Study of the Plasticization Effect of 1-Ethyl-3-methylimidazolium Acetate in TPS/PVA Biodegradable Blends Produced by Melt-Mixing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
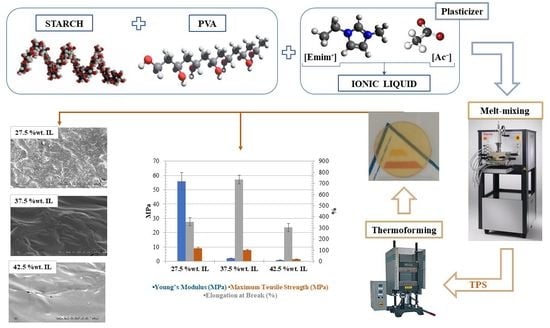
2.2. Preparation of the Starch/PVA Films Using the Ionic Liquid as Plasticized
2.3. Characterization of the Films
2.3.1. Scanning Electron Microscopy (SEM) Analysis
2.3.2. Mechanical Properties
2.3.3. Hydration Properties
2.3.4. Migration Degree
2.3.5. Attenuated Total Reflection-Fourier Transform Infrared (ATR-FTIR) Spectroscopy
2.3.6. X-ray Diffraction (XRD) Studies
2.3.7. Dynamic Mechanical Analysis (DMA)
3. Results and Discussion
3.1. Scanning Electron Microscopy (SEM) Analysis
3.2. Mechanical Properties
3.3. Hydration Properties
3.4. Migration Degree
3.5. Attenuated Total Reflection-Fourier Transform Infrared (ATR-FTIR) Spectroscopy
3.6. X-ray Diffraction (XRD) Studies
3.7. Dynamic Mechanical Analysis (DMA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wypych, G. (Ed.) Plasticizers Use and Selection for Specific Polymers. In Handbook of Plasticizers; ChemTec Publishing: Scarborough, ON, Canada, 2012; pp. 307–419. [Google Scholar]
- Shi, W.; Damodaran, K.; Nulwala, H.B.; Luebke, D.R. Theoretical and Experimental Studies of Water Interaction in Acetate Based Ionic Liquids. Phys. Chem. Chem. Phys. 2012, 14, 15897–15908. [Google Scholar] [CrossRef] [PubMed]
- Ghandi, K. A Review of Ionic Liquids, Their Limits and Applications. Green Sustain. Chem. 2014, 4, 44–53. [Google Scholar] [CrossRef] [Green Version]
- Crowhurst, L.; Mawdsley, P.R.; Perez-Arlandis, J.M.; Salter, P.A.; Welton, T. Solvent-Solute Interactions in Ionic Liquids. Phys. Chem. Chem. Phys. 2003, 5, 2790–2794. [Google Scholar] [CrossRef]
- Ren, F.; Wang, J.; Xie, F.; Zan, K.; Wang, S.; Wang, S. Applications of Ionic Liquids in Starch Chemistry: A Review. Green Chem. 2020, 22, 2162–2183. [Google Scholar] [CrossRef]
- Montalbán, M.G.; Hidalgo, J.M.; Collado-González, M.; Díaz Baños, F.G.; Víllora, G. Assessing Chemical Toxicity of Ionic Liquids on Vibrio Fischeri: Correlation with Structure and Composition. Chemosphere 2016, 155, 405–414. [Google Scholar] [CrossRef]
- Díaz Alvarez, J.C.; Martínez Rey, R.; Barrero Acosta, R. Ionic Liquids: Physicochemical Properties and Potential Application in Upgrading of Heavy Crude Oils. Rev. Ion 2012, 25, 61–87. [Google Scholar]
- Wilpiszewska, K.; Spychaj, T. Ionic Liquids: Media for Starch Dissolution, Plasticization and Modification. Carbohydr. Polym. 2011, 86, 424–428. [Google Scholar] [CrossRef]
- Rahman, M.; Brazel, C.S. Ionic Liquids: New Generation Stable Plasticizers for Poly(Vinyl Chloride). Polym. Degrad. Stab. 2006, 91, 3371–3382. [Google Scholar] [CrossRef]
- Scott, M.P.; Brazel, C.S.; Benton, M.G.; Mays, J.W.; Holbrey, J.D.; Rogers, R.D. Application of Ionic Liquids as Plasticizers for Poly(Methyl Methacrylate). Chem. Commun. 2002, 2, 1370–1371. [Google Scholar] [CrossRef]
- Wong, S.I.; Lin, H.; Sunarso, J.; Wong, B.T.; Jia, B. Optimization of Ionic-Liquid Based Electrolyte Concentration for High-Energy Density Graphene Supercapacitors. Appl. Mater. Today 2020, 18, 100522. [Google Scholar] [CrossRef]
