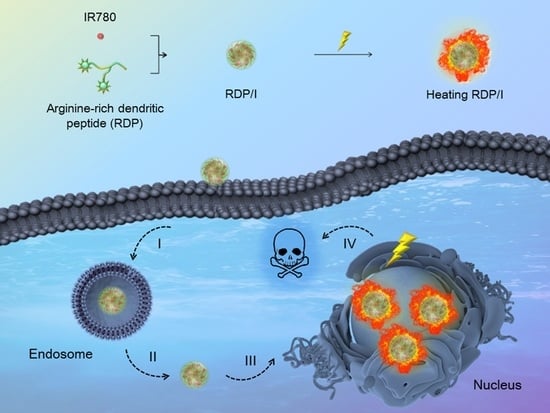
Nucleus-Targeting Nanoplatform Based on Dendritic Peptide for Precise Photothermal Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Lines
2.3. Synthesis of RDP
2.4. Determination of Critical Micelle Concentration (CMC)
2.5. Preparation of RDP/I
2.6. Characterization of RDP/I
2.7. Release Behavior In Vitro
2.8. Photothermal Efficiency Tests
2.9. Cytotoxicity Assay In Vitro
2.10. In Vitro Wound-Healing Assay
2.11. Intracellular Uptake of RDP/I
2.12. Tumor Suppression Study
3. Results and Discussion
3.1. Characterization of RDP
3.2. Characterization of RDP/I
3.3. Photothermal Efficiency Tests
3.4. Cytotoxicity Assay In Vitro
3.5. In Vitro Wound-Healing Assay
3.6. Intracellular Uptake ofRDP/I
3.7. Tumor Suppression Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Jing, R.W.; Wang, Q.; Chen, L.; Li, G.T.; Li, R.B.; Zhang, L.J.; Zhang, H.B.; Zuo, B.F.; Seow, Y.Q.; Qiao, X.; et al. Functional imaging and targeted drug delivery in mice and patient tumors with a cell nucleolus-localizing and tumor-targeting peptide. Biomaterials 2022, 289, 121758. [Google Scholar] [CrossRef]
- Du, W.; Du, S.B.; Dong, X.; Bai, H.; Jiang, J.M.; Hao, S.P.; Yang, F.; Xiao, Q.C.; Zhang, B.; Ge, J.Y.; et al. Biodegradable silica nanocapsules enable efficient nuclear-targeted delivery of native proteins for cancer therapy. Biomaterials 2023, 294, 122000. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.J.; Xu, W.H.; Xiao, P.H.; Kang, M.M.; Yan, D.Y.; Wen, H.F.; Song, N.; Wang, D.; Tang, B.Z. Molecular engineering of high-performance aggregation-induced emission photosensitizers to boost cancer theranostics mediated by acid-triggered nucleus-targeted nanovectors. ACS Nano 2021, 15, 10689–10699. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.S.; Huang, Y.; Wang, B.; Ma, L.L.; Karges, J.; Xiao, H.H. Photo-reduction with NIR light of nucleus-targeting PtIV nanoparticles for combined tumor-targeted chemotherapy and photodynamic immunotherapy. Angew. Chem. Int. Ed. 2022, 61, 202201486. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Yang, X.; Luo, X.P.; Xue, X.L.; Qian, C.G.; Sun, M.J. Photoactivated nanosheets accelerate nucleus access of cisplatin for drug-resistant cancer therapy. Adv. Funct. Mater. 2020, 30, 2001546. [Google Scholar] [CrossRef]
- Chen, S.; Fan, J.; Qiu, W.; Liu, F.; Yan, G.; Zeng, X.; Zhang, X. A cellular/intranuclear dual-targeting nanoplatform based on gold nanostar for accurate tumor photothermal therapy. J. Mater. Chem. B 2018, 6, 1543–1551. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Shen, F.Z.; Cai, Z.H.; Pan, W.Z.; Yin, Y.M.; Deng, X.; Zhang, X.; Machuki, J.O.; Yu, Y.Y.; Yang, D.Z.; et al. Target-induced core-satellite nanostructure assembly strategy for dual-signal-on fluorescence imaging and raman quantification of intracellular microrna guided photothermal therapy. Small 2020, 16, 2005511. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, K.X.; Wang, Y.Y.; Jiang, W.X.; Cheng, H.; Wang, Q.W.; Xiang, T.T.; Zhang, Z.Z.; Liu, J.J.; Shi, J.J. Intracellular self-assembly driven nucleus-targeted photo-immune stimulator with chromatin decompaction function for robust innate and adaptive antitumor immunity. Adv. Funct. Mater. 2022, 32, 2108883. [Google Scholar] [CrossRef]
