Suitability of Black Soldier Fly Frass as Soil Amendment and Implication for Organic Waste Hygienization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Black Soldier Fly Frass Collection
2.2. Frass and Soil Analyses
2.3. Preparation of Media
2.4. Pathogen Quantification/Assessment of Microbial Colonization in Frass and Soil
2.5. Statistical Analyses
3. Results and Discussion
3.1. Assessment of Microbial Load in Frass and Frass-Amended Soils
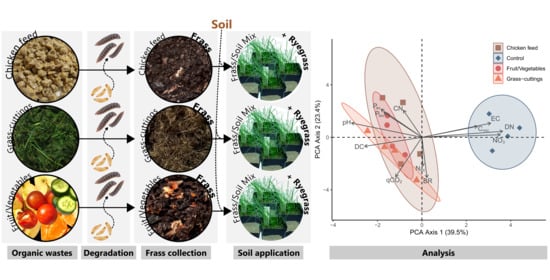
3.2. Black Soldier Fly Frass Properties, Soil Quality and Plant Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. Global Food Losses and Food Waste: Extent, Causes and Prevention; Food and Agriculture Organization of the United Nations: Rome, Italy, 2011; ISBN 978-92-5-107205-9. [Google Scholar]
- United Nations. World Population Prospects. The 2017 Revision; Department of Economic and Social Affairs—Population Division: New York, NY, USA, 2017; p. 53. [Google Scholar]
- European Commission. 811: Green Paper on the Management of Bio-Waste in the European Union; Commission of the European Communities: Brussels, Belgium, 2008. [Google Scholar]
- Pastor, B.; Velasquez, Y.; Gobbi, P.; Rojo, S. Conversion of organic wastes into fly larval biomass: Bottlenecks and challenges. J. Insects Food Feed 2015, 1, 179–193. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Klocke, M.; Schluter, O. Insect biodiversity: Underutilized bioresource for sustainable applications in life sciences. Reg. Environ. Chang. 2017, 17, 1445–1454. [Google Scholar] [CrossRef]
- Sogari, G.; Amato, M.; Biasato, I.; Chiesa, S.; Gasco, L. The potential role of insects as feed: A multi-perspective review. Animals 2019, 9, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhury, A.R.; Ashok, K.N.; Srinivas, K.; Arutchelvan, V.; Thota, K.R.; Ravi, S.N.; Sandeep, K.D.; Goutham, R.M. Black soldier fly larvae, a viable opportunity for entrepreneurship. Acta Sci. Agric. 2018, 2, 11–20. [Google Scholar]
- Klammsteiner, T.; Walter, A.; Pan, H.; Gassner, M.; Heussler, C.D.; Schermer, M.; Insam, H. On everyone’s lips: Insects for food and feed. In Proceedings of the 5th Austrian Citizen Science Conference, Obergurgl, Austria, 26—28 June 2019; Volume 366, p. 6. [Google Scholar]
- Quilliam, R.S.; Nuku-Adeku, C.; Maquart, P.; Little, D.; Newton, R.; Murray, F. Integrating insect frass biofertilisers into sustainable peri-urban agro-food systems. J. Insects Food Feed 2020, 1–8. [Google Scholar] [CrossRef]
- Beesigamukama, D.; Mochoge, B.; Korir, N.K.; Fiaboe, K.K.M.; Nakimbugwe, D.; Khamis, F.M.; Dubois, T.; Subramanian, S.; Wangu, M.M.; Ekesi, S.; et al. Biochar and gypsum amendment of agro-industrial waste for enhanced black soldier fly larval biomass and quality frass fertilizer. PLoS ONE 2020, 15, e238154. [Google Scholar] [CrossRef]
- Beesigamukama, D.; Mochoge, B.; Korir, N.K.; Fiaboe, K.K.M.; Nakimbugwe, D.; Khamis, F.M.; Subramanian, S.; Dubois, T.; Musyoka, M.W.; Ekesi, S.; et al. Exploring Black Soldier Fly Frass as Novel Fertilizer for Improved Growth, Yield, and Nitrogen Use Efficiency of Maize Under Field Conditions. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef]
- Diener, S.; Zurbrügg, C.; Tockner, K. Conversion of organic material by black soldier fly larvae: Establishing optimal feeding rates. Waste Manag. Res. 2009, 27, 603–610. [Google Scholar] [CrossRef]
- Alattar, M.; Alattar, F.; Popa, R. Effects of microaerobic fermentation and black soldier fly larvae food scrap processing residues on the growth of corn plants (Zea mays). Plant Sci. Today 2016, 3, 57–62. [Google Scholar] [CrossRef]
- Choi, Y.-C.; Choi, J.-Y.; Kim, J.-G.; Kim, M.-S.; Kim, W.-T.; Park, K.-H.; Bae, S.-W.; Jeong, G.-S. Potential usage of food waste as a natural fertilizer after digestion by Hermetia illucens (Diptera: Stratiomyidae). Int. J. Ind. Entomol. 2009, 19, 171–174. [Google Scholar]
- Sarpong, D.; Oduro-Kwarteng, S.; Gyasi, S.F.; Buamah, R.; Donkor, E.; Awuah, E.; Baah, M.K. Biodegradation by composting of municipal organic solid waste into organic fertilizer using the black soldier fly (Hermetia illucens) (Diptera: Stratiomyidae) larvae. Int. J. Recycl. Org. Waste Agric. 2019, 8, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Beesigamukama, D.; Mochoge, B.; Korir, N.; Musyoka, M.W.; Fiaboe, K.K.M.; Nakimbugwe, D.; Khamis, F.M.; Subramanian, S.; Dubois, T.; Ekesi, S.; et al. Nitrogen Fertilizer Equivalence of Black Soldier Fly Frass Fertilizer and Synchrony of Nitrogen Mineralization for Maize Production. Agronomy 2020, 10, 1395. [Google Scholar] [CrossRef]
- Klammsteiner, T.; Walter, A.; Bogataj, T.; Heussler, C.D.; Stres, B.; Steiner, F.M.; Schlick-Steiner, B.C.; Arthofer, W.; Insam, H. The core gut microbiome of black soldier fly (Hermetia illucens) larvae raised on low-bioburden diets. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Osimani, A.; Milanović, V.; Cardinali, F.; Garofalo, C.; Clementi, F.; Pasquini, M.; Riolo, P.; Ruschioni, S.; Isidoro, N.; Loreto, N.; et al. The bacterial biota of laboratory-reared edible mealworms (Tenebrio molitor L.): From feed to frass. Int. J. Food Microbiol. 2018, 272, 49–60. [Google Scholar] [CrossRef]
- Wang, H.; Rehman, K.; Liu, X.; Yang, Q.; Zheng, L.; Li, W.; Cai, M.; Li, Q.; Zhang, J.; Yu, Z. Insect biorefinery: A green approach for conversion of crop residues into biodiesel and protein. Biotechnol. Biofuels 2017, 10, 304. [Google Scholar] [CrossRef] [Green Version]
- Erickson, M.C.; Islam, M.; Sheppard, C.; Liao, J.; Doyle, M.P. Reduction of Escherichia coli o157:h7 and Salmonella enterica serovar enteritidis in chicken manure by larvae of the black soldier fly. J. Food Prot. 2004, 67, 685–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lalander, C.; Diener, S.; Magri, M.E.; Zurbrugg, C.; Lindstrom, A.; Vinneras, B. Faecal sludge management with the larvae of the black soldier fly (Hermetia illucens)—From a hygiene aspect. Sci. Total Environ. 2013, 458–460, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Tomberlin, J.K.; Brady, J.A.; Sanford, M.R.; Yu, Z. Black soldier fly (Diptera: Stratiomyidae) larvae reduce Escherichia coli in dairy manure. Environ. Entomol. 2008, 37, 1525–1530. [Google Scholar] [CrossRef] [PubMed]
- Vogel, H.; Müller, A.; Heckel, D.G.; Gutzeit, H.; Vilcinskas, A. Nutritional immunology: Diversification and diet-dependent expression of antimicrobial peptides in the black soldier fly Hermetia illucens. Dev. Comp. Immunol. 2018, 78, 141–148. [Google Scholar] [CrossRef]
- Zhang, J.; Bisch-Knaden, S.; Fandino, R.A.; Yan, S.; Obiero, G.F.; Grosse-Wilde, E.; Hansson, B.S.; Knaden, M. The olfactory co-receptor IR8a governs larval-frass mediated competition avoidance in a hawkmoth. Proc. Natl. Acad. Sci. USA 2019, 116, 21828–21833. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.G.; Li, X.; Gao, Y.L.; Liu, Y.; Dong, W.X.; Xiao, C. Oviposition deterrents in larval frass of potato tuberworm moth, Phthorimaea operculella (Lepidoptera: Gelechiidae). Neotrop. Entomol. 2019, 48, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Blomquist, G.J.; Figueroa-Teran, R.; Aw, M.; Song, M.; Gorzalski, A.; Abbott, N.L.; Chang, E.; Tittiger, C. Pheromone production in bark beetles. Insect Biochem. Mol. Biol. 2010, 40, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Lorenzana, L.R.J. Frass volatiles as attractant to the mango pulp weevil (Sternochetus frigidus (Fabr.) (Coleoptera: Curculionidae)). Philipp. Agric. Sci. 2014, 97, 385–390. [Google Scholar]
- Mitchell, R.F.; Hanks, L.M. Insect frass as a pathway for transmission of bacterial wilt of cucurbits. Environ. Entomol. 2009, 38, 395–403. [Google Scholar] [CrossRef]
- Roy, K.; Ewing, C.P.; Hughes, M.A.; Keith, L.; Bennett, G.M. Presence and viability of Ceratocystis lukuohia in ambrosia beetle frass from Rapid ʻŌhiʻa Death-affected Metrosideros polymorpha trees on Hawai’i Island. For. Pathol. 2019, 49, e12476. [Google Scholar] [CrossRef] [Green Version]
- Khisti, U.V.; Kathade, S.A.; Aswani, M.A.; Anand, P.K.; Bipinraj, N.K. Isolation and identification of saccharomyces cerevisiae from caterpillar frass and their probiotic characterization. Biosci. Biotechnol. Res. Asia 2019, 16, 179–186. [Google Scholar] [CrossRef]
- Frost, C.J.; Hunter, M.D. Recycling of nitrogen in herbivore feces: Plant recovery, herbivore assimilation, soil retention, and leaching losses. Oecologia 2007, 151, 42–53. [Google Scholar] [CrossRef]
- Frost, C.J.; Hunter, M.D. Insect canopy herbivory and frass deposition affect soil nutrient dynamics and export in oak mesocosms. Ecology 2004, 85, 3335–3347. [Google Scholar] [CrossRef]
- Yang, S.-S.; Chen, Y.; Kang, J.-H.; Xie, T.-R.; He, L.; Xing, D.-F.; Ren, N.-Q.; Ho, S.-H.; Wu, W.-M. Generation of high-efficient biochar for dye adsorption using frass of yellow mealworms (larvae of Tenebrio molitor Linnaeus) fed with wheat straw for insect biomass production. J. Clean Prod. 2019, 227, 33–47. [Google Scholar] [CrossRef]
- Schmitt, E.; de Vries, W. Potential benefits of using Hermetia illucens frass as a soil amendment on food production and for environmental impact reduction. Curr. Opin. Green Sustain. Chem. 2020. [Google Scholar] [CrossRef]
- Goberna, M.; Podmirseg, S.M.; Waldhuber, S.; Knapp, B.A.; García, C.; Insam, H. Pathogenic bacteria and mineral N in soils following the land spreading of biogas digestates and fresh manure. Appl. Soil Ecol. 2011, 49, 18–25. [Google Scholar] [CrossRef]
- Kandeler, E. Nitrate. In Methods in Soil Biology; Schinner, F., Öhlinger, R., Kandeler, E., Margesin, R., Eds.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 408–410. ISBN 978-3-642-60966-4. [Google Scholar]
- Kandeler, E. Ammonium. In Methods in Soil Biology; Schinner, F., Öhlinger, R., Kandeler, E., Margesin, R., Eds.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 406–408. ISBN 978-3-642-60966-4. [Google Scholar]
- Illmer, P. Total, organic, inorganic and plant available phosphorus. In Methods in Soil Biology; Schinner, F., Öhlinger, R., Kandeler, E., Margesin, R., Eds.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 412–416. ISBN 978-3-642-60966-4. [Google Scholar]
- Heinemeyer, O.; Insam, H.; Kaiser, E.A.; Walenzik, G. Soil microbial biomass and respiration measurements: An automated technique based on infra-red gas analysis. Plant Soil 1989, 116, 191–195. [Google Scholar] [CrossRef]
- Anderson, T.-H.; Domsch, K.H. The metabolic quotient for CO2 (qCO2) as a specific activity parameter to assess the effects of environmental conditions, such as ph, on the microbial biomass of forest soils. Soil Biol. Biochem. 1993, 25, 393–395. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; ISBN 3-900051-07-0.
