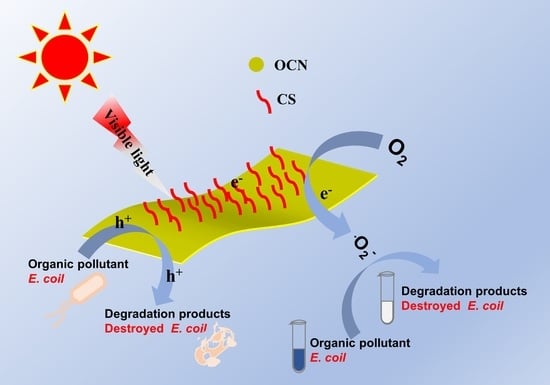
Chitosan-Grafted Carbon Oxynitride Nanoparticles: Investigation of Photocatalytic Degradation and Antibacterial Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of CS-Grafted OCN
2.3. Characterization
2.4. Reactive Oxygen Species (ROS) Measurements
2.5. Antibacterial Activity
2.6. Photocatalytic Performance Assays
3. Results and Discussion
3.1. Structure and Composition of Prepared CS-OCN
3.2. Photocatalytic Performances of Prepared Materials
3.2.1. Optical Performance
3.2.2. Photocatalytic Decomposition
3.2.3. Photocatalytic Antibacterial
3.2.4. Determination of ROS
3.2.5. Photoelectrochemical Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, C.; Xie, M.; Kong, L.; Lu, W.; Feng, Z.; Zhan, J. Mn3O4 nanodots loaded g-C3N4 nanosheets for catalytic membrane degradation of organic contaminants. J. Hazard. Mater. 2020, 390, 122146. [Google Scholar] [CrossRef]
- Jones, V.J.; Rose, N.L.; Self, A.E.; Solovieva, N.; Yang, H. Evidence of global pollution and recent environmental change in Kamchatka, Russia. Glob. Planet Change 2015, 134, 82–90. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Zhang, C.; Lai, C.; Zeng, G.; Huang, D.; Cheng, M.; Wang, J.; Chen, F.; Zhou, C.; Xiong, W. BiOX (X = Cl, Br, I) photocatalytic nanomaterials: Applications for fuels and environmental management. Adv. Colloid Interface Sci. 2018, 254, 76–93. [Google Scholar] [CrossRef]
- Chen, G.; He, S.; Shi, G.; Ma, Y.; Ruan, C.; Jin, X.; Chen, Q.; Liu, X.; Dai, H.; Chen, X. In-situ immobilization of ZIF-67 on wood aerogel for effective removal of tetracycline from water. Chem. Eng. J. 2021, 423, 130184. [Google Scholar] [CrossRef]
- Li, C.; Sun, X.; Zhu, Y.; Liang, W.; Nie, Y.; Shi, W.; Ai, S. Core-shell structural nitrogen-doped carbon foam loaded with nano zero-valent iron for simultaneous remediation of Cd (II) and NAP in water and soil: Kinetics, mechanism, and environmental evaluation. Sci. Total Environ. 2022, 832, 155091. [Google Scholar] [CrossRef] [PubMed]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, B.; Ma, J.; Ning, P. Novel synthesis of aluminum hydroxide gel-coated nano zero-valent iron and studies of its activity in flocculation-enhanced removal of tetracycline. J. Environ. Sci. 2020, 89, 194–205. [Google Scholar] [CrossRef]
- Priyadharsan, A.; Vasanthakumar, V.; Karthikeyan, S.; Raj, V.; Shanavas, S.; Anbarasan, P.M. Multi-functional properties of ternary CeO2/SnO2/rGO nanocomposites: Visible light driven photocatalyst and heavy metal removal. J. Photochem. Photobiol. A Chem. 2017, 346, 32–45. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Zhou, C.; Xiong, W.; Zeng, G.; Huang, D.; Zhang, C.; Wang, W.; Song, B.; Tang, X. Recent advances in application of graphitic carbon nitride-based catalysts for degrading organic contaminants in water through advanced oxidation processes beyond photocatalysis: A critical review. Water Res. 2020, 184, 116200. [Google Scholar] [CrossRef]
- Ikram, M.; Haider, A.; Naz, S.; Bari, M.A.; Haider, J.; Ul-Hamid, A.; Nabgan, W.; Imran, M.; Nazir, G.; Ali, S. Chitosan and carbon nitride doped barium hydroxide nanoparticles served as dye degrader and bactericidal potential: A molecular docking study. Int. J. Biol. Macromol. 2023, 224, 938–949. [Google Scholar] [CrossRef]