- Montalbán, M.G.; Collado-González, M.; Lozano-Pérez, A.A.; Díaz Baños, F.G.; Víllora, G. Extraction of Organic Compounds Involved in the Kinetic Resolution of Rac-2-Pentanol from n-Hexane by Imidazolium-Based Ionic Liquids: Liquid-Liquid Equilibrium. J. Mol. Liq. 2018, 252, 445–453. [Google Scholar] [CrossRef]
- Rojas, O.G.; Hall, S.R. On the Synergistic Interaction of an Ionic Liquid and Biopolymers in the Synthesis of Strontium Niobate. Mater. Chem. Phys. 2017, 202, 220–224. [Google Scholar] [CrossRef] [Green Version]
- Lucas, M.; MacDonald, B.A.; Wagner, G.L.; Joyce, S.A.; Rector, K.D. Ionic Liquid Pretreatment of Poplar Wood at Room Temperature: Swelling and Incorporation of Nanoparticles. ACS Appl. Mater. Interfaces 2010, 2, 2198–2205. [Google Scholar] [CrossRef] [PubMed]
- Domene-López, D.; Guillén, M.M.; Martin-Gullon, I.; García-Quesada, J.C.; Montalbán, M.G. Study of the Behavior of Biodegradable Starch/Polyvinyl Alcohol/Rosin Blends. Carbohydr. Polym. 2018, 202, 299–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Use, and Fate of All Plastics Ever Made. Sci. Adv. 2017, 3, 25–29. [Google Scholar] [CrossRef] [Green Version]
- Kaza, S.; Yao, L.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; World Bank Publications: Washington, DC, USA, 2018. [Google Scholar]
- Scaffaro, R.; Citarrella, M.C.; Gulino, E.F.; Morreale, M. Hedysarum Coronarium-Based Green Composites Prepared by Compression Molding and Fused Deposition Modeling. Materials 2022, 15, 465. [Google Scholar] [CrossRef]
- Kumar, M.; Xiong, X.; He, M.; Tsang, D.C.W.; Gupta, J.; Khan, E.; Harrad, S.; Hou, D.; Ok, Y.S.; Bolan, N.S. Microplastics as Pollutants in Agricultural Soils. Environ. Pollut. 2020, 265, 114980. [Google Scholar] [CrossRef]
- Li, W.; Wufuer, R.; Duo, J.; Wang, S.; Luo, Y.; Zhang, D.; Pan, X. Microplastics in Agricultural Soils: Extraction and Characterization after Different Periods of Polythene Film Mulching in an Arid Region. Sci. Total Environ. 2020, 749, 141420. [Google Scholar] [CrossRef]
- Lusher, A.L.; Bråte, I.L.N.; Munno, K.; Hurley, R.R.; Welden, N.A. Is It or Isn’t It: The Importance of Visual Classification in Microplastic Characterization. Appl. Spectrosc. 2020, 74, 1139–1153. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Oh, Y.; Yun, J.; Yoo, E.; Jung, D.; Park, K.S.; Oh, K.K.; Lee, S.H. Characterization of Blended Cellulose/Biopolymer Films Prepared Using Ionic Liquid. Cellulose 2020, 27, 5101–5119. [Google Scholar] [CrossRef]
- Zan, K.; Wang, J.; Ren, F.; Yu, J.; Wang, S.; Xie, F.; Wang, S. Structural Disorganization of Cereal, Tuber and Bean Starches in Aqueous Ionic Liquid at Room Temperature: Role of Starch Granule Surface Structure. Carbohydr. Polym. 2021, 258, 117677. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ren, F.; Yu, J.; Copeland, L.; Wang, S.; Wang, S. Toward a Better Understanding of Different Dissolution Behavior of Starches in Aqueous Ionic Liquids at Room Temperature. ACS Omega 2019, 4, 11312–11319. [Google Scholar] [CrossRef] [PubMed]
