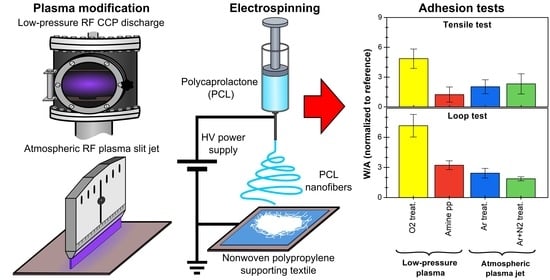
Enhanced Adhesion of Electrospun Polycaprolactone Nanofibers to Plasma-Modified Polypropylene Fabric
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plasma Modifications of PP Spunbond Fabric
2.2. Electrospinning of PCL Nanofibers
2.3. Surface Characterization
2.4. Adhesion Testing of PCL NFs to PP Fabric
3. Results and Discussion
3.1. Characterization of As-Modified PP Fabric
3.2. Time Stability of PP Fabric Plasma Modifications
3.3. Adhesion of PCL NFs to PP Fabric
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barnes, C.; Sell, S.; Knapp, D.; Walpoth, B.; Brand, D.; Bowlin, G. Preliminary investigation of electrospun collagen and polydioxanone for vascular tissue engineering applications. Int. J. Electrospun Nanofibers Appl. 2007, 1, 73–87. [Google Scholar]
- Welle, A.; Kröger, M.; Döring, M.; Niederer, K.; Pindel, E.; Chronakis, I. Electrospun aliphatic polycarbonates as tailored tissue scaffold materials. Biomaterials 2007, 28, 2211–2219. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, J.; Ramakrishna, S. Biocompatible Nanofiber Matrices for the Engineering of a Dermal Substitute for Skin Regeneration. Tissue Eng. 2005, 11, 847–854. [Google Scholar] [CrossRef]
- Chen, J.; Chu, B.; Hsiao, B.S. Mineralization of hydroxyapatite in electrospun nanofibrous poly(L-lactic acid) scaffolds. J. Biomed. Mater. Res. Part A 2006, 79, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Mohammadalipour, M.; Asadolahi, M.; Mohammadalipour, Z.; Behzad, T.; Karbasi, S. Plasma surface modification of electrospun polyhydroxybutyrate (PHB) nanofibers to investigate their performance in bone tissue engineering. Int. J. Biol. Macromol. 2023, 230, 123167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, X.; Zeng, L.; Zhang, J.; Zuo, J.; Zou, J.; Ding, J.; Chen, X. Polymer Fiber Scaffolds for Bone and Cartilage issue Engineering. Adv. Funct. Mater. 2019, 29, 1903279. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, C.; Wang, Q.; Zhou, F.; Li, T.; Wang, B.; Su, W.; Shang, D.; Wu, S. A simple, quick, and cost-effective strategy to fabricate polycaprolactone/silk fibroin nanofiber yarns for biotextile-based tissue scaffold application. Eur. Polym. J. 2023, 186, 111863. [Google Scholar] [CrossRef]
- Senthamizhan, A.; Balusamy, B.; Uyar, T. Recent progress on designing electrospun nanofibers for colorimetric biosensing applications. Curr. Opin. Biomed. Eng. 2020, 13, 1–8. [Google Scholar] [CrossRef]
- Unal, B.; Yalcinkaya, E.E.; Demirkol, D.O.; Timur, S. An electrospun nanofiber matrix based on organo-clay for biosensors: PVA/PAMAM-Montmorillonite. Appl. Surf. Sci. 2018, 444, 542–551. [Google Scholar] [CrossRef]
- Stafiniak, A.; Boratyński, B.; Baranowska-Korczyc, A.; Szyszka, A.; Krasowska, M.R.; Prażmowska, J.; Fronc, K.; Elbaum, D.; Paszkiewicz, R.; Tłaczała, M. A novel electrospun ZnO nanofibers biosensor fabrication. Sens. Actuators B Chem. 2011, 160, 1413–1418. [Google Scholar] [CrossRef]
- Qiu, J.; Yu, T.; Zhang, W.; Zhao, Z.; Zhang, Y.; Ye, G.; Zhao, Y.; Du, X.; Liu, X.; Yang, L.; et al. A Bioinspired, Durable, and Nondisposable Transparent Graphene Skin Electrode for Electrophysiological Signal Detection. ACS Mater. Lett. 2020, 2, 999–1007. [Google Scholar] [CrossRef]
- Faraji, S.; Nowroozi, N.; Nouralishahi, A.; Shabani Shayeh, J. Electrospun poly-caprolactone/graphene oxide/quercetin nanofibrous scaffold for wound dressing: Evaluation of biological and structural properties. Life Sci. 2020, 257, 118062. [Google Scholar] [CrossRef]