- Dobson, R.S.; Burgess, J.E. Biological treatment of precious metal refinery wastewater: A review. Miner. Eng. 2007, 20, 519–532. [Google Scholar] [CrossRef]
- Rahman, A.; Warsi, M.F.; Shakir, I.; Shahid, M.; Zulfiqar, S. Fabrication of Ce3+ substituted nickel ferrite-reduced graphene oxide heterojunction with high photocatalytic activity under visible light irradiation. J. Hazard. Mater. 2020, 394, 122593. [Google Scholar] [CrossRef]
- Rahman, A.; Aadil, M.; Akhtar, M.; Warsi, M.F.; Jamil, A.; Shakir, I.; Shahid, M. Magnetically recyclable Ni1-xCdxCeyFe2-yO4-rGO nanocomposite photocatalyst for visible light driven photocatalysis. Ceram. Int. 2020, 46, 13517–13526. [Google Scholar] [CrossRef]
- Em, S.; Yedigenov, M.; Khamkhash, L.; Atabaev, S.; Molkenova, A.; Poulopoulos, S.G.; Atabaev, T.S. Uncovering the Role of Surface-Attached Ag Nanoparticles in Photodegradation Improvement of Rhodamine B by ZnO-Ag Nanorods. Nanomaterials 2022, 12, 2882. [Google Scholar] [CrossRef] [PubMed]
- Dodoo-Arhin, D.; Asiedu, T.; Agyei-Tuffour, B.; Nyankson, E.; Obada, D.; Mwabora, J.M. Photocatalytic degradation of Rhodamine dyes using zinc oxide nanoparticles. Mater. Today Proc. 2021, 38, 809–815. [Google Scholar] [CrossRef]
- Bari, A.; Ikram, M.; Haider, A.; Ul-Hamid, A.; Haider, J.; Shahzadi, I.; Nazir, G.; Shahzadi, A.; Imran, M.; Ghaffar, A. Evaluation of bactericidal potential and catalytic dye degradation of multiple morphology based chitosan/polyvinylpyrrolidone-doped bismuth oxide nanostructures. Nanoscale Adv. 2022, 4, 2713–2728. [Google Scholar] [CrossRef] [PubMed]
- Tichapondwa, S.M.; Newman, J.P.; Kubheka, O. Effect of TiO2 phase on the photocatalytic degradation of methylene blue dye. Phys. Chem. Earth 2020, 118, 102900. [Google Scholar] [CrossRef]
- Poolwong, J.; Kiatboonyarit, T.; Achiwawanich, S.; Butburee, T.; Khemthong, P.; Kityakarn, S. Three-dimensional hierarchical porous TiO2 for enhanced adsorption and photocatalytic degradation of remazol dye. Nanomaterials 2021, 11, 1715. [Google Scholar] [CrossRef]
- Wang, W.; Yu, Y.; An, T.; Li, G.; Yip, H.Y.; Yu, J.C.; Wong, P.K. Visible-light-driven photocatalytic inactivation of E. coli K-12 by bismuth vanadate nanotubes: Bactericidal performance and mechanism. Environ. Sci. Technol. 2012, 46, 4599–4606. [Google Scholar] [CrossRef]
- Sundaram, I.M.; Kalimuthu, S.; Ponniah, G. Highly active ZnO modified g-C3N4 Nanocomposite for dye degradation under UV and Visible Light with enhanced stability and antimicrobial activity. Compos. Commun. 2017, 5, 64–71. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Preparation and enhanced visible-light photocatalytic H2-production activity of graphene/C3N4 composites. J. Phys. Chem. C 2011, 115, 7355–7363. [Google Scholar] [CrossRef]
- Sakuna, P.; Ketwong, P.; Ohtani, B.; Trakulmututa, J.; Kobkeatthawin, T.; Luengnaruemitchai, A.; Smith, S.M. The Influence of Metal-Doped Graphitic Carbon Nitride on Photocatalytic Conversion of Acetic Acid to Carbon Dioxide. Front. Chem. 2022, 10, 162. [Google Scholar] [CrossRef]