- Chen, M.Y.; Juengpanich, S.; Li, S.J.; Topatana, W.; Lu, Z.Y.; Zheng, Q.; Cao, J.S.; Hu, J.H.; Chan, E.; Hou, L.D.; et al. Bortezomib-encapsulated dual responsive copolymeric nanoparticles for gallbladder cancer targeted therapy. Adv. Sci. 2022, 9, 2103895. [Google Scholar] [CrossRef]
- Wang, K.N.; Liu, L.Y.; Mao, D.; Hou, M.X.; Tan, C.P.; Mao, Z.W.; Liu, B. A nuclear-targeted AIE photosensitizer for enzyme inhibition and photosensitization in cancer cell ablation. Angew. Chem. Int. Ed. 2022, 61, 202114600. [Google Scholar]
- Tu, Z.X.; Donskyi, I.S.; Qiao, H.S.; Zhu, Z.L.; Unger, W.E.S.; Hackenberger, C.P.R.; Chen, W.; Adeli, M.; Haag, R. Graphene oxide-cyclic R10 peptide nuclear translocation nanoplatforms for the surmounting of multiple-drug resistance. Adv. Funct. Mater. 2020, 30, 2000933. [Google Scholar] [CrossRef]
- Tang, L.; Zhang, A.N.; Zhang, Z.Y.; Zhao, Q.Q.; Li, J.; Mei, Y.J.; Yin, Y.; Wang, W. Multifunctional inorganic nanomaterials for cancer photoimmunotherapy. Cancer Commun. 2022, 42, 141–163. [Google Scholar] [CrossRef] [PubMed]
- Dilliard, S.A.; Siegwart, D.J. Passive, active and endogenous organ-targeted lipid and polymer nanoparticles for delivery of genetic drugs. Nat. Rev. Mater. 2023, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.J.; Zhao, H.J.; Wang, S.; Fan, Z.W.; Ma, Y.; Yin, Y.M.; Wang, W.; Xi, R.M.; Meng, M. A tumor-targeting near-infrared heptamethine cyanine photosensitizer with twisted molecular structure for enhanced imaging-guided cancer phototherapy. J. Am. Chem. Soc. 2021, 143, 20828–20836. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Du, X.F.; Liu, B.; Li, C.R.; Long, J.; Zhao, M.X.; Yao, Z.Y.; Liang, X.J.; Lai, Y.X. Engineering Supramolecular Nanomedicine for Targeted Near Infrared-triggered Mitochondrial Dysfunction to Potentiate Cisplatin for Efficient Chemophototherapy. ACS Nano 2022, 16, 1421–1435. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; He, X.D.; Wang, Z.G.; Wang, Y.P.; Liu, S.; Li, X.Y.; Huang, Y.B. Combining PD-L1 inhibitors with immunogenic cell death triggered by chemo-photothermal therapy via a thermosensitive liposome system to stimulate tumor-specific immunological response. Nanoscale 2021, 13, 12966–12978. [Google Scholar] [CrossRef]
- Li, Z.; Chu, Z.Y.; Yang, J.; Qian, H.S.; Xu, J.M.; Chen, B.J.; Tian, T.; Chen, H.; Xu, Y.S.; Wang, F. Immunogenic cell death augmented by manganese zinc sulfide nanoparticles for metastatic melanoma immunotherapy. ACS Nano 2022, 16, 15471–15483. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Zou, Y.T.; Yang, M.; Mei, S.; Liu, X.H.; Han, H.Y.; Zhang, C.D.; Niu, M.M. Highly potent, selective, biostable, and cell-permeable cyclic d-peptide for dual-targeting therapy of lung cancer. J. Am. Chem. Soc. 2022, 144, 7117–7128. [Google Scholar] [CrossRef]
