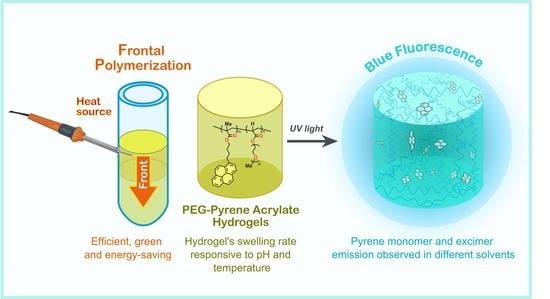
Facile Obtainment of Fluorescent PEG Hydrogels Bearing Pyrene Groups by Frontal Polymerization
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Notes
2.2. Characterization of PybuMA Comonomer and the Obtained PEGPy Hydrogels
2.3. Synthesis of 4-(Pyren-1-yl)Butyl Methacrylate (PybuMA)
2.4. Frontal Polymerization
2.5. Swelling Measurements
3. Results and Discussion
3.1. Synthesis of the PybuMA Monomer
3.2. Frontal Polymerization of PEGPy Hydrogel
3.3. Characterization of the Obtained Hydrogels
3.4. Thermal Properties of the Obtained Hydrogels
3.5. Swelling Behavior
3.6. Optical Properties of PEGPy Hydrogels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.S.; Khademhosseini, A. Advances in Engineering Hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, X.; Zhou, J.; Shi, J.; Xu, B. Supramolecular Hydrogelators and Hydrogels: From Soft Matter to Molecular Biomaterials. Chem. Rev. 2015, 115, 13165–13307. [Google Scholar] [CrossRef]
- Mehwish, N.; Dou, X.; Zhao, Y.; Feng, C.-L. Supramolecular Fluorescent Hydrogelators as Bio-Imaging Probes. Mater. Horiz. 2018, 6, 14–44. [Google Scholar] [CrossRef]
- Khurana, B.; Gierlich, P.; Meindl, A.; Gomes-da-Silva, L.C.; Senge, M.O. Hydrogels: Soft Matters in Photomedicine. Photochem. Photobiol. Sci. 2019, 18, 2613–2656. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Cheng, Y.; Tong, J.; Zhang, L.; Wei, Y.; Tian, M. Recent Advances in Thermo-Sensitive Hydrogels for Drug Delivery. J. Mater. Chem. B 2021, 9, 2979–2992. [Google Scholar] [CrossRef] [PubMed]
- Köse, K.; Mavlan, M.; Uzun, L.; Youngblood, J.P. Cholesterol Removal via Cyclodextrin-Decoration on Cellulose Nanocrystal (CNC)-Grafted Poly(HEMA-GMA) Nanocomposite Adsorbent. Cellulose 2021, 28, 471–487. [Google Scholar] [CrossRef]
- Köse, K.; Mavlan, M.; Nuruddin, M.; Gómez, A.M.U.; Youngblood, J.P. Modification of Glycidyl Methacrylate Based Cryogels by Cellulose Nanocrystals and Determination of Dye Adsorption Performance. Cellulose 2022, 29, 1623–1636. [Google Scholar] [CrossRef]
- Akveran, G.A.; Köse, K.; Köse, D.A. Solvent Effect on Endosulfan Adsorption onto Polymeric Arginine-Methacrylate Cryogels. Environ. Sci. Pollut. Res. 2018, 25, 25458–25467. [Google Scholar] [CrossRef]
- Roy, A.; Manna, K.; Pal, S. Recent Advances in Various Stimuli-Responsive Hydrogels: From Synthetic Designs to Emerging Healthcare Applications. Mater. Chem. Front. 2022, 6, 2338–2385. [Google Scholar] [CrossRef]
- Cheng, Q.; Hao, A.; Xing, P. Stimulus-Responsive Luminescent Hydrogels: Design and Applications. Adv. Colloid Interface Sci. 2020, 286, 102301. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Young, D.J.; Loh, X.J. Fluorescent Gels: A Review of Synthesis, Properties, Applications and Challenges. Mater. Chem. Front. 2019, 3, 1489–1502. [Google Scholar] [CrossRef]
- Wei, S.; Li, Z.; Lu, W.; Liu, H.; Zhang, J.; Chen, T.; Tang, B.Z. Multicolor Fluorescent Polymeric Hydrogels. Angew. Chem. Int. Ed. 2021, 60, 8608–8624. [Google Scholar] [CrossRef]