- Balusamy, B.; Senthamizhan, A.; Uyar, T. 8-Electrospun nanofibrous materials for wound healing applications. In Electrospun Materials for Tissue Engineering and Biomedical Applications; Uyar, T., Kny, E., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 147–177. [Google Scholar] [CrossRef]
- Li, M.; Qiu, W.; Wang, Q.; Li, N.; Liu, L.; Wang, X.; Yu, J.; Li, X.; Li, F.; Wu, D. Nitric Oxide-Releasing Tryptophan-Based Poly(ester urea)s Electrospun Composite Nanofiber Mats with Antibacterial and Antibiofilm Activities for Infected Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 15911–15926. [Google Scholar] [CrossRef]
- Deng, Z.; Mu, H.; Jiang, L.; Xi, W.; Xu, X.; Zheng, W. Preparation and characterization of electrospun PLGA-SF nanofibers as a potential drug delivery system. Mater. Chem. Phys. 2022, 289, 126452. [Google Scholar] [CrossRef]
- Agarwal, S.; Greiner, A.; Wendorff, J. Functional materials by electrospinning of polymers. Prog. Polym. Sci. 2013, 38, 963–991. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Si, Y.; Han, Y.; Wu, T.; Iqbal, M.I.; Fei, B.; Li, R.K.Y.; Hu, J.; Qu, J. Recent Progress in Protective Membranes Fabricated via Electrospinning: Advanced Materials, Biomimetic Structures, and Functional Applications. Adv. Mater. 2022, 34, 2107938. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Asadian, M.; Chan, K.V.; Norouzi, M.; Grande, S.; Cools, P.; Morent, R.; Geyter, N.D. Fabrication and plasma modification of nanofibrous tissue engineering scaffolds. Nanomaterials 2020, 10, 119. [Google Scholar] [CrossRef] [Green Version]
- Yoo, H.S.; Kim, T.G.; Park, T.G. Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Nanofibers Regen. Med. Drug Deliv. 2009, 61, 1033–1042. [Google Scholar] [CrossRef]
- Bridges, A.W.; García, A.J. Anti-Inflammatory Polymeric Coatings for Implantable Biomaterials and Devices. J. Diabetes Sci. Technol. 2008, 2, 984–994. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef] [Green Version]
- Varesano, A.; Rombaldoni, F.; Tonetti, C.; Mauro, S.D.; Mazzuchetti, G. Chemical treatments for improving adhesion between electrospun nanofibers and fabrics. J. Appl. Polym. Sci. 2014, 131, 39766. [Google Scholar] [CrossRef]
- Amini, G.; Gharehaghaji, A.A. Improving adhesion of electrospun nanofiber mats to supporting substrate by using adhesive bonding. Int. J. Adhes. Adhes. 2018, 86, 40–44. [Google Scholar] [CrossRef]
- Liu, W.; Zhan, J.; Su, Y.; Wu, T.; Wu, C.; Ramakrishna, S.; Mo, X.; Al-Deyab, S.S.; El-Newehy, M. Effects of plasma treatment to nanofibers on initial cell adhesion and cell morphology. Colloids Surf. Biointerfaces 2014, 113, 101–106. [Google Scholar] [CrossRef]
- Yan, D.; Jones, J.; Yuan, X.Y.; Xu, X.H.; Sheng, J.; Lee, J.C.M.; Ma, G.Q.; Yu, Q.S. Plasma treatment of electrospun PCL random nanofiber meshes (NFMs) for biological property improvement. J. Biomed. Mater. Res. Part A 2013, 101, 963–972. [Google Scholar] [CrossRef]
- Duque Sánchez, L.; Brack, N.; Postma, A.; Pigram, P.J.; Meagher, L. Surface modification of electrospun fibres for biomedical applications: A focus on radical polymerization methods. Biomaterials 2016, 106, 24–45. [Google Scholar] [CrossRef]
- Park, K.; Ju, Y.M.; Son, J.S.; Ahn, K.D.; Han, D.K. Surface modification of biodegradable electrospun nanofiber scaffolds and their interaction with fibroblasts. J. Biomater. Sci. Polym. Ed. 2007, 18, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Chatelier, R.C.; Xie, X.; Gengenbach, T.R.; Griesser, H.J. Quantitative Analysis of Polymer Surface Restructuring. Langmuir 1995, 11, 2576–2584. [Google Scholar] [CrossRef]
- Ruiz, J.C.; St-Georges-Robillard, A.; Thérésy, C.; Lerouge, S.; Wertheimer, M.R. Fabrication and Characterisation of Amine-Rich Organic Thin Films: Focus on Stability. Plasma Process. Polym. 2010, 7, 737–753. [Google Scholar] [CrossRef]