- Ma, T.; Chen, R.; Lv, N.N.; Chen, Y.; Qin, H.M.; Jiang, H.; Zhu, J.T. Size-transformable bicomponent peptide nanoparticles for deep tumor penetration and photo-chemo combined antitumor therapy. Small 2022, 18, 2106291. [Google Scholar] [CrossRef]
- Zhu, J.W.; Tian, J.; Yang, C.; Chen, J.P.; Wu, L.H.; Fan, M.N.; Cai, X.J. L-Arg-rich amphiphilic dendritic peptide as a versatile NO donor for NO/photodynamic synergistic treatment of bacterial infections and promoting wound healing. Small 2021, 17, 2101495. [Google Scholar] [CrossRef]
- Chen, S.; Fan, J.X.; Qiu, W.X.; Liu, L.H.; Cheng, H.; Liu, F.; Yan, G.P.; Zhang, X.Z. Self-assembly drug delivery system based on programmable dendritic peptide applied in multidrug resistance tumor therapy. Macromol. Rapid. Commun. 2017, 38, 1700409. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, Y.X.; Wang, J.X.; Luo, X.; Huang, Y.D.; Xu, J.L.; Yan, G.P.; Chen, S.; Zhang, X.Z. A multi-functional drug delivery system based on dendritic peptide for tumor nuclear accurate targeting therapy. Acta Polym. Sin. 2018, 6, 682–691. [Google Scholar]
- Chen, S.; Fan, J.X.; Liu, X.H.; Zhang, M.K.; Liu, F.; Zeng, X.; Yan, G.P.; Zhang, X.Z. A self-delivery system based on an amphiphilic proapoptotic peptide for tumor targeting therapy. J. Mater. Chem. B 2019, 7, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhou, J.; Fu, Y.; Zheng, Y.; Shen, W.; Zhou, J.; Yin, T. Deeply Infiltrating iRGD-graphene oxide for the geting-based antimigration. Adv. Healthc. Mater. 2021, 10, 2100536. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, J.; Ma, X.; Liu, F.; Yan, G. Cationic peptide-modified gold nanostars as efficient delivery platform for rna interference antitumor therapy. Polymers 2021, 13, 3764. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wu, B.; Wu, Y.; Song, X.; Zhang, S.; Liu, Z. Camouflaging nanoparticles with brain metastatic tumor cell membranes: A new strategy to traverse blood–brain barrier for imaging and therapy of brain tumors. Adv. Funct. Mater. 2020, 30, 1909369. [Google Scholar] [CrossRef]
- Liu, T.; Xiong, C.F.; Zhang, L.J.; Jiao, G.H.; Shi, H.; Feng, J.; Zhang, X.Z. Boosting doxorubicin-induced mitochondria apoptosis for the monodrug-mediated combination of chemotherapy and chemodynamic therapy. Adv. Healthc. Mater. 2023, 12, 2202045. [Google Scholar] [CrossRef]
- Du, Y.Q.; Zhang, R.; Yang, J.N.; Liu, S.K.; Zhou, J.L.; Zhao, R.X.; He, F.; Zhang, Y.Q.; Yang, P.P.; Lin, J. A “closed-loop” therapeutic strategy based on mutually reinforced ferroptosis and immunotherapy. Adv. Funct. Mater. 2022, 32, 2111784. [Google Scholar] [CrossRef]