- Sankri, A.; Arhaliass, A.; Dez, I.; Gaumont, A.C.; Grohens, Y.; Lourdin, D.; Pillin, I.; Rolland-Sabaté, A.; Leroy, E. Thermoplastic Starch Plasticized by an Ionic Liquid. Carbohydr. Polym. 2010, 82, 256–263. [Google Scholar] [CrossRef]
- Zhang, B.; Xie, F.; Shamshina, J.L.; Rogers, R.D.; McNally, T.; Wang, D.K.; Halley, P.J.; Truss, R.W.; Zhao, S.; Chen, L. Facile Preparation of Starch-Based Electroconductive Films with Ionic Liquid. ACS Sustain. Chem. Eng. 2017, 5, 5457–5467. [Google Scholar] [CrossRef] [Green Version]
- Bendaoud, A.; Chalamet, Y. Plasticizing Effect of Ionic Liquid on Cellulose Acetate Obtained by Melt Processing. Carbohydr. Polym. 2014, 108, 75–82. [Google Scholar] [CrossRef]
- Mahmood, H.; Moniruzzaman, M.; Yusup, S.; Welton, T. Ionic Liquids Assisted Processing of Renewable Resources for the Fabrication of Biodegradable Composite Materials. Green Chem. 2017, 19, 2051–2075. [Google Scholar] [CrossRef] [Green Version]
- Mateyawa, S.; Xie, D.F.; Truss, R.W.; Halley, P.J.; Nicholson, T.M.; Shamshina, J.L.; Rogers, R.D.; Boehm, M.W.; McNally, T. Effect of the Ionic Liquid 1-Ethyl-3-Methylimidazolium Acetate on the Phase Transition of Starch: Dissolution or Gelatinization? Carbohydr. Polym. 2013, 94, 520–530. [Google Scholar] [CrossRef] [Green Version]
- Zdanowicz, M.; Spychaj, T. Ionic Liquids as Starch Plasticizers or Solvents. Polimery 2011, 56, 861–864. [Google Scholar] [CrossRef]
- Yang, X.; Qiao, C.; Li, Y.; Li, T. Dissolution and Resourcfulization of Biopolymers in Ionic Liquids. React. Funct. Polym. 2016, 100, 181–190. [Google Scholar] [CrossRef]
- Luchese, C.L.; Benelli, P.; Spada, J.C.; Tessaro, I.C. Impact of the Starch Source on the Physicochemical Properties and Biodegradability of Different Starch-Based Films. J. Appl. Polym. Sci. 2018, 135, 46564. [Google Scholar] [CrossRef]
- Lopez-Rubio, A.; Flanagan, B.M.; Shrestha, A.K.; Gidley, M.J.; Gilbert, E.P. Molecular Rearrangement of Starch during in vitro Digestion: Toward a Better Understanding of Enzyme Resistant Starch Formation in Processed Starches. Biomacromolecules 2008, 9, 1951–1958. [Google Scholar] [CrossRef] [PubMed]
- Lekube, B.M.; Fahrngruber, B.; Kozich, M.; Wastyn, M.; Burgstaller, C. Influence of Processing on the Mechanical Properties and Morphology of Starch-Based Blends for Film Applications. J. Appl. Polym. Sci. 2019, 136, 47990. [Google Scholar] [CrossRef]
- Valero-Valdivieso, M.F.; Ortegón, Y.; Uscategui, Y. Biopolímeros: Avances y Perspectivas. Dyna 2013, 80, 171–180. [Google Scholar]
- Biliaderis, C.G.; Lazaridou, A.; Arvanitoyannis, I. Glass Transition and Physical Properties of Polyol-Plasticized Pullulan-Starch Blends at Low Moisture. Carbohydr. Polym. 1999, 40, 29–47. [Google Scholar] [CrossRef]
- Talja, R.A.; Helén, H.; Roos, Y.H.; Jouppila, K. Effect of Various Polyols and Polyol Contents on Physical and Mechanical Properties of Potato Starch-Based Films. Carbohydr. Polym. 2007, 67, 288–295. [Google Scholar] [CrossRef]
- Sreekumar, P.A.; Al-Harthi, M.A.; De, S.K. Effect of Glycerol on Thermal and Mechanical Properties of Polyvinyl Alcohol/Starch Blends. J. Appl. Polym. Sci. 2012, 123, 135–142. [Google Scholar] [CrossRef]
- Da Róz, A.L.; Carvalho, A.J.F.; Gandini, A.; Curvelo, A.A.S. The Effect of Plasticizers on Thermoplastic Starch Compositions Obtained by Melt Processing. Carbohydr. Polym. 2006, 63, 417–424. [Google Scholar] [CrossRef]
- Xie, F.; Flanagan, B.M.; Li, M.; Sangwan, P.; Truss, R.W.; Halley, P.J.; Strounina, E.V.; Whittaker, A.K.; Gidley, M.J.; Dean, K.M.; et al. Characteristics of Starch-Based Films Plasticised by Glycerol and by the Ionic Liquid 1-Ethyl-3-Methylimidazolium Acetate: A Comparative Study. Carbohydr. Polym. 2014, 111, 841–848. [Google Scholar] [CrossRef] [Green Version]
- Domene-López, D.; Delgado-Marín, J.J.; García-Quesada, J.C.; Martín-Gullón, I.; Montalbán, M.G. Electroconductive Starch/Multi-Walled Carbon Nanotube Films Plasticized by 1-Ethyl-3-Methylimidazolium Acetate. Carbohydr. Polym. 2020, 229, 115545. [Google Scholar] [CrossRef]
- Abera, G.; Woldeyes, B.; Demash, H.D.; Miyake, G. The Effect of Plasticizers on Thermoplastic Starch Films Developed from the Indigenous Ethiopian Tuber Crop Anchote (Coccinia abyssinica) Starch. Int. J. Biol. Macromol. 2020, 155, 581–587. [Google Scholar] [CrossRef]
- Gomez-Coma, L.; Garea, A.; Irabien, A. Carbon Dioxide Capture by [Emim][Ac] Ionic Liquid in a Polysulfone Hollow Fiber Membrane Contactor. Int. J. Greenh. Gas Control 2016, 52, 401–409. [Google Scholar] [CrossRef] [Green Version]
- Ostadjoo, S.; Berton, P.; Shamshina, J.L.; Rogers, R.D. Scaling-up Ionic Liquid-Based Technologies: How Much Do We Care about Their Toxicity? Prima Facie Information on 1-Ethyl-3-Methylimidazolium Acetate. Toxicol. Sci. 2018, 161, 249–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Coma, L.; Garea, A.; Irabien, A. Non-Dispersive Absorption of CO2 in [Emim][EtSO4] and [Emim][Ac]: Temperature Influence. Sep. Purif. Technol. 2014, 132, 120–125. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, X.; Yan, C.; Cao, Y.; Mu, T. The Dynamic Process of Atmospheric Water Sorption in [EMIM][Ac] and Mixtures of [EMIM][Ac] with Biopolymers and CO2 Capture in These Systems. J. Phys. Chem. B 2014, 118, 11523–11536. [Google Scholar] [CrossRef] [PubMed]
- Domene-López, D.; Delgado-Marín, J.J.; Martin-Gullon, I.; García-Quesada, J.C.; Montalbán, M.G. Comparative Study on Properties of Starch Films Obtained from Potato, Corn and Wheat Using 1-Ethyl-3-Methylimidazolium Acetate as Plasticizer. Int. J. Biol. Macromol. 2019, 135, 845–854. [Google Scholar] [CrossRef]
- Decaen, P.; Rolland-Sabaté, A.; Colomines, G.; Guilois, S.; Lourdin, D.; Della Valle, G.; Leroy, E. Influence of Ionic Plasticizers on the Processing and Viscosity of Starch Melts. Carbohydr. Polym. 2020, 230, 115591. [Google Scholar] [CrossRef]
- Tan, X.; Li, X.; Chen, L.; Xie, F. Solubility of Starch and Microcrystalline Cellulose in 1-Ethyl-3-Methylimidazolium Acetate Ionic Liquid and Solution Rheological Properties. Phys. Chem. Chem. Phys. 2016, 18, 27584–27593. [Google Scholar] [CrossRef]
- Liu, K.; Tan, X.; Li, X.; Chen, L.; Xie, F. Characterization of Regenerated Starch from 1-Ethyl-3-Methylimidazolium Acetate Ionic Liquid with Different Anti-Solvents. J. Polym. Sci. Part B Polym. Phys. 2018, 56, 1231–1238. [Google Scholar] [CrossRef]