- Siow, K.S.; Britcher, L.; Kumar, S.; Griesser, H.J. Plasma Methods for the Generation of Chemically Reactive Surfaces for Biomolecule Immobilization and Cell Colonization - A Review. Plasma Process. Polym. 2006, 3, 392–418. [Google Scholar] [CrossRef]
- Rombaldoni, F.; Mahmood, K.; Varesano, A.; Songia, M.B.; Aluigi, A.; Vineis, C.; Mazzuchetti, G. Adhesion enhancement of electrospun nanofiber mats to polypropylene nonwoven fabric by low-temperature oxygen plasma treatment. Surf. Coat. Technol. 2013, 216, 178–184. [Google Scholar] [CrossRef]
- Pavliňák, D.; Galmiz, O.; Pavliňáková, V.; Poláček, P.; Kelar, J.; Stupavská, M.; Černák, M. Application of dielectric barrier plasma treatment in the nanofiber processing. Mater. Today Commun. 2018, 16, 330–338. [Google Scholar] [CrossRef]
- Vitchuli, N.; Shi, Q.; Nowak, J.; Nawalakhe, R.; Sieber, M.; Bourham, M.; McCord, M.; Zhang, X. Plasma-electrospinning hybrid process and plasma pretreatment to improve adhesive properties of nanofibers on fabric surface. Plasma Chem. Plasma Process. 2012, 32, 275–291. [Google Scholar] [CrossRef]
- Nawalakhe, R.; Shi, Q.; Vitchuli, N.; Noar, J.; Caldwell, J.M.; Breidt, F.; Bourham, M.A.; Zhang, X.; McCord, M.G. Novel atmospheric plasma enhanced chitosan nanofiber/gauze composite wound dressings. J. Appl. Polym. Sci. 2013, 129, 916–923. [Google Scholar] [CrossRef]
- Nawalakhe, R.; Shi, Q.; Vitchuli, N.; Bourham, M.A.; Zhang, X.; McCord, M.G. Plasma-Assisted Preparation of High-Performance Chitosan Nanofibers/Gauze Composite Bandages. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 709–717. [Google Scholar] [CrossRef]
- Shi, Q.; Vitchuli, N.; Nowak, J.; Jiang, S.; Caldwell, J.M.; Breidt, F.; Bourham, M.; Zhang, X.; McCord, M. Multifunctional and durable nanofiber-fabric-layered composite for protective application. J. Appl. Polym. Sci. 2013, 128, 1219–1226. [Google Scholar] [CrossRef]
- Jelínek, P.; Polášková, K.; Jeník, F.; Jeníková, Z.; Dostál, L.; Dvořáková, E.; Cerman, J.; Šourková, H.; Buršíková, V.; Špatenka, P.; et al. Effects of additives on atmospheric pressure gliding arc applied to the modification of polypropylene. Surf. Coat. Technol. 2019, 372, 45–55. [Google Scholar] [CrossRef]
- Polášková, K.; Klíma, M.; Jeníková, Z.; Blahová, L.; Zajíčková, L. Effect of Low Molecular Weight Oxidized Materials and Nitrogen Groups on Adhesive Joints of Polypropylene Treated by a Cold Atmospheric Plasma Jet. Polymers 2021, 13, 4396. [Google Scholar] [CrossRef]
- Polášková, K.; Nečas, D.; Dostál, L.; Klíma, M.; Fiala, P.; Zajíčková, L. Self-organization phenomena in cold atmospheric pressure plasma slit jet. Plasma Sources Sci. Technol. 2022, 31, 125014. [Google Scholar] [CrossRef]
- Guo, Y.; Guo, Y.; He, W.; Zhao, Y.; Shen, R.; Liu, J.; Wang, J. PET/TPU nanofiber composite filters with high interfacial adhesion strength based on one-step co-electrospinning. Powder Technol. 2021, 387, 136–145. [Google Scholar] [CrossRef]
- Tiu, B.D.B.; Delparastan, P.; Ney, M.R.; Gerst, M.; Messersmith, P.B. Enhanced Adhesion and Cohesion of Bioinspired Dry/Wet Pressure-Sensitive Adhesives. ACS Appl. Mater. Interfaces 2019, 11, 28296–28306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivals, I.; Personnaz, L.; Creton, C.; Simal, F.; Roose, P.; Van Es, S. A Statistical Method for the Prediction of the Loop Tack and the Peel of PSAs from Probe test Measurements. Meas. Sci. Technol. 2005, 16, 2020. [Google Scholar] [CrossRef] [Green Version]