- Su, G.; Li, Z.; Dai, R. Recent Advances in Applied Fluorescent Polymeric Gels. ACS Appl. Polym. Mater. 2022, 4, 3131–3152. [Google Scholar] [CrossRef]
- Jin, R.; Kong, D.; Yan, X.; Zhao, X.; Li, H.; Liu, F.; Sun, P.; Lin, Y.; Lu, G. Integrating Target-Responsive Hydrogels with Smartphone for On-Site Ppb-Level Quantitation of Organophosphate Pesticides. ACS Appl. Mater. Interfaces 2019, 11, 27605–27614. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhou, M.; Yang, C. Fluorescent Hydrogel Waveguide for On-Site Detection of Heavy Metal Ions. Sci. Rep. 2017, 7, 7902. [Google Scholar] [CrossRef] [Green Version]
- Shibata, H.; Heo, Y.J.; Okitsu, T.; Matsunaga, Y.; Kawanishi, T.; Takeuchi, S. Injectable Hydrogel Microbeads for Fluorescence-Based in Vivo Continuous Glucose Monitoring. Proc. Natl. Acad. Sci. USA 2010, 107, 17894–17898. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Chauhan, S.; Geng, J.; Qin, Y.; Watson, D.F.; Lovell, J.F. Implantable Tin Porphyrin-PEG Hydrogels with PH-Responsive Fluorescence. Biomacromolecules 2017, 18, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Winnik, F.M. Photophysics of Preassociated Pyrenes in Aqueous Polymer Solutions and in Other Organized Media. Chem. Rev. 1993, 24, 587–614. [Google Scholar] [CrossRef]
- Thoma, J.L.; Duhamel, J. Characterization of the Local Volume Probed by the Side-Chain Ends of Poly(Oligo(Ethylene Glycol) 1-Pyrenemethyl Ether Methacrylate) Bottle Brushes in Solution Using Pyrene Excimer Fluorescence. Macromolecules 2021, 54, 9341–9350. [Google Scholar] [CrossRef]
- Duhamel, J. Internal Dynamics of Dendritic Molecules Probed by Pyrene Excimer Formation. Polymers 2012, 4, 211–239. [Google Scholar] [CrossRef] [Green Version]
- Thoma, J.L.; McNelles, S.A.; Adronov, A.; Duhamel, J. Direct Measure of the Local Concentration of Pyrenyl Groups in Pyrene-Labeled Dendrons Derived from the Rate of Fluorescence Collisional Quenching. Polymers 2020, 12, 2919. [Google Scholar] [CrossRef]
- Porcu, P.; Vonlanthen, M.; Ruiu, A.; González-Méndez, I.; Rivera, E. Energy Transfer in Dendritic Systems Having Pyrene Peripheral Groups as Donors and Different Acceptor Groups. Polymers 2018, 10, 1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueira-Duarte, T.M.; Müllen, K. Pyrene-Based Materials for Organic Electronics. Chem. Rev. 2011, 111, 7260–7314. [Google Scholar] [CrossRef] [PubMed]
- Bains, G.; Patel, A.B.; Narayanaswami, V. Pyrene: A Probe to Study Protein Conformation and Conformational Changes. Molecules 2011, 16, 7909–7935. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Panja, A.; Halder, D.; Bairi, P.; Nandi, A.K. Isomerization-Induced Excimer Formation of Pyrene-Based Acylhydrazone Controlled by Light- and Solvent-Sensing Aromatic Analytes. J. Phys. Chem. B 2021, 125, 13804–13816. [Google Scholar] [CrossRef]
- Lee, W.-F.; Liu, P.-Y. Preparation and Properties of Novel Photoluminescent Thermosensitive Hydrogels Containing a Pyrene Group. J. Polym. Res. 2017, 24, 33. [Google Scholar] [CrossRef]