- Gunawardene, O.H.P.; Amaraweera, S.M.; Wannikayaka, W.M.D.B.; Fernando, N.M.L.; Gunathilake, C.A.; Manamperi, W.A.; Kulatunga, A.K.; Manipura, A. Role of Compatibilization of Phthalic Acid in Cassava Starch/Poly Vinyl Alcohol Thin Films. In Proceedings of the 12th International Conference on Structural Engineering and Construction Management: ICSECM 2021, Kandy, Sri Lanka, 2–5 December 2021; Springer Nature: Singapore, 2022; pp. 665–689. [Google Scholar]
- Narancic, T.; Cerrone, F.; Beagan, N.; O’Connor, K.E. Recent Advances in Bioplastics: Application and Biodegradation. Polymers 2020, 12, 920. [Google Scholar] [CrossRef] [Green Version]
- Gulino, E.F.; Citarrella, M.C.; Maio, A.; Scaffaro, R. An Innovative Route to Prepare in Situ Graded Crosslinked PVA Graphene Electrospun Mats for Drug Release. Compos. Part A 2022, 155, 106827. [Google Scholar] [CrossRef]
- Gaaz, T.S.; Sulong, A.B.; Akhtar, M.N.; Kadhum, A.A.H.; Mohamad, A.B.; Al-amiery, A.A. Properties and Applications of Polyvinyl Alcohol, Halloysite Nanotubes and Their Nanocomposites. Molecules 2015, 20, 22833–22847. [Google Scholar] [CrossRef] [Green Version]
- Domene-López, D.; García-Quesada, J.C.; Martin-Gullon, I.; Montalbán, M.G. Influence of Starch Composition and Molecular Weight on Physicochemical Properties of Biodegradable Films. Polymers 2019, 11, 1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eaton, M.D.; Domene-López, D.; Wang, Q.; Montalbán, M.G.; Martin-Gullon, I.; Shull, K.R.; Martin-Gullon, I.; Shull, K.R. Exploring the Effect of Humidity on Thermoplastic Starch Films Using the Quartz Crystal Microbalance. Carbohydr. Polym. 2021, 261, 117727. [Google Scholar] [CrossRef] [PubMed]
- ASTM D882-12; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM International: West Conshohocken, PA, USA, 2012. Available online: https://www.astm.org/DATABASE.CART/HISTORICAL/D882-02.htm (accessed on 28 March 2018).
- Jaramillo, C.M.; González Seligra, P.; Goyanes, S.; Bernal, C.; Famá, L. Biofilms Based on Cassava Starch Containing Extract of Yerba Mate as Antioxidant and Plasticizer. Starch/Staerke 2015, 67, 780–789. [Google Scholar] [CrossRef]
- Marcilla, A.; García, S.; García-Quesada, J.C. Study of the Migration of PVC Plasticizers. J. Anal. Appl. Pyrolysis 2004, 71, 457–463. [Google Scholar] [CrossRef]
- Dai, L.; Zhang, J.; Cheng, F. Effects of Starches from Different Botanical Sources and Modification Methods on Physicochemical Properties of Starch-Based Edible Films. Int. J. Biol. Macromol. 2019, 132, 897–905. [Google Scholar] [CrossRef]
- Sciarini, L.S.; Rolland-Sabaté, A.; Guilois, S.; Decaen, P.; Leroya, E.; Le Bail, P. Understanding the Destructuration of Starch in Water–Ionic Liquid Mixtures. Green Chem. 2015, 17, 291–299. [Google Scholar] [CrossRef]
- Zhong, Y.; Li, Y.; Liang, W.; Liu, L.; Li, S.; Xue, J.; Guo, D. Comparison of Gelatinization Method, Starch Concentration, and Plasticizer on Physical Properties of High-Amylose Starch Films. J. Food Process Eng. 2018, 41, 1–8. [Google Scholar] [CrossRef]
- Otero-Mato, J.M.; Lesch, V.; Montes-Campos, H.; Smiatek, J.; Diddens, D.; Cabeza, O.; Gallego, L.J.; Varela, L.M. Solvation in Ionic Liquid-Water Mixtures: A Computational Study. J. Mol. Liq. 2019, 292, 111273. [Google Scholar] [CrossRef]
- Ismail, S.; Mansor, N.; Majeed, Z.; Man, Z. Effect of Water and [Emim][OAc] as Plasticizer on Gelatinization of Starch. Procedia Eng. 2016, 148, 524–529. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Gao, B.; Zhang, S. Adjusting Hydrogen Bond by Lever Principle to Achieve High Performance Starch-Based Biodegradable Films with Low Migration Quantity. Carbohydr. Polym. 2022, 298, 120107. [Google Scholar] [CrossRef] [PubMed]
- Edhirej, A.; Sapuan, S.M.; Jawaid, M.; Zahari, N.I. Cassava/Sugar Palm Fiber Reinforced Cassava Starch Hybrid Composites: Physical, Thermal and Structural Properties. Int. J. Biol. Macromol. 2017, 101, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xie, F.; Yu, L.; Chen, L.; Li, L. Thermal Processing of Starch-Based Polymers. Prog. Polym. Sci. 2009, 34, 1348–1368. [Google Scholar] [CrossRef]
- Li, C.; Hu, Y.; Li, E. Effects of Amylose and Amylopectin Chain-Length Distribution on the Kinetics of Long-Term Rice Starch Retrogradation. Food Hydrocoll. 2021, 111, 106239. [Google Scholar] [CrossRef]
- Schmitt, H.; Guidez, A.; Prashantha, K.; Soulestin, J.; Lacrampe, M.F.; Krawczak, P. Studies on the Effect of Storage Time and Plasticizers on the Structural Variations in Thermoplastic Starch. Carbohydr. Polym. 2015, 115, 364–372. [Google Scholar] [CrossRef]
- Nazrin, A.; Sapuan, S.M.; Ilyas, R.A. Water Barrier and Mechanical Properties of Sugar Palm Crystalline Nanocellulose Reinforced Thermoplastic Sugar Palm Starch (TPS)/Poly(Lactic Acid) (PLA) Blend Bionanocomposites. Nanotechnol. Rev. 2021, 10, 431–442. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, L.; Xie, F.; Li, X.; Truss, R.W.; Halley, P.J.; Shamshina, J.L.; Rogers, R.D.; McNally, T. Understanding the Structural Disorganization of Starch in Water-Ionic Liquid Solutions. Phys. Chem. Chem. Phys. 2015, 17, 13860–13871. [Google Scholar] [CrossRef] [Green Version]
- Delgado, J.M.; Rodes, A.; Orts, J.M. B3LYP and in Situ ATR-SEDIRAS Study of the Infrared Behavior and Bonding Mode of Adsorbed Acetate Anions on Silver Thin-Film Electrodes. J. Phys. Chem. C 2007, 111, 14476–14483. [Google Scholar] [CrossRef]
- Zhang, B.; Xie, F.; Zhang, T.; Chen, L.; Li, X.; Truss, R.W.; Halley, P.J.; Shamshina, J.L.; McNally, T.; Rogers, R.D. Different Characteristic Effects of Ageing on Starch-Based Films Plasticised by 1-Ethyl-3-Methylimidazolium Acetate and by Glycerol. Carbohydr. Polym. 2016, 146, 67–79. [Google Scholar] [CrossRef] [Green Version]
- Das, K.; Ray, D.; Bandyopadhyay, N.R.; Gupta, A.; Sengupta, S.; Sahoo, S.; Mohanty, A.; Misra, M. Preparation and Characterization of Cross-Linked Starch/Poly(Vinyl Alcohol) Green Films with Low Moisture Absorption. Ind. Eng. Chem. Res. 2010, 49, 2176–2185. [Google Scholar] [CrossRef]