- Niu, P.; Yang, Y.; Jimmy, C.Y.; Liu, G.; Cheng, H.-M. Switching the selectivity of the photoreduction reaction of carbon dioxide by controlling the band structure of a g-C3N4 photocatalyst. Chem. Commun. 2014, 50, 10837–10840. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.-H.; Liu, R.-S.; Hsiao, M. Graphitic carbon nitride-based nanocomposites and their biological applications: A review. Nanoscale 2019, 11, 14993–15003. [Google Scholar] [CrossRef]
- Shen, C.; Chen, C.; Wen, T.; Zhao, Z.; Wang, X.; Xu, A. Superior adsorption capacity of g-C3N4 for heavy metal ions from aqueous solutions. J. Colloid Interface Sci. 2015, 456, 7–14. [Google Scholar] [CrossRef]
- Hu, R.; Wang, X.; Dai, S.; Shao, D.; Hayat, T.; Alsaedi, A. Application of graphitic carbon nitride for the removal of Pb (II) and aniline from aqueous solutions. Chem. Eng. J. 2015, 260, 469–477. [Google Scholar] [CrossRef]
- Thomas, R.T.; Sandhyarani, N. Template free synthesis of graphitic carbon nitride/titania mesoflowers. RSC Adv. 2015, 5, 72683–72690. [Google Scholar] [CrossRef]
- Niu, P.; Zhang, L.; Liu, G.; Cheng, H.M. Graphene-like carbon nitride nanosheets for improved photocatalytic activities. Adv. Funct. Mater. 2012, 22, 4763–4770. [Google Scholar] [CrossRef]
- Huang, D.; Chen, S.; Zeng, G.; Gong, X.; Zhou, C.; Cheng, M.; Xue, W.; Yan, X.; Li, J. Artificial Z-scheme photocatalytic system: What have been done and where to go? Coord. Chem. Rev. 2019, 385, 44–80. [Google Scholar] [CrossRef]
- Kavitha, R.; Nithya, P.M.; Kumar, S.G. Noble metal deposited graphitic carbon nitride based heterojunction photocatalysts. Appl. Surf. Sci. 2020, 508, 145142. [Google Scholar] [CrossRef]
- Zhang, M.; Wen, J.; Zhang, S.; Zhai, Y. Tremella-like porous carbon nitride co-doped with oxygen and carbon towards efficient visible-light-driven purification of wastewater. Sep. Purif. Technol. 2021, 257, 117984. [Google Scholar] [CrossRef]
- Liu, C.; Tang, Y.; Huo, P.; Chen, F. Novel AgCl/CNTs/g-C3N4 nanocomposite with high photocatalytic and antibacterial activity. Mater. Lett. 2019, 257, 126708. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Z.; Yang, Y.; Li, H. Construction of carbon quantum dots sensitized porous carbon nitride/titanium dioxide nanosheets for enhancing visible light photocatalytic degradation of tetracycline. J. Environ. Chem. Eng. 2022, 10, 108083. [Google Scholar] [CrossRef]
- Adnan, M.A.M.; Phoon, B.L.; Julkapli, N.M. Mitigation of pollutants by chitosan/metallic oxide photocatalyst: A review. J. Clean. Prod. 2020, 261, 121190. [Google Scholar] [CrossRef]
- Qi, H.; Ji, X.; Shi, C.; Ma, R.; Huang, Z.; Guo, M.; Li, J.; Guo, Z. Bio-templated 3D porous graphitic carbon nitride hybrid aerogel with enhanced charge carrier separation for efficient removal of hazardous organic pollutants. J. Colloid Interface Sci. 2019, 556, 366–375. [Google Scholar] [CrossRef]
- Xing, Q.; Zhang, M.; Xu, X.; Zhang, B.; Duan, Y.; Wei, Y.; Wang, J.; Li, M. Rapid photocatalytic inactivation of E. coli by polyethyleneimine grafted O-doped g-C3N4: Synergetic effects of the boosted reactive oxygen species production and adhesion performance. Appl. Surf. Sci. 2022, 573, 151496. [Google Scholar] [CrossRef]
- Hou, J.; Liu, S.; Jiang, X.; Waterhouse, G.I.N.; Zhang, Z.-M.; Yu, L.-M. Polyaniline/graphite carbon nitride composite coatings with outstanding photo-induced anodic antifouling and antibacterial properties under visible light. Prog. Org. Coat. 2021, 154, 106203. [Google Scholar] [CrossRef]
- Sun, L.; Liu, C.; Li, J.; Zhou, Y.; Wang, H.; Huo, P.; Ma, C.; Yan, Y. Fast electron transfer and enhanced visible light photocatalytic activity by using poly-o-phenylenediamine modified AgCl/g-C3N4 nanosheets. Chin. J. Catal. 2019, 40, 80–94. [Google Scholar] [CrossRef]
- Karthikeyan, K.T.; Nithya, A.; Jothivenkatachalam, K. Photocatalytic and antimicrobial activities of chitosan-TiO2 nanocomposite. Int. J. Biol. Macromol. 2017, 104, 1762–1773. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, L.; Hu, D.; Yan, Q.; Wang, Z.; Li, S.; Chen, C.; Xue, Q. Swelling induced regeneration of TiO2-impregnated chitosan adsorbents under visible light. Carbohydr. Polym. 2016, 140, 433–441. [Google Scholar] [CrossRef]
- Choudhary, P.; Bahuguna, A.; Kumar, A.; Dhankhar, S.S.; Nagaraja, C.M.; Krishnan, V. Oxidized graphitic carbon nitride as a sustainable metal-free catalyst for hydrogen transfer reactions under mild conditions. Green Chem. 2020, 22, 5084–5095. [Google Scholar] [CrossRef]
- Deeksha, B.; Sadanand, V.; Hariram, N.; Rajulu, A.V. Preparation and properties of cellulose nanocomposite fabrics with in situ generated silver nanoparticles by bioreduction method. J. Bioresour. Bioprod. 2021, 6, 75–81. [Google Scholar] [CrossRef]
- Xing, Y.; Wang, X.; Hao, S.; Zhang, X.; Wang, X.; Ma, W.; Zhao, G.; Xu, X. Recent advances in the improvement of g-C3N4 based photocatalytic materials. Chin. Chem. Lett. 2021, 32, 13–20. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Prashanth, K.V.H.; Tharanathan, R.N. Crosslinked chitosan—Preparation and characterization. Carbohydr. Res. 2006, 341, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, X.; Thomas, A.; Fu, X. Metal-containing carbon nitride compounds: A new functional organic–metal hybrid material. Adv. Mater. 2009, 21, 1609–1612. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, J.; Zhang, G.; Huang, J.; Liu, P.; Antonietti, M.; Wang, X. Synthesis of bulk and nanoporous carbon nitride polymers from ammonium thiocyanate for photocatalytic hydrogen evolution. J. Mater. Chem. A 2011, 21, 13032–13039. [Google Scholar] [CrossRef]
- Shalom, M.; Inal, S.; Fettkenhauer, C.; Neher, D.; Antonietti, M. Improving carbon nitride photocatalysis by supramolecular preorganization of monomers. J. Am. Chem. Soc. 2013, 135, 7118–7121. [Google Scholar] [CrossRef]
- Shen, Q.; Wei, L.; Bibi, R.; Wang, K.; Hao, D.; Zhou, J.; Li, N. Boosting photocatalytic degradation of tetracycline under visible light over hierarchical carbon nitride microrods with carbon vacancies. J. Hazard. Mater. 2021, 413, 125376. [Google Scholar] [CrossRef]
- Lu, Y.; Ji, C.; Li, Y.; Qu, R.; Sun, C.; Zhang, Y. Facile one-pot synthesis of C and g-C3N4 composites with enhanced photocatalytic activity using hydroxymethylated melamine as carbon source and soft template. Mater. Lett. 2018, 211, 78–81. [Google Scholar] [CrossRef]
- Wei, F.; Liu, Y.; Zhao, H.; Ren, X.; Liu, J.; Hasan, T.; Chen, L.; Li, Y.; Su, B.-L. Oxygen self-doped g-C3N4 with tunable electronic band structure for unprecedentedly enhanced photocatalytic performance. Nanoscale 2018, 10, 4515–4522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, H.; Zhang, X.; Wu, J.; Li, N.; Zheng, Y.; Tao, X. Fragmented phosphorus-doped graphitic carbon nitride nanoflakes with broad sub-bandgap absorption for highly efficient visible-light photocatalytic hydrogen evolution. Appl. Catal. B 2018, 225, 397–405. [Google Scholar] [CrossRef]
- Thomas, A.; Fischer, A.; Goettmann, F.; Antonietti, M.; Müller, J.-O.; Schlögl, R.; Carlsson, J.M. Graphitic carbon nitride materials: Variation of structure and morphology and their use as metal-free catalysts. J. Mater. Chem. 2008, 18, 4893–4908. [Google Scholar] [CrossRef] [Green Version]
- Gong, H.; Hao, X.; Jin, Z.; Ma, Q. WP modified S-scheme Zn0.5Cd0.5 S/WO3 for efficient photocatalytic hydrogen production. New J. Chem. 2019, 43, 19159–19171. [Google Scholar] [CrossRef]
- Song, X.; Zhang, X.; Li, X.; Che, H.; Huo, P.; Ma, C.; Yan, Y.; Yang, G. Enhanced light utilization efficiency and fast charge transfer for excellent CO2 photoreduction activity by constructing defect structures in carbon nitride. J. Colloid Interface Sci. 2020, 578, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Che, H.; Che, G.; Zhou, P.; Liu, C.; Dong, H.; Li, C.; Song, N.; Li, C. Nitrogen doped carbon ribbons modified g-C3N4 for markedly enhanced photocatalytic H2-production in visible to near-infrared region. Chem. Eng. J. 2020, 382, 122870. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, J.; Macyk, W.; Wageh, S.; Al-Ghamdi, A.A.; Wang, L. C3N4/PDA S-Scheme Heterojunction with Enhanced Photocatalytic H2O2 Production Performance and Its Mechanism. Adv. Sustain. Syst. 2023, 7, 2200113. [Google Scholar] [CrossRef]
- Wu, Q.; Jiang, B.; Weng, Y.; Liu, J.; Li, S.; Hu, Y.; Yang, K.; Liang, Z.; Zhang, L.; Zhang, Y. 3-Carboxybenzoboroxole functionalized polyethylenimine modified magnetic graphene oxide nanocomposites for human plasma glycoproteins enrichment under physiological conditions. Anal. Chem. 2018, 90, 2671–2677. [Google Scholar] [CrossRef]
- Zhao, C.; Yan, Q.; Wang, S.; Dong, P.; Zhang, L. Regenerable g-C3N4–chitosan beads with enhanced photocatalytic activity and stability. RSC Adv. 2018, 8, 27516–27524. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zhang, X.; Yu, X.; Gao, F.; Shen, Z.; Zhang, X.; Ge, S.; Liu, J.; Gu, Z.; Chen, C. An all-organic semiconductor C3N4/PDINH heterostructure with advanced antibacterial photocatalytic therapy activity. Adv. Mater. 2019, 31, 1901965. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Gao, X.; Fan, H.; Zhang, M.; Waterhouse, G.I.; Zhu, S. Green and recyclable graphitic carbon nitride/chitosan/polyvinyl alcohol photocatalytic films with efficient antibacterial activity for fruit packaging. Int. J. Biol. Macromol. 2023, 236, 123974. [Google Scholar] [CrossRef]
- Shen, H.; Durkin, D.P.; Aiello, A.; Diba, T.; Lafleur, J.; Zara, J.M.; Shen, Y.; Shuai, D. Photocatalytic graphitic carbon nitride-chitosan composites for pathogenic biofilm control under visible light irradiation. J. Hazard. Mater. 2021, 408, 124890. [Google Scholar] [CrossRef]
- Kong, X.; Liu, X.; Zheng, Y.; Chu, X.; Zhang, Y.; Wu, S. Graphitic carbon nitride-based materials for photocatalytic antibacterial application. Mater. Sci. Eng. R Rep. 2021, 145, 100610. [Google Scholar] [CrossRef]