- Plaut, R.H.; Williams, N.L.; Dillard, D.A. Elastic analysis of the loop tack test for pressure sensitive adhesives. J. Adhes. 2001, 76, 37–53. [Google Scholar] [CrossRef]
- Štrbková, L.; Manakhov, A.; Zajíčková, L.; Stoica, A.; Veselý, P.; Chmelík, R. The adhesion of normal human dermal fibroblasts to the cyclopropylamine plasma polymers studied by holographic microscopy. Surf. Coat. Technol. 2016, 295, 70–77. [Google Scholar] [CrossRef] [Green Version]
- Michlíček, M.; Blahová, L.; Dvořáková, E.; Nečas, D.; Zajíčková, L. Deposition penetration depth and sticking probability in plasma polymerization of cyclopropylamine. Appl. Surf. Sci. 2021, 540, 147979. [Google Scholar] [CrossRef]
- Kupka, V.; Dvoráková, E.; Manakhov, A.; Michlíček, M.; Petruš, J.; Vojtová, L.; Zajíčková, L. Well-blended PCL/PEO electrospun nanofibers with functional properties enhanced by plasma processing. Polymers 2020, 12, 1403. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers, the Scienta ESCA300 Database; John Wiley & Sons: Chichester, UK, 1992; p. 295. [Google Scholar]
- Nemcakova, I.; Blahova, L.; Rysanek, P.; Blanquer, A.; Bacakova, L.; Zajíčková, L. Behaviour of Vascular Smooth Muscle Cells on Amine Plasma-Coated Materials with Various Chemical Structures and Morphologies. Int. J. Mol. Sci. 2020, 21, 9467. [Google Scholar] [CrossRef]
- Morent, R.; Geyter, N.D.; Gengembre, L.; Leys, C.; Payen, E.; Vlierberghe, S.V.; Schacht, E. Surface treatment of a polypropylene film with a nitrogen DBD at medium pressure. Eur. Phys. J. Appl. Phys. 2008, 43, 289–294. [Google Scholar] [CrossRef] [Green Version]
- Sarani, A.; Nikiforov, A.Y.; Geyter, N.D.; Morent, R.; Leys, C. Surface modification of polypropylene with an atmospheric pressure plasma jet sustained in argon and an argon/water vapour mixture. Appl. Surf. Sci. 2011, 257, 8737–8741. [Google Scholar] [CrossRef]
- Buchtelová, M.; Blahová, L.; Nečas, D.; Křížková, P.; Bartošíková, J.; Medalová, J.; Kolská, Z.; Hegemann, D.; Zajíčková, L. Insight into peculiar adhesion of cells to plasma-chemically prepared multifunctional “amino-glue” surfaces. Plasma Process. Polym. 2023, 20, e2200157. [Google Scholar] [CrossRef]
- Gengenbach, T.R.; Griesser, H.J. Aging of 1,3-diaminopropane plasma-deposited polymer films: Mechanisms and reaction pathways. J. Polym. Sci. Part A Polym. Chem. 1999, 37, 2191–2206. [Google Scholar] [CrossRef]
- Girard-Lauriault, P.L.; Dietrich, P.M.; Gross, T.; Wirth, T.; Unger, W.E. Chemical characterization of the long-term ageing of nitrogen-rich plasma polymer films under various ambient conditions. Plasma Process. Polym. 2013, 10, 388–395. [Google Scholar] [CrossRef]
- Vandenbossche, M.; Hegemann, D. Recent approaches to reduce aging phenomena in oxygen- and nitrogen-containing plasma polymer films: An overview. Curr. Opin. Solid State Mater. Sci. 2018, 22, 26–38. [Google Scholar] [CrossRef]
- Dorai, R.; Kushner, M.J. A model for plasma modification of polypropylene using atmospheric pressure discharges. J. Phys. D Appl. Phys. 2003, 36, 666. [Google Scholar] [CrossRef] [Green Version]
- Strobel, M.; Strobel, J.M.; Jones, V.; Lechuga, H.; Lyons, C.S. Effect on wettability of the topography and oxidation state of biaxially oriented poly (propylene) film. J. Adhes. Sci. Technol. 2019, 33, 1644–1657. [Google Scholar] [CrossRef]
- Mortazavi, M.; Nosonovsky, M. A model for diffusion-driven hydrophobic recovery in plasma treated polymers. Appl. Surf. Sci. 2012, 258, 6876–6883. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janů, L.; Dvořáková, E.; Polášková, K.; Buchtelová, M.; Ryšánek, P.; Chlup, Z.; Kruml, T.; Galmiz, O.; Nečas, D.; Zajíčková, L. Enhanced Adhesion of Electrospun Polycaprolactone Nanofibers to Plasma-Modