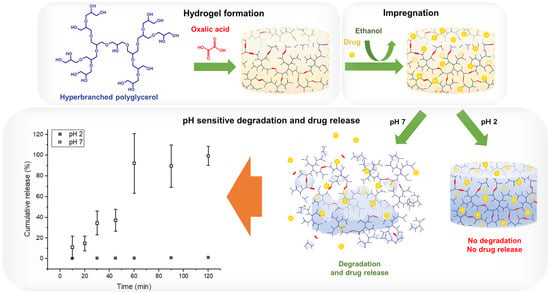
pH-Sensitive Degradable Oxalic Acid Crosslinked Hyperbranched Polyglycerol Hydrogel for Controlled Drug Release
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Study of Hydrogel Formation
Measurement of Gel Percent
2.3. Optical Microscopy
2.4. Fourier Transformed Infrared Spectroscopy
2.5. Swelling Test
2.6. Thermal Behavior
2.7. Drug Impregnation
2.8. Drug Release
3. Results
3.1. Study of Hydrogel Formation
3.2. Optical Microscopy
3.3. Fourier Transformed Infrared Spectroscopy
3.4. Swelling Test
3.5. Thermal Behavior
3.6. Drug Impregnation
3.7. Drug Release
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schmaljohann, D. Thermo- and PH-Responsive Polymers in Drug Delivery. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Yahya, R.; Hassan, A.; Yar, M.; Azzahari, A.D.; Selvanathan, V.; Sonsudin, F.; Abouloula, C.N. PH Sensitive Hydrogels in Drug Delivery: Brief History, Properties, Swelling, and Release Mechanism, Material Selection and Applications. Polymers 2017, 9, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ofridam, F.; Tarhini, M.; Lebaz, N.; Gagnière, É.; Mangin, D.; Elaissari, A. PH-Sensitive Polymers: Classification and Some Fine Potential Applications. Polym. Adv. Technol. 2021, 32, 1455–1484. [Google Scholar] [CrossRef]
- Hendi, A.; Hassan, M.U.; Elsherif, M.; Alqattan, B.; Park, S.; Yetisen, A.K.; Butt, H. Healthcare Applications of PH-Sensitive Hydrogel-Based Devices: A Review. Int. J. Nanomed. 2020, 15, 3887–3901. [Google Scholar] [CrossRef]
- Kocak, G.; Tuncer, C.; Bütün, V. PH-Responsive Polymers. Polym. Chem. 2017, 8, 144–176. [Google Scholar] [CrossRef]
- Barbosa, L.R.S.; Ortore, M.G.; Spinozzi, F.; Mariani, P.; Bernstorff, S.; Itri, R. The Importance of Protein-Protein Interactions on the PH-Induced Conformational Changes of Bovine Serum Albumin: A Small-Angle x-Ray Scattering Study. Biophys. J. 2010, 98, 147–157. [Google Scholar] [CrossRef]
- Raghuwanshi, V.S.; Yu, B.; Browne, C.; Garnier, G. Reversible PH Responsive Bovine Serum Albumin Hydrogel Sponge Nanolayer. Front. Bioeng. Biotechnol. 2020, 8, 573. [Google Scholar] [CrossRef]
- He, L.; Fullenkamp, D.E.; Rivera, J.G.; Messersmith, P.B. PH Responsive Self-Healing Hydrogels Formed by Boronate-Catechol Complexation. Chem. Commun. 2011, 47, 7497–7499. [Google Scholar] [CrossRef] [Green Version]
- Gujarathi, N.A.; Rane, B.R.; Patel, J.K. PH Sensitive Polyelectrolyte Complex of O-Carboxymethyl Chitosan and Poly (Acrylic Acid) Cross-Linked with Calcium for Sustained Delivery of Acid Susceptible Drugs. Int. J. Pharm. 2012, 436, 418–425. [Google Scholar] [CrossRef]
- Hezaveh, H.; Muhamad, I.I. Controlled Drug Release via Minimization of Burst Release in PH-Response Kappa-Carrageenan/Polyvinyl Alcohol Hydrogels. Chem. Eng. Res. Des. 2013, 91, 508–519. [Google Scholar] [CrossRef]
- Karimi, M.; Ghasemi, A.; Sahandi Zangabad, P.; Rahighi, R.; Moosavi Basri, S.M.; Mirshekari, H.; Amiri, M.; Shafaei Pishabad, Z.; Aslani, A.; Bozorgomid, M.; et al. Smart Micro/Nanoparticles in Stimulus-Responsive Drug/Gene Delivery Systems. Chem. Soc. Rev. 2016, 45, 1457–1501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-Based Hydrogels for Controlled, Localized Drug Delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Bal, T.; Panpalia, S.G.; Rajora, A.D.; Ghosh, B.D. Preparation and Characterization of Microwave Irradiated PH-Sensitive Polyacrylamide Grafted Flax Seed Mucilage Graft Copolymeric Hydrogel (PFLSM-g-PAM-Cl-MBA) and Its Evaluation as Effective Polymeric Scaffold. Sustain. Chem. Pharm. 2021, 22, 100479. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hui, P.C.L.; Siu, W.S.; Kan, C.W.; Leung, P.C.; Wanxue, C.; Chiou, J.C. Influence of PH-Responsive Compounds Synthesized from Chitosan and Hyaluronic Acid on Dual-Responsive (PH/Temperature) Hydrogel Drug Delivery Systems of Cortex Moutan. Int. J. Biol. Macromol. 2021, 168, 163–174. [Google Scholar] [CrossRef]
- Wang, T.; Yi, W.; Zhang, Y.; Wu, H.; Fan, H.; Zhao, J.; Wang, S. Sodium Alginate Hydrogel Containing Platelet-Rich Plasma for Wound Healing. Colloids Surf. B Biointerfaces 2023, 222, 113096. [Google Scholar] [CrossRef]
- Stiriba, S.E.; Frey, H.; Haag, R. Dendritic Polymers in Biomedical Applications: From Potential to Clinical Use in Diagnostics and Therapy. Angew. Chem.—Int. Ed. 2002, 41, 1329–1334. [Google Scholar] [CrossRef]
- Kumari, M.; Prasad, S.; Fruk, L.; Parshad, B. Polyglycerol-Based Hydrogels and Nanogels: From Synthesis to Applications. Future Med. Chem. 2021, 13, 419–438. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Wu, Z.; Gao, X.; Cheng, C.; Wang, Z.; Li, C. A Hydrotropic β-Cyclodextrin Grafted Hyperbranched Polyglycerol Co-Polymer for Hydrophobic Drug Delivery. Acta Biomater. 2011, 7, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Alrobaian, M.; Almalki, W.H.; Mahnashi, M.H.; Alyami, B.A.; Alqarni, A.O.; Alqahtani, Y.S.; Alharbi, K.S.; Alghamdi, S.; Panda, S.K.; et al. Superbranched Polyglycerol Nanostructures as Drug Delivery and Theranostics Tools for Cancer Treatment. Drug Discov. Today 2021, 26, 1006–1017. [Google Scholar] [CrossRef]
- Cuneo, T.; Gao, H. Recent Advances on Synthesis and Biomaterials Applications of Hyperbranched Polymers. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2020, 12, e1640. [Google Scholar] [CrossRef]
- Frey, H.; Haag, R. Dendritic Polyglycerol: A New Versatile Biocompatible Material. Rev. Mol. Biotechnol. 2002, 90, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Abbina, S.; Vappala, S.; Kumar, P.; Siren, E.M.J.; La, C.C.; Abbasi, U.; Brooks, D.E.; Kizhakkedathu, J.N. Hyperbranched Polyglycerols: Recent Advances in Synthesis, Biocompatibility and Biomedical Applications. J. Mater. Chem. B 2017, 5, 9249–9277. [Google Scholar] [CrossRef]
- Pouyan, P.; Cherri, M.; Haag, R. Polyglycerols as Multi-Functional Platforms: Synthesis and Biomedical Applications. Polymers 2022, 14, 2684. [Google Scholar] [CrossRef] [PubMed]
- Kainthan, R.K.; Janzen, J.; Levin, E.; Devine, D.V.; Brooks, D.E. Biocompatibility Testing of Branched and Linear Polyglycidol. Biomacromolecules 2006, 7, 703–709. [Google Scholar] [CrossRef]
- Imran Ul-Haq, M.; Lai, B.F.L.; Chapanian, R.; Kizhakkedathu, J.N. Influence of Architecture of High Molecular Weight Linear and Branched Polyglycerols on Their Biocompatibility and Biodistribution. Biomaterials 2012, 33, 9135–9147. [Google Scholar] [CrossRef]
- Yeh, P.Y.J.; Kainthan, R.K.; Zou, Y.; Chiao, M.; Kizhakkedathu, J.N. Self-Assembled Monothiol-Terminated Hyperbranched Polyglycerols on a Gold Surface: A Comparative Study on the Structure, Morphology, and Protein Adsorption Characteristics with Linear Poly(Ethylene Glycol)S. Langmuir 2008, 24, 4907–4916. [Google Scholar] [CrossRef]
- Taylor, D.K.; Jayes, F.L.; House, A.J.; Ochieng, M.A. Temperature-Responsive Biocompatible Copolymers Incorporating Hyperbranched Polyglycerols for Adjustable Functionality. J. Funct. Biomater. 2011, 2, 173–194. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Achazi, K.; Steinhilber, D.; Kratz, F.; Dernedde, J.; Haag, R. A Facile Approach for Dual-Responsive Prodrug Nanogels Based on Dendritic Polyglycerols with Minimal Leaching. J. Control. Release 2014, 174, 209–216. [Google Scholar] [CrossRef]
- Steinhilber, D.; Sisson, A.L.; Mangoldt, D.; Welker, P.; Licha, K.; Haag, R. Synthesis, Reductive Cleavage, and Cellular Interaction Studies of Biodegradable, Polyglycerol Nanogels. Adv. Funct. Mater. 2010, 20, 4133–4138. [Google Scholar] [CrossRef] [Green Version]
- Mabey, W.; Mill, T. Critical Review of Hydrolysis of Organic Compounds in Water Under Environmental Conditions. J. Phys. Chem. Ref. Data 1978, 7, 383–415. [Google Scholar] [CrossRef] [Green Version]
- Fayle, S.E.; Gerrard, J.A.; Simmons, L.; Meade, S.J.; Reid, E.A.; Johnston, A.C. Crosslinkage of Proteins by Dehydroascorbic Acid and Its Degradation Products. Food Chem. 2000, 70, 193–198. [Google Scholar] [CrossRef]
- Moghadas, B.; Solouk, A.; Sadeghi, D. Development of Chitosan Membrane Using Non-Toxic Crosslinkers for Potential Wound Dressing Applications. Polym. Bull. 2021, 78, 4919–4929. [Google Scholar] [CrossRef]
- Jóźwiak, T.; Filipkowska, U.; Szymczyk, P.; Rodziewicz, J.; Mielcarek, A. Effect of Ionic and Covalent Crosslinking Agents on Properties of Chitosan Beads and Sorption Effectiveness of Reactive Black 5 Dye. React. Funct. Polym. 2017, 114, 58–74. [Google Scholar] [CrossRef]
- Popescu, I.; Constantin, M.; Pelin, I.M.; Suflet, D.M.; Ichim, D.L.; Daraba, O.M.; Fundueanu, G. Eco-Friendly Synthesized PVA/Chitosan/Oxalic Acid Nanocomposite Hydrogels Embedding Silver Nanoparticles as Antibacterial Materials. Gels 2022, 8, 268. [Google Scholar] [CrossRef] [PubMed]
- Mitra, T.; Sailakshmi, G.; Gnanamani, A.; Mandal, A.B. Di-Carboxylic Acid Cross-Linking Interactions Improves Thermal Stability and Mechanical Strength of Reconstituted Type i Collagen: Part I. Oxalic Acid. J. Therm. Anal. Calorim. 2011, 105, 325–330. [Google Scholar] [CrossRef]
- Enache, A.A.; Serbezeanu, D.; Vlad-Bubulac, T.; Ipate, A.M.; Suflet, D.M.; Drobotă, M.; Barbălată-Mândru, M.; Udrea, R.M.; Rîmbu, C.M. Tunable Properties via Composition Modulations of Poly(Vinyl Alcohol)/Xanthan Gum/Oxalic Acid Hydrogels. Materials 2022, 15, 2657. [Google Scholar] [CrossRef] [PubMed]
- Murcia Valderrama, M.A.; van Putten, R.J.; Gruter, G.J.M. The Potential of Oxalic—and Glycolic Acid Based Polyesters (Review). Towards CO2 as a Feedstock (Carbon Capture and Utilization—CCU). Eur. Polym. J. 2019, 119, 445–468. [Google Scholar] [CrossRef]
- Schuler, E.; Demetriou, M.; Shiju, N.R.; Gruter, G.J.M. Towards Sustainable Oxalic Acid from CO2 and Biomass. ChemSusChem 2021, 14, 3636–3664. [Google Scholar] [CrossRef]
- Sunder, A.; Hanselmann, R.; Frey, H.; Mülhaupt, R. Controlled Synthesis of Hyperbranched Polyglycerols by Ring-Opening Multibranching Polymerization. Macromolecules 1999, 32, 4240–4246. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of Solute Release from Porous Hydrophilic Polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Costa, P.; Lobo, J.M.S. Modelling and Comparison of Dissolution Profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seligra, P.G.; Medina Jaramillo, C.; Famá, L.; Goyanes, S. Biodegradable and Non-Retrogradable Eco-Films Based on Starch–Glycerol with Citric Acid as Crosslinking Agent. Carbohydr. Polym. 2016, 138, 66–74. [Google Scholar] [CrossRef] [Green Version]
- Demitri, C.; Del Sole, R.; Scalera, F.; Sannino, A.; Vasapollo, G.; Maffezzoli, A.; Ambrosio, L.; Nicolais, L. Novel Superabsorbent Cellulose-Based Hydrogels Crosslinked with Citric Acid. J. Appl. Polym. Sci. 2008, 110, 2453–2460. [Google Scholar] [CrossRef]
- Gohil, J.M.; Ray, P. Studies on Oxalic Acid as a Crosslinker of Polyvinyl Alcohol. Polym. Polym. Compos. 2009, 17, 403–410. [Google Scholar] [CrossRef]
- Das, P.; Ray, S.K.; Kuila, S.B.; Samanta, H.S.; Singha, N.R. Systematic Choice of Crosslinker and Filler for Pervaporation Membrane: A Case Study with Dehydration of Isopropyl Alcohol-Water Mixtures by Polyvinyl Alcohol Membranes. Sep. Purif. Technol. 2011, 81, 159–173. [Google Scholar] [CrossRef]
- Bruice, P.Y. Organic Chemistry, 4th ed.; Pearson Prentice Hall: São Paulo, Brazil, 2006. [Google Scholar]
- Callister, W.D.; Rethwisch, D.G. Ciência e Engenharia de Materiais: Uma Introdução, 10th ed.; LTC: Rio de Janeiro, Brazil, 2020; ISBN 8521637284. [Google Scholar]
- Buchtova, N.; Budtova, T. Cellulose Aero-, Cryo- and Xerogels: Towards Understanding of Morphology Control. Cellulose 2016, 23, 2585–2595. [Google Scholar] [CrossRef]
- Maia, L.P. Poligliceróis Hiperramificados Modificados Para Encapsulamento de Fármacos Em CO2-Sc: Síntese, Comportamento Em CO2-Sc, Encapsulamento e Liberação; Universidade de São Paulo: São Paulo, Brazil, 2016. [Google Scholar]
- Solano-Delgado, L.C.; Bravo-Sanabria, C.A.; Ardila-Suárez, C.; Ramírez-Caballero, G.E. Stimuli-Responsive Hydrogels Based on Polyglycerol Crosslinked with Citric and Fatty Acids. Int. J. Polym. Sci. 2018, 2018, 3267361. [Google Scholar] [CrossRef] [Green Version]
- Laftah, W.A.; Hashim, S.; Ibrahim, A.N.; Laftah, W.A.; Hashim, S.; Ibrahim, A.N. Polymer Hydrogels: A Review Polymer Hydrogels: A Review. Polym. Plast. Technol. Eng. 2011, 50, 1475–1486. [Google Scholar] [CrossRef]
- Rasia, G.M. Síntese e Funcionalização de Hidrogéis de Poli(Álcool Vinílico); Universidade Federal do Rio Grande do Sul: Porto Alegre, Brazil, 2014. [Google Scholar]
- Erceg, T.; Stupar, A.; Cvetinov, M.; Vasić, V.; Ristić, I. Investigation the Correlation between Chemical Structure and Swelling, Thermal and Flocculation Properties of Carboxymethylcellulose Hydrogels. J. Appl. Polym. Sci. 2021, 138, 50240. [Google Scholar] [CrossRef]
- Lu, H.; Feng, S. Supramolecular Silicone Elastomers with Healable and Hydrophobic Properties Crosslinked by “Salt-Forming Vulcanization”. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 903–911. [Google Scholar] [CrossRef]
- Kahar, A.W.M.; Ismail, H.; Hamid, A.A. The Correlation between Crosslink Density and Thermal Properties of High-Density Polyethylene/Natural Rubber/Thermoplastic Tapioca Starch Blends Prepared via Dynamic Vulcanisation Approach. J. Therm. Anal. Calorim. 2016, 123, 301–308. [Google Scholar] [CrossRef]
- Dilaver, M.; Yurdakoc, K. Fumaric Acid Cross-Linked Carboxymethylcellulose/Poly(Vinyl Alcohol) Hydrogels. Polym. Bull. 2016, 73, 2661–2675. [Google Scholar] [CrossRef]
- Wang, S.; Song, Z.; Wang, J.; Dong, Y.; Wu, M. Solubilities of Ibuprofen in Different Pure Solvents. J. Chem. Eng. Data 2010, 5, 5283–5285. [Google Scholar] [CrossRef]
- Rashid, A.; White, E.T.; Howes, T.; Litster, J.D.; Marziano, I. Effect of Solvent Composition and Temperature on the Solubility of Ibuprofen in Aqueous Ethanol. J. Chem. Eng. Data 2014, 59, 2699–2703. [Google Scholar] [CrossRef]
- Shishir, M.R.I.; Xie, L.; Sun, C.; Zheng, X.; Chen, W. Advances in Micro and Nano-Encapsulation of Bioactive Compounds Using Biopolymer and Lipid-Based Transporters. Trends Food Sci. Technol. 2018, 78, 34–60. [Google Scholar] [CrossRef]
- Maia-Obi, L.P.; Vidinha, P.; Gomes, H.; Camino, R. Non-Inclusion Complexation of Peracetylated b-Cyclodextrin with Ibuprofen in Supercritical Carbon Dioxide. J. Supercrit. Fluids 2021, 169, 105098. [Google Scholar] [CrossRef]
- Rasenack, N.; Müller, B.W. Ibuprofen Crystals with Optimized Properties. Int. J. Pharm. 2002, 245, 9–24. [Google Scholar] [CrossRef]
- Cano, H.; Gabas, N.; Canselier, J.P. Experimental Study on the Ibuprofen Crystal Growth Morphology in Solution. J. Cryst. Growth 2001, 224, 335–341. [Google Scholar] [CrossRef]
- Shaw, L.R.; Irwin, W.J.; Grattan, T.J.; Conway, B.R. The Effect of Selected Water-Soluble Excipients on the Dissolution of Paracetamol and Ibuprofen. Drug Dev. Ind. Pharm. 2005, 31, 515–525. [Google Scholar] [CrossRef]
- Qiu, X.; Leporatti, S.; Donath, E.; Mo, H. Studies on the Drug Release Properties of Polysaccharide Multilayers Encapsulated Ibuprofen Microparticles. Langmuir 2001, 17, 5375–5380. [Google Scholar] [CrossRef]
- París, R.; Quijada-Garrido, I. Swelling and Hydrolytic Degradation Behaviour of PH-Responsive Hydrogels of Poly[(N-Isopropylacrylamide)-Co-(Methacrylicacid)] Crosslinked by Biodegradable Polycaprolactone Chains. Polym. Int. 2009, 58, 362–367. [Google Scholar] [CrossRef]
- Sharma, R.C.; Sharma, M.M. Kinetics of Alkaline Hydrolysis of Esters. II. Unsaturated Esters and Oxalic Esters. Bull. Chem. Soc. Jpn. 1970, 43, 642–645. [Google Scholar] [CrossRef] [Green Version]
- Raafat, A.I. Gelatin Based PH-Sensitive Hydrogels for Colon-Specific Oral Drug Delivery: Synthesis, Characterization, and In Vitro Release Study. J. Appl. Polym. Sci. 2010, 118, 2642–2649. [Google Scholar] [CrossRef]
- Frutos, G.; Prior-Cabanillas, A.; París, R.; Quijada-Garrido, I. A Novel Controlled Drug Delivery System Based on PH-Responsive Hydrogels Included in Soft Gelatin Capsules. Acta Biomater. 2010, 6, 4650–4656. [Google Scholar] [CrossRef] [PubMed]
- Abuhelwa, A.Y.; Foster, D.J.R.; Upton, R.N. Research Article A Quantitative Review and Meta-Models of the Variability and Factors Affecting Oral Drug Absorption—Part I: Gastrointestinal PH. AAPS J. 2016, 18, 1309–1321. [Google Scholar] [CrossRef]











| Sample | OA (% w/w) | [HPG] (g mL−1) | Pre-Cure | Cure | |
|---|---|---|---|---|---|
| t (h) | t (h) | T (°C) | |||
| 1 | 10 | 0.13 | 18 | 24 | 60 |
| 2 | 10 | 0.13 | 2.5 | 24 | 80 |
| 3 | 20 | 0.26 | 1.3 | 24 | 60 |
| 4 | 20 | 0.26 | 3.0 | 3.7 | 60 |
| 5 | 20 | 0.26 | 3.0 | 7.7 | 60 |
| 6 (HPG-OA1) | 20 | 0.26 | 3.0 | 13.5 | 60 |
| 7 | 20 | 0.50 | 2.3 | 2.5 | 60 |
| 8 | 20 | 0.50 | 2.3 | 6.5 | 60 |
| 9 (HPG-OA2) | 20 | 0.50 | 2.3 | 13.5 | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Campos, B.A.; da Silva, N.C.B.; Moda, L.S.; Vidinha, P.; Maia-Obi, L.P. pH-Sensitive Degradable Oxalic Acid Crosslinked Hyperbranched Polyglycerol Hydrogel for Controlled Drug Release. Polymers 2023, 15, 1795. https://doi.org/10.3390/polym15071795
de Campos BA, da Silva NCB, Moda LS, Vidinha P, Maia-Obi LP. pH-Sensitive Degradable Oxalic Acid Crosslinked Hyperbranched Polyglycerol Hydrogel for Controlled Drug Release. Polymers. 2023; 15(7):1795. https://doi.org/10.3390/polym15071795
Chicago/Turabian Stylede Campos, Bianca Andrade, Natalia Cristina Borges da Silva, Lucas Szmgel Moda, Pedro Vidinha, and Lígia Passos Maia-Obi. 2023. "pH-Sensitive Degradable Oxalic Acid Crosslinked Hyperbranched Polyglycerol Hydrogel for Controlled Drug Release" Polymers 15, no. 7: 1795. https://doi.org/10.3390/polym15071795