- Shi, L.; Wang, T.; Zhang, H.; Chang, K.; Meng, X.; Liu, H.; Ye, J. An amine-functionalized iron (III) metal–organic framework as efficient visible-light photocatalyst for Cr (VI) reduction. Adv. Sci. 2015, 2, 1500006. [Google Scholar] [CrossRef]
- Iqbal, S.; Bahadur, A.; Anwer, S.; Ali, S.; Irfan, R.M.; Li, H.; Shoaib, M.; Raheel, M.; Anjum, T.A.; Zulqarnain, M. Effect of temperature and reaction time on the morphology of L-cysteine surface capped chalcocite (Cu2S) snowflakes dendrites nanoleaves and photodegradation study of methyl orange dye under visible light. Colloids Surf. A 2020, 601, 124984. [Google Scholar] [CrossRef]
- Bahadur, A.; Hussain, W.; Iqbal, S.; Ullah, F.; Shoaib, M.; Liu, G.; Feng, K. A morphology controlled surface sulfurized CoMn2O4 microspike electrocatalyst for water splitting with excellent OER rate for binder-free electrocatalytic oxygen evolution. J. Mater. Chem. A 2021, 9, 12255–12264. [Google Scholar] [CrossRef]
- Yang, L.; Huang, J.; Cao, L.; Shi, L.; Yu, Q.; Kong, X.; Jie, Y. pH-regulated template-free assembly of Sb4O5Cl2 hollow microsphere crystallites with self-narrowed bandgap and optimized photocatalytic performance. Sci. Rep. 2016, 6, 27765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Zeng, X.; Hu, X.; Hu, J.; Wang, Z.; Yin, Y.; Sun, C.; Zhang, X. Two-dimensional g-C3N4/TiO2 nanocomposites as vertical Z-scheme heterojunction for improved photocatalytic water disinfection. Catal. Today 2019, 335, 243–251. [Google Scholar] [CrossRef]
- Guo, H.; Niu, C.G.; Feng, C.Y.; Liang, C.; Zhang, L.; Wen, X.J.; Yang, Y.; Liu, H.-Y.; Li, L.; Lin, L.S. Steering exciton dissociation and charge migration in green synthetic oxygen-substituted ultrathin porous graphitic carbon nitride for boosted photocatalytic reactive oxygen species generation. Chem. Eng. J. 2020, 385, 123919. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, Y.; Wang, W.; Yang, Y.; Zhou, C.; Wang, L.; Lei, L.; He, D.; Luo, H.; Huang, D. Formation of Mo2C/hollow tubular g-C3N4 hybrids with favorable charge transfer channels for excellent visible-light-photocatalytic performance. Appl. Surf. Sci. 2020, 527, 146757. [Google Scholar] [CrossRef]
- Hong, Y.; Meng, Y.; Zhang, G.; Yin, B.; Zhao, Y.; Shi, W.; Li, C. Facile fabrication of stable metal-free CQDs/g-C3N4 heterojunctions with efficiently enhanced visible-light photocatalytic activity. Sep. Purifi. Technol. 2016, 171, 229–237. [Google Scholar] [CrossRef]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, X.; Luan, J.; Song, T.; Sun, H.; Dai, Y.; Yu, J.; Tian, H. Chitosan-Grafted Carbon Oxynitride Nanoparticles: Investigation of Photocatalytic Degradation and Antibacterial Activity. Polymers 2023, 15, 1688. https://doi.org/10.3390/polym15071688
Bai X, Luan J, Song T, Sun H, Dai Y, Yu J, Tian H. Chitosan-Grafted Carbon Oxynitride Nanoparticles: Investigation of Photocatalytic Degradation and Antibacterial Activity. Polymers. 2023; 15(7):1688. https://doi.org/10.3390/polym15071688
Chicago/Turabian StyleBai, Xuemei, Jingmin Luan, Tingting Song, Haifeng Sun, Yuhua Dai, Jianxiang Yu, and Huafeng Tian. 2023. "Chitosan-Grafted Carbon Oxynitride Nanoparticles: Investigation of Photocatalytic Degradation and Antibacterial Activity" Polymers 15, no. 7: 1688. https://doi.org/10.3390/polym15071688