- Hathout, R.M.; Metwally, A.A.; El-Ahmady, S.H.; Metwally, E.S.; Ghonim, N.A.; Bayoumy, S.A.; Erfan, T.; Ashraf, R.; Fadel, M.; El-Kholy, A.I.; et al. Dual stimuli-responsive polypyrrole nanoparticles for anticancer therapy. J. Drug Deliv. Sci. Technol. 2018, 47, 176–180. [Google Scholar] [CrossRef]
- Cheng, K.; Liu, B.; Zhang, X.S.; Zhang, R.Y.; Zhang, F.; Ashraf, G.; Fan, G.Q.; Tian, M.Y.; Sun, X.; Yuan, J.; et al. Biomimetic material degradation for synergistic enhanced therapy by regulating endogenous energy metabolism imaging under hypothermia. Nat. Commun. 2022, 13, 4567. [Google Scholar] [CrossRef]
- Chen, S.; Fan, J.X.; Zheng, D.W.; Liu, F.; Zeng, X.; Yan, G.P.; Zhang, X.Z. A multi-functional drug delivery system based on polyphenols for efficient tumor inhibition and metastasis prevention. Biomater. Sci. 2020, 8, 702–711. [Google Scholar] [CrossRef]
- Zou, M.Z.; Li, Z.H.; Bai, X.F.; Liu, C.J.; Zhang, X.Z. Hybrid vesicles based on autologous tumor cell membrane and bacterial outer membrane to enhance innate immune response and personalized tumor immunotherapy. Nano Lett. 2021, 21, 8609–8618. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Lei, Q.; Qiu, W.X.; Liu, L.H.; Zheng, D.W.; Fan, J.X.; Rong, L.; Sun, Y.X.; Zhang, X.Z. Mitochondria-targeting "Nanoheater" for enhanced photothermal/chemo-therapy. Biomaterials 2017, 117, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Mai, Z.Y.; Zhong, J.; Zhang, J.S.; Chen, G.M.; Tang, Y.; Ma, W.; Li, G.; Feng, Z.Z.; Li, F.Z.; Liang, X.J.; et al. Carrier-free immunotherapeutic nano-booster with dual synergistic effects based on glutaminase inhibition combined with photodynamic therapy. ACS Nano 2023, 17, 1583–1596. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.R.; He, R.Y.; Liu, Q.; Lin, M.; Dong, Y.Q.; Li, K.; Tang, B.Z.; Liu, B.; Xu, F. Near-infrared light-regulated cancer theranostic nanoplatform based on aggregation-induced emission luminogen encapsulated upconversion nanoparticles. Theranostics 2019, 9, 246–264. [Google Scholar] [CrossRef]






| Peptide | M (Calculated) | m/z (Found) |
|---|---|---|
| CRRK(RRCG(Fmoc))2 | 1949.92 | [M + 5H]5+/5: 391.2 [M + 4H]4+/4: 488.5 [M + 3H]3+/3: 650.8 [M + 2H]2+/2: 976.3 |
| Sample | Hydrodynamic Diameter (nm) | Zeta Potential (mV) |
|---|---|---|
| RDP/I | 137.2 ± 0.6 | +30.2 ± 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.-S.; Ma, X.-Y.; Zheng, S.-Y.; Chen, S.; Fan, J.-X.; Liu, F.; Yan, G.-P. Nucleus-Targeting Nanoplatform Based on Dendritic Peptide for Precise Photothermal Therapy. Polymers 2023, 15, 1753. https://doi.org/10.3390/polym15071753
Wang W-S, Ma X-Y, Zheng S-Y, Chen S, Fan J-X, Liu F, Yan G-P. Nucleus-Targeting Nanoplatform Based on Dendritic Peptide for Precise Photothermal Therapy. Polymers. 2023; 15(7):1753. https://doi.org/10.3390/polym15071753
Chicago/Turabian StyleWang, Wen-Song, Xiao-Yu Ma, Si-Yao Zheng, Si Chen, Jin-Xuan Fan, Fan Liu, and Guo-Ping Yan. 2023. "Nucleus-Targeting Nanoplatform Based on Dendritic Peptide for Precise Photothermal Therapy" Polymers 15, no. 7: 1753. https://doi.org/10.3390/polym15071753