- Phetwarotai, W.; Potiyaraj, P.; Aht-Ong, D. Characteristics of Biodegradable Polylactide/Gelatinized Starch Films: Effects of Starch, Plasticizer, and Compatibilizer. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Bendaoud, A.; Chalamet, Y. Effects of Relative Humidity and Ionic Liquids on the Water Content and Glass Transition of Plasticized Starch. Carbohydr. Polym. 2013, 97, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Bhagabati, P.; Hazarika, D.; Katiyar, V. Tailor-Made Ultra-Crystalline, High Molecular Weight Poly(ε-Caprolactone) Films with Improved Oxygen Gas Barrier and Optical Properties: A Facile and Scalable Approach. Int. J. Biol. Macromol. 2019, 124, 1040–1052. [Google Scholar] [CrossRef]
- Madrigal, L.; Sandoval, A.J.; Müller, A.J. Effects of Corn Oil on Glass Transition Temperatures of Cassava Starch. Carbohydr. Polym. 2011, 85, 875–884. [Google Scholar] [CrossRef]
- Sreekumar, P.A.; Al-Harthi, M.A.; De, S.K. Studies on Compatibility of Biodegradable Starch/Polyvinyl Alcohol Blends. Polym. Eng. Sci. 2012, 52, 2167–2172. [Google Scholar] [CrossRef]
- López, O.V.; Lecot, C.J.; Zaritzky, N.E.; García, M.A. Biodegradable Packages Development from Starch Based Heat Sealable Films. J. Food Eng. 2011, 105, 254–263. [Google Scholar] [CrossRef]
- Zhang, S.; He, Y.; Yin, Y.; Jiang, G. Fabrication of Innovative Thermoplastic Starch Bio-Elastomer to Achieve High Toughness Poly(Butylene Succinate) Composites. Carbohydr. Polym. 2019, 206, 827–836. [Google Scholar] [CrossRef]
- Jha, P.; Dharmalingam, K.; Nishizu, T.; Katsuno, N.; Anandalakshmi, R. Effect of Amylose–Amylopectin Ratios on Physical, Mechanical, and Thermal Properties of Starch-Based Bionanocomposite Films Incorporated with CMC and Nanoclay. Starch/Staerke 2020, 72, 1900121. [Google Scholar] [CrossRef]







| Sample | Water Content (%) | Solubility in Water (%) |
|---|---|---|
| IL_27.5 | 9.41 ± 0.25 | 35.2 ± 0.2 |
| IL_30 | 9.30 ± 0.26 | 37.0 ± 0.4 |
| IL_32.5 | 9.93 ± 0.29 | 40.8 ± 0.2 |
| IL_37.5 | 9.97 ± 0.16 | 49.3 ± 1.2 |
| IL_40 | 11.4 ± 0.2 | 50.8 ± 1.0 |
| IL_42.5 | 14.9 ± 0.9 | 57.6 ± 1.5 |
| Films Sample | |||||||
|---|---|---|---|---|---|---|---|
| Starch_Pure | IL_27.5 | IL_30 | IL_32.5 | IL_37.5 | IL_40 | IL_42.5 | |
| R995/1022 | 1.39 | 0.824 | 0.673 | 0.659 | 0.637 | 0.613 | 0.536 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, J.M.; Montalbán, M.G.; Domene-López, D.; Martín-Gullón, I.; García-Quesada, J.C. Study of the Plasticization Effect of 1-Ethyl-3-methylimidazolium Acetate in TPS/PVA Biodegradable Blends Produced by Melt-Mixing. Polymers 2023, 15, 1788. https://doi.org/10.3390/polym15071788
Castro JM, Montalbán MG, Domene-López D, Martín-Gullón I, García-Quesada JC. Study of the Plasticization Effect of 1-Ethyl-3-methylimidazolium Acetate in TPS/PVA Biodegradable Blends Produced by Melt-Mixing. Polymers. 2023; 15(7):1788. https://doi.org/10.3390/polym15071788
Chicago/Turabian StyleCastro, Jennifer M., Mercedes G. Montalbán, Daniel Domene-López, Ignacio Martín-Gullón, and Juan C. García-Quesada. 2023. "Study of the Plasticization Effect of 1-Ethyl-3-methylimidazolium Acetate in TPS/PVA Biodegradable Blends Produced by Melt-Mixing" Polymers 15, no. 7: 1788. https://doi.org/10.3390/polym15071788