- Rasch, D.; Göstl, R. Gated Photoreactivity of Pyrene Copolymers in Multiresponsive Cross-Linked StarPEG-Hydrogels. ACS Polym. Au 2021, 1, 59–66. [Google Scholar] [CrossRef]
- Li, X.; Huang, X.; Mutlu, H.; Malik, S.; Theato, P. Conductive Hydrogel Composites with Autonomous Self-Healing Properties. Soft Matter 2020, 16, 10969–10976. [Google Scholar] [CrossRef]
- Gou, Z.; Wang, A.; Tian, M.; Zuo, Y. Pyrene-Based Monomer-Excimer Dual Response Organosilicon Polymer for the Selective Detection of 2,4,6-Trinitrotoluene (TNT) and 2,4,6-Trinitrophenol (TNP). Mater. Chem. Front. 2022, 6, 607–612. [Google Scholar] [CrossRef]
- Suslick, B.A.; Hemmer, J.; Groce, B.R.; Stawiasz, K.J.; Geubelle, P.H.; Malucelli, G.; Mariani, A.; Moore, J.S.; Pojman, J.A.; Sottos, N.R. Frontal Polymerizations: From Chemical Perspectives to Macroscopic Properties and Applications. Chem. Rev. 2023, 123, 3237–3298. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Shen, H.-X.; Liu, C.; Wang, C.; Zhu, L.; Chen, S. Advances in Frontal Polymerization Strategy: From Fundamentals to Applications. Prog. Polym. Sci. 2022, 127, 101514. [Google Scholar] [CrossRef]
- Nason, C.; Roper, T.; Hoyle, C.; Pojman, J.A. UV-Induced Frontal Polymerization of Multifunctional (Meth)Acrylates. Macromolecules 2005, 38, 5506–5512. [Google Scholar] [CrossRef]
- Pojman, J.A.; Ilyashenko, V.M.; Khan, A.M. Free-Radical Frontal Polymerization: Self-Propagating Thermal Reaction Waves. J. Chem. Soc. Faraday Trans. 1996, 92, 2825–2837. [Google Scholar] [CrossRef]
- Pojman, J.A.; Curtis, G.; Ilyashenko, V.M. Frontal Polymerization in Solution. J. Am. Chem. Soc. 1996, 118, 3783–3784. [Google Scholar] [CrossRef]
- Bynum, S.; Tullier, M.; Garcia, C.M.; Guidry, J.; Runnoe, E.; Pojman, J.A. The Effect of Acrylate Functionality on Frontal Polymerization Velocity and Temperature. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 982–988. [Google Scholar] [CrossRef]
- Robertson, I.D.; Yourdkhani, M.; Centellas, P.J.; Aw, J.E.; Ivanoff, D.G.; Goli, E.; Lloyd, E.M.; Dean, L.M.; Sottos, N.R.; Geubelle, P.H.; et al. Rapid Energy-Efficient Manufacturing of Polymers and Composites via Frontal Polymerization. Nature 2018, 557, 223–227. [Google Scholar] [CrossRef]
- Nuvoli, L.; Sanna, D.; Alzari, V.; Nuvoli, D.; Sanna, V.; Malfatti, L.; Mariani, A. Double Responsive Copolymer Hydrogels Prepared by Frontal Polymerization. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 2166–2170. [Google Scholar] [CrossRef]
- Sanna, R.; Alzari, V.; Nuvoli, D.; Scognamillo, S.; Marceddu, S.; Mariani, A. Polymer Hydrogels of 2-hydroxyethyl Acrylate and Acrylic Acid Obtained by Frontal Polymerization. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 1515–1520. [Google Scholar] [CrossRef]
- Sanna, D.; Alzari, V.; Nuvoli, D.; Nuvoli, L.; Rassu, M.; Sanna, V.; Mariani, A. β-Cyclodextrin-Based Supramolecular Poly(N-Isopropylacrylamide) Hydrogels Prepared by Frontal Polymerization. Carbohydr. Polym. 2017, 166, 249–255. [Google Scholar] [CrossRef]
- Zaragoza-Galán, G.; Fowler, M.; Rein, R.; Solladié, N.; Duhamel, J.; Rivera, E. Fluorescence Resonance Energy Transfer in Partially and Fully Labeled Pyrene Dendronized Porphyrins Studied with Model Free Analysis. J. Phys. Chem. C 2014, 118, 8280–8294. [Google Scholar] [CrossRef]
- García-Rodríguez, A.; Porcu, P.; Estrada-Montaño, A.S.; Vonlanthen, M.; Martínez-Serrano, R.D.; Zaragoza-Galán, G.; Rivera, E. Design of Novel Conjugated Systems Bearing Donor-Acceptor Groups (Pyrene-Bodipy): Optical, Photophysical Properties and Energy Transfer. Dyes Pigm. 2021, 185, 108925. [Google Scholar] [CrossRef]
- Bañales-Leal, Y.; García-Rodríguez, A.; Cuétara-Guadarrama, F.; Vonlanthen, M.; Sorroza-Martínez, K.; Morales-Morales, D.; Rivera, E. Design of Flexible Dendritic Systems Bearing Donor-Acceptor Groups (Pyrene-Porphyrin) for FRET Applications. Dyes Pigm. 2021, 191, 109382. [Google Scholar] [CrossRef]
- Ramírez-Fuentes, Y.S.; Illescas, J.; Gelover-Santiago, A.; Rivera, E. Luminescent Polymers Containing Pyrenyl Groups Prepared by Frontal Polymerization of Di(Ethylene Glycol) Ethyl Ether Acrylate Using Trigonox-23 as Initiator. Mater. Chem. Phys. 2012, 135, 772–779. [Google Scholar] [CrossRef]
- Illescas, J.; Ramírez-Fuentes, Y.S.; Zaragoza-Galán, G.; Porcu, P.; Mariani, A.; Rivera, E. PEGDA-based Luminescent Polymers Prepared by Frontal Polymerization. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 2890–2897. [Google Scholar] [CrossRef]
- Martínez-Serrano, R.D.; Ugone, V.; Porcu, P.; Vonlanthen, M.; Sorroza-Martínez, K.; Cuétara-Guadarrama, F.; Illescas, J.; Zhu, X.-X.; Rivera, E. Novel Porphyrin-Containing Hydrogels Obtained by Frontal Polymerization: Synthesis, Characterization and Optical Properties. Polymer 2022, 247, 124785. [Google Scholar] [CrossRef]
- Esquivel-Guzmán, J.A.; Zhang, H.; Mohanty, C.; Liu, X.; Rivera, E.; Illescas, J.; Lavertu, M.; Zhu, X.X. Thermoresponsive Copolymers Based on Synthetic Porphyrin Derivatives. MRS Adv. 2022, 7, 1126–1132. [Google Scholar] [CrossRef]









| Sample | PybuMA (mol%) | Vf (cm min−1) | Tmax (°C) |
|---|---|---|---|
| PEGMA | 0.0 | 0.47 | 95.9 |
| PEGPy-1 | 0.6 | 0.27 | 80.6 |
| PEGPy-2 | 1.2 | 0.32 | 83.9 |
| PEGPy-3 | 2.5 | 0.39 | 87.5 |
| PEGPy-4 | 5.0 | 0.47 | 89.5 |
| PEGPy-5 | 7.0 | 0.51 | 84.1 |
| PEGPy-6 | 10.0 | 0.37 | 86.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Serrano, R.D.; Cuétara-Guadarrama, F.; Vonlanthen, M.; Illescas, J.; Zhu, X.-X.; Rivera, E. Facile Obtainment of Fluorescent PEG Hydrogels Bearing Pyrene Groups by Frontal Polymerization. Polymers 2023, 15, 1687. https://doi.org/10.3390/polym15071687
Martínez-Serrano RD, Cuétara-Guadarrama F, Vonlanthen M, Illescas J, Zhu X-X, Rivera E. Facile Obtainment of Fluorescent PEG Hydrogels Bearing Pyrene Groups by Frontal Polymerization. Polymers. 2023; 15(7):1687. https://doi.org/10.3390/polym15071687
Chicago/Turabian StyleMartínez-Serrano, Ricardo D., Fabián Cuétara-Guadarrama, Mireille Vonlanthen, Javier Illescas, Xiao-Xia Zhu, and Ernesto Rivera. 2023. "Facile Obtainment of Fluorescent PEG Hydrogels Bearing Pyrene Groups by Frontal Polymerization" Polymers 15, no. 7: 1687. https://doi.org/10.3390/polym15071687