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- De Smet, J.; Wynants, E.; Cos, P.; Campenhout, L.V. Microbial community dynamics during rearing of black soldier fly larvae (Hermetia illucens) and its impact on exploitation potential. Appl. Environ. Microbiol. 2018, 84, e2722-17. [Google Scholar] [CrossRef] [Green Version]
- Huang, K.; Xia, H.; Cui, G.; Li, F. Effects of earthworms on nitrification and ammonia oxidizers in vermicomposting systems for recycling of fruit and vegetable wastes. Sci. Total Environ. 2017, 578, 337–345. [Google Scholar] [CrossRef] [Green Version]
- Fielding, D.J.; Trainor, E.; Zhang, M. Diet influences rates of carbon and nitrogen mineralization from decomposing grasshopper frass and cadavers. Biol. Fertil. Soils 2013, 49, 537–544. [Google Scholar] [CrossRef]
- McTavish, M.J.; Smenderovac, E.; Gunn, J.; Murphy, S.D. Insect defoliators in recovering industrial landscapes: Effects of landscape degradation and remediation near an abandoned metal smelter on gypsy moth (Lepidoptera: Lymantriidae) feeding, frass production, and frass properties. Environ. Entomol. 2019, 48, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Poveda, J.; Jiménez-Gómez, A.; Saati-Santamaría, Z.; Usategui-Martín, R.; Rivas, R.; García-Fraile, P. Mealworm frass as a potential biofertilizer and abiotic stress tolerance-inductor in plants. Appl. Soil Ecol. 2019, 142, 110–122. [Google Scholar] [CrossRef]
- Gómez-Brandón, M.; Juárez, M.F.-D.; Zangerle, M.; Insam, H. Effects of digestate on soil chemical and microbiological properties: A comparative study with compost and vermicompost. J. Hazard. Mater. 2016, 302, 267–274. [Google Scholar] [CrossRef]
- Podmirseg, S.M.; Waldhuber, S.; Knapp, B.A.; Insam, H.; Goberna, M. Robustness of the autochthonous microbial soil community after amendment of cattle manure or its digestate. Biol. Fertil. Soils 2019, 55, 565–576. [Google Scholar] [CrossRef] [Green Version]
- Raaijmakers, J.M.; Mazzola, M. Soil immune responses. Science 2016, 352, 1392–1393. [Google Scholar] [CrossRef]
- Zhu, B.; Gutknecht, J.L.M.; Herman, D.J.; Keck, D.C.; Firestone, M.K.; Cheng, W. Rhizosphere priming effects on soil carbon and nitrogen mineralization. Soil Biol. Biochem. 2014, 76, 183–192. [Google Scholar] [CrossRef] [Green Version]
- Sharp, R.G. A review of the applications of chitin and its derivatives in agriculture to modify plant-microbial interactions and improve crop yields. Agronomy 2013, 3, 757–793. [Google Scholar] [CrossRef]
- Tharanathan, R.N.; Kittur, F.S. Chitin—The undisputed biomolecule of great potential. Crit. Rev. Food Sci. Nutr. 2003, 43, 61–87. [Google Scholar] [CrossRef] [PubMed]
- Dioha, I.; Ikeme, C.H.; Nafiu, T. Effect of carbon to nitrogen ratio on biogas production. Int. Res. J. Nat. Sci. 2013, 1, 1–10. [Google Scholar]
- Wang, L.; Li, Y.; Prasher, S.O.; Yan, B.; Ou, Y.; Cui, H.; Cui, Y. Organic matter, a critical factor to immobilize phosphorus, copper, and zinc during composting under various initial C/N ratios. Bioresour. Technol. 2019, 289, 121745. [Google Scholar] [CrossRef] [PubMed]
- Borkott, H.; Insam, H. Symbiosis with bacteria enhances the use of chitin by the springtail, Folsomia candida (Collembola). Biol. Fertil. Soils 1990, 9, 126–129. [Google Scholar] [CrossRef]
- Insam, H.; Merschak, P. Nitrogen leaching from forest soil cores after amending organic recycling products and fertilizers. Waste Manag. Res. 1997, 15, 277–292. [Google Scholar] [CrossRef]
- Spohn, M. Microbial respiration per unit microbial biomass depends on litter layer carbon-to-nitrogen ratio. Biogeosciences 2015, 12, 817–823. [Google Scholar] [CrossRef] [Green Version]
- Leita, L.; De Nobili, M.; Mondini, C.; Muhlbachova, G.; Marchiol, L.; Bragato, G.; Contin, M. Influence of inorganic and organic fertilization on soil microbial biomass, metabolic quotient and heavy metal bioavailability. Biol. Fertil. Soils 1999, 28, 371–376. [Google Scholar] [CrossRef]
- Ros, M.; Klammer, S.; Knapp, B.; Aichberger, K.; Insam, H. Long-term effects of compost amendment of soil on functional and structural diversity and microbial activity. Soil Use Manag. 2006, 22, 209–218. [Google Scholar] [CrossRef]
- Tittonell, P.; Corbeels, M.; van Wijk, M.T.; Vanlauwe, B.; Giller, K.E. Combining Organic and Mineral Fertilizers for Integrated Soil Fertility Management in Smallholder Farming Systems of Kenya: Explorations Using the Crop-Soil Model FIELD. Agron. J. 2008, 100, 1511–1526. [Google Scholar] [CrossRef]
- Fageria, V.D. Nutrient Interactions in Crop Plants. J. Plant Nutr. 2001, 24, 1269–1290. [Google Scholar] [CrossRef]
- Mertenat, A.; Diener, S.; Zurbrügg, C. Black soldier fly biowaste treatment—Assessment of global warming potential. Waste Manag. 2019, 84, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Bulak, P.; Proc, K.; Pawłowska, M.; Kasprzycka, A.; Berus, W.; Bieganowski, A. Biogas generation from insects breeding post production wastes. J. Clean Prod. 2020, 244, 118777. [Google Scholar] [CrossRef]
- Lalander, C.; Nordberg, A.; Vinneras, B. A comparison in product-value potential in four treatment strategies for food waste and faeces—Assessing composting, fly larvae composting and anaerobic digestion. Glob. Chang. Biol. Bioenergy 2018, 10, 84–91. [Google Scholar] [CrossRef] [Green Version]



| CF | GC | FV | |
|---|---|---|---|
| Pupation rate [%] | 55.2 ± 5.3 b | 98.7 ± 2.0 c | 22.4 ± 2.0 a |
| Prepupae fresh weight [mg] | 198 ± 10 | 167 ± 11 | 165 ± 31 |
| Prepupae dry weight [%] | 32.6 ± 1.9 | 29.6 ± 1.9 | 32.2 ± 9.3 |
| Prepupae water content [%] | 67.4 ± 3.7 | 70.4 ± 5.6 | 67.8 ± 9.9 |
| Prepupae organic content [%] | 27.6 ± 1.6 | 26.5 ± 1.8 | 31.4 ± 8.4 |
| Prepupae inorganic content [%] | 5.1 ± 0.3 c | 3.1 ± 0.2 b | 0.9 ± 0.9 a |
| Frass residues [%] | 43.7 ± 1.0 c | 46.0 ± 1.5 b | 28.5 ± 0.5 a |
| Parameter | Value |
|---|---|
| pH | 7.3 ± 0.4 |
| EC [µs cm-1] | 78.1 ± 2.7 |
| VS [g kg-1] | 78.5 ± 26.6 |
| Ctot [g kg-1] | 40 |
| Ntot [g kg-1] | 1.7 |
| Ptot [mg kg-1] | 823 ± 190 |
| Pav [mg kg-1] | 6.88 ± 1.28 |
| CF-F | GC-F | FV-F | |
|---|---|---|---|
| pH | 6.22 ± 0.14 C | 5.40 ± 0.03 A | 5.58 ± 0.01 B |
| EC [mS cm-1] | 5.67 ± 0.27 c | 3.06 ± 0.03 b | 2.36 ± 0.11 a |
| Dry matter [%] | 90.9 ± 0.0 | 89.9 ± 0.0 | 90.4 ± 0.0 |
| Ctot [g kg-1] | 479 ± 8 B | 443 ± 6 A | 488 ± 4 B |
| Ntot [g kg-1] | 25.9 ± 0.9 b | 24.4 ± 0.2 b | 18.3 ± 1.2 a |
| C:N ratio | 18.5 ± 0.3 a | 18.2 ± 0.4 a | 26.6 ± 1.7 b |
| VS [g kg-1] | 910 ± 7 c | 825 ± 9 a | 873 ± 4 b |
| C-S | CF-S | GC-S | FV-S | |
|---|---|---|---|---|
| pH CaCl2 | 7.53 ± 0.02 a | 7.57 ± 0.01 ab | 7.58 ± 0.03 b | 7.58 ± 0.02 b |
| EC [µS cm-1] | 95.5 ± 1.8 B | 79.8 ± 6.4 A | 77.3 ± 2.9 A | 81.5 ± 7.8 A |
| VS [g kg-1] | 37.3 ± 1.1 | 35.4 ± 1.3 | 38.4 ± 2.0 | 37.8 ± 2.1 |
| Ctot [g kg-1] | 17.9 ± 3.2 | 21.7 ± 4. | 22.5 ± 6.1 | 20.6 ± 5.2 |
| Ntot [g kg-1] | 0.98 ± 0.35 | 0.99 ± 0.51 | 1.17 ± 0.39 | 0.98 ± 0.44 |
| C:N ratio | 20.3 ± 8.1 | 26.2 ± 11.7 | 21.0 ± 9.8 | 22.1 ± 9.0 |
| NH4+ [mg kg-1] | 0.57 ± 0.13 | 0.58 ± 0.06 | 0.58 ± 0.08 | 0.61 ± 0.14 |
| NO3- [mg kg-1] | 45.2 ± 4.1 b | 15.4 ± 3.3 a | 17.0 ± 3.5 a | 12.1 ± 4.4 a |
| DOC [mg kg-1] | 48.3 ± 3.8 | 50.2 ± 1.5 | 48.5 ± 1.9 | 51.8 ± 1.3 |
| DC [mg kg-1] | 95.6 ± 1.6 a | 104.7 ± 1.4 b | 103.5 ±4.0 b | 112.1 ± 2.0 c |
| DN [mg kg-1] | 35.6 ± 3.9 C | 17.0 ± 1.5 B | 15.3 ± 1.8 AB | 15.0 ± 0.6 A |
| Pav [mg kg-1] | 5.2 ± 0.4 | 6.1 ± 1.0 | 6.1 ± 1.4 | 5.8 ± 0.9 |
| Ptot [mg kg-1] | 783 ± 46 ab | 866 ± 35 b | 757 ± 33 a | 721 ± 45 a |
| P bioavailability [%] | 67 ± 8 | 70 ± 12 | 81 ± 23 | 80 ± 12 |
| BR [µg CO2 g-1 dw h-1] | 5.6 ± 0.15 | 4.6 ± 1.5 | 6.7 ± 0.7 | 5.6 ± 0.5 |
| Cmic [µg CO2 g-1 dw soil] | 416.1 ± 103.5 | 276.4 ± 38.7 | 279.0 ± 22.7 | 336.2 ± 16.1 |
| qCO2 [µg CO2-C h-1/µg-1 C mic] | 14.7 ± 4.7 | 16.4 ± 4.3 | 24.5 ± 4.5 | 16.6 ± 1.4 |
| Plant biomass [mg dw] | 85 ± 7 | 80 ± 6 | 74 ± 4 | 75 ± 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klammsteiner, T.; Turan, V.; Fernández-Delgado Juárez, M.; Oberegger, S.; Insam, H. Suitability of Black Soldier Fly Frass as Soil Amendment and Implication for Organic Waste Hygienization. Agronomy 2020, 10, 1578. https://doi.org/10.3390/agronomy10101578
Klammsteiner T, Turan V, Fernández-Delgado Juárez M, Oberegger S, Insam H. Suitability of Black Soldier Fly Frass as Soil Amendment and Implication for Organic Waste Hygienization. Agronomy. 2020; 10(10):1578. https://doi.org/10.3390/agronomy10101578
Chicago/Turabian StyleKlammsteiner, Thomas, Veysel Turan, Marina Fernández-Delgado Juárez, Simon Oberegger, and Heribert Insam. 2020. "Suitability of Black Soldier Fly Frass as Soil Amendment and Implication for Organic Waste Hygienization" Agronomy 10, no. 10: 1578. https://doi.org/10.3390/agronomy10101578