered for EOs and was the result of the differences in EOs compositions and abundances of the major compounds, since the most active EOs were those showing also the higher antioxidant activity. In order to better understand the relationships between the biological properties (results from the disk diffusion assay, antioxidant properties, and total phenol content) and the amounts of the most abundant compounds in EOs, a PCA analysis was performed. As a result, the chemical composition and quantification of the specific compounds in EOs were correlated with the biological activity. The analysis allowed better understanding the significant factors responsible for the antioxidant and antibacterial properties (thymol, carvacrol, γ-terpinene, p-cymene) activity and for the antifungal effect (thymol, carvacrol, γ-terpinene, p-cymene, pulegone, and iso-menthone). Although the presence of compounds with known antimicrobial properties, such as camphor and eucalyptol [
41,
42] EOs from
S. fruticosa subsp.
thomasii samples (Sf1 and Sf2), did not show significant effect against the tested microorganisms. Differently from Chaturvedi et al. [
42], in this study, the EO containing camphor did not show an antifungal effect despite it being present in combination with eucalyptol in Sf1 and Sf. Similarly to
S. fruticosa subsp.
thomasii, the Sm1 from
S. montana subsp.
montana has no effect on fungal strains and showed a completely opposite behavior to Sm2. This difference in effect for the
S. montana subsp.
montana samples could be explained by the impact of environmental and plant community conditions on the chemical composition of EOs [
32]. Indeed, Sm2 had a higher content of thymol and carvacrol than Sm1, which was ineffective against microorganisms, but lower than Tc1 and Tc2 and even showing comparable MIC values, which may be due to a synergistic effect of all compounds detected even if occurring at low concentrations. It has been reported that most of the antioxidant activity from plant sources are derived from phenolic phytochemicals [
43] such as thymol and carvacrol, which were proven to be potent antioxidants [
44]. In contrast, other usually abundant phytochemicals such as γ-terpinene, α-pinene, β-pinene, limonene, linalol, eucalyptol, pulegone, and menthone have extremely low and almost nonexistent antioxidant activities [
44,
45]. However, other authors found a strong association between the monoterpene γ-terpinene and the antimicrobial and antioxidant properties of
Satureja thymbra [
46]. In our study, EOs Tc1 and Tc2, characterized by the highest thymol (413.2 and 484.5 µg/mL, respectively) and carvacrol (261.3 and 163.5 µg/mL, respectively) amounts, were characterized by the highest antioxidant activity as EO Sm2, although this sample contained a significantly lower concentration of thymol (53.4 µg/mL). This result can be explained by the different composition of Sm2 with respect to Tc1 and Tc2: as reported in Perrino et al. [
32], thymol represented 46.1% of the total compounds in Sm2, while in Tc1 and Tc2, thymol and carvacrol accounted for 31–35% and 26–17%, respectively, thus not representing the predominant compounds. Moreover, Sm2 was characterized by the presence of γ-terpinene to the same extent (14.57%) of Tc1 and Tc2 (14.95% and 17.71%, respectively) although lower amounts were quantified among the three EOs (14.9, 178.7, and 202.9 µg/mL). We can suppose that the strong antimicrobial activity of
T. capitata samples was the result of the dominance of phenolic compounds thymol and carvacrol and the monoterpene γ-terpinene, which are all known for their strong bioactivity [
46,
47,
48].
In contrast, the EOs from S. fruticosa subsp. thomasii did not show biological activity. Sf1 was rich in eucalyptol (541.2 µg/mL), camphor (232.7 µg/mL), α-myrcene (50 µg/m), α-pinene (36.2 µg/m), and caryophyllene (E) (15.5 µg/mL). Sf2 mainly differed from Sf1 in the higher eucalyptol (671.9 µg/mL) content and the presence of a low amount of carvacrol (6.6 µg/mL), but no differences in the biological activity between the two samples were observed. Moreover, EOs from S. fruticosa subsp. thomasii had no effect on A. niger and had a weak activity against B. subtilis, which was mainly due to the predominance of eucalyptol and camphor, which are only active in extremely high amounts.
In the case of
C. suaveolens, pulegone was the predominant compound in both samples: it is a monoterpene ketone, typical of
Lamiaceae EOs and with commercial value because of its important antimicrobial activities [
49,
50]. The most known sources of pulegone are
Mentha pulegium L.,
Hedeoma pulegioides L. Pers., and genus
Calamintha Mill. and
Acinos Mill., which show a high content of pulegone (59.9–96.9%) [
51,
52]. Both EO samples did not show antioxidant properties due to the lack of phenolic compounds.
Therefore, it can be supposed that the biological activity of EOs is not only due to the higher concentration of specific compounds, but a different combination of compounds can act in a synergistic way, thus affecting the biological properties of EOs.
Regarding the antimicrobial activity of EOs, a comparison with MIC values reported in the literature is not possible, since the antimicrobial susceptibility mainly depends on the test used other than the target strains. However, some similarities were found with published data. In particular, Moukhles et al. [
53] demonstrated that
T. capitata was active against
B. subtilis. Additional articles showcased the strength of
Thymbra L. species against
A. niger and numerous fungal and bacterial strains such as
Aspergillus fumigatus,
Penicillium funiculosum,
Bacillus cereus,
Listeria monocytogenes,
Salmonella enterica, and
Escherichia coli [
54,
55,
56].
S. montana subsp.
montana had lower MIC results in the literature against
B. subtilis (0.25%) and
A. niger (from 0.06 to 0.5%) [
57]. The antimicrobial effect was apparent on a wide range of microorganisms such as
Bacillus cereus,
Staphylococcus aureus,
Streptococcus faecalis,
Escherichia coli,
Salmonella typhi,
Aspergillus fumigates,
Pseudomonas aeruginosa,
Enterecoccus feacalis, and
Saccharomyces cerevisiae [
57,
58]. However, it is important to indicate a deficiency in comparable data; with the exception of
T. capitata, the tested species are rare and/or endemic and are found in specific places around the Mediterranean basin. Rather few data are available for
S. fruticosa subsp.
thomasii and
C. suaveolens. Additionally, it is necessary to consider the differences in the methodological approaches, making the comparison between findings rather inaccurate.
Several studies have been performed to extend bread shelf-life using essential oils, as recently reviewed by some authors [
7,
10,
59]. EOs can be applied as ingredients in the bread formulation, as additives in the packaging or in combination with other technologies (hurdle technology) to prolong the product shelf-life. Recently, Skendi et al. [
28] tested the effect of three aromatic plants belonging to the
Lamiaceae family (oregano, thyme, and Satureja) as EOs or in dry form directly in the bread formulation. According to our results, the authors detected carvacrol, α-pinene, p-cymene, and γ-terpinene as main components and observed that both forms of addition (essential oil and dry material) resulted in a higher efficacy against the
Penicillium spp. strain compared to
Aspergillus niger. The use of EOs at a concentration of 50 µl/100 g flour allowed reducing the fungal growth on the bread surface with respect to the control. Even if a comparison with our results cannot provide a realistic prevision of the actual efficacy of the EOs, we suppose that the combination of different preserving technologies, such as the use of sourdough, metabolites from lactic acid bacteria, and/or packaging can induce a delay in the microbial growth on bread. As an example, Debonne et al. [
8] tested different concentrations of EO from
Thymus zygis in sourdough bread to inhibit the growth of
A. niger and
P. paneum, but the promising performance observed in vitro were not confirmed in bread. This result was attributed to the food matrix effect, which caused a not homogeneous distribution of the oil. However, other studies [
59] demonstrated that large phenolic compounds such as thymol and eugenol (thyme, cinnamon, and clove) show a higher antifungal effect if they are applied directly to the medium, whereas small molecules such as allyl isothiocyanate and citral (mustard and lemongrass) were most efficient when added as volatiles.
S. montana subsp.
montana and
T. capitata, which are generally used as spices and food preservatives, mainly in meat and fruit products, for their antimicrobial properties [
60,
61,
62,
63,
64,
65] were shown in the current study to be the most active against fungi and bacteria and have the highest the antioxidant properties. The low MIC values observed for the studied EOs, mainly against fungal strains, can be exploited in the preservation of bakery products, which are frequently contaminated by strains belonging to the species tested in the current study. Moreover, the results of the present study highlight the antifungal efficacy of a scarcely studied species,
C. suaveolens, which was rich in pulegone, which is a monoterpene ketone associated to the inhibition of wood-decay fungi [
66].
The most actives were those from two species, S. montana subsp. montana and T. capitata, which can be exploited as bio-preservatives for bakery products. Moreover, the research provides additional scientific evidence on the potential uses of some common Lamiaceae species for food uses, in order to obtain effective conservation, protection, and valorization of these species. Data relevant to C. suaveolens and S. fruticosa subsp. thomasii, both species of conservation interest and limited geographic distribution, provide new information on the chemical composition of their essential oils and on the potential of C. suaveolens as a source of antifungal compounds.
Factors as temperature, rainfall, altitude, soil features, and habitat conditions have a qualitative and quantitative impact on Lamiaceae species essential oils. This variability does not permit a comparison between essential oils composition. Nevertheless, exploring the essential oils composition is possible to understand how environmental and plant communities condition affect the second metabolism of plants. In turns, this can allow recreating the optimal conditions for domesticating plants that have to produce specific essential oils.
The results suggest that using very low concentrations of EOs in different antifungal approaches such as the hurdle technology can result in the efficacious fungal inhibition. The hurdle technology (or combination technology) is a concept that relies mainly on the simultaneous manipulation and optimization of a series of preservation factors (or hurdles) and their interaction (hurdle effect) in food.
Further studies are necessary to demonstrate the efficacy of the EOs deriving from aromatic plants of the Lamiaceae family when applied to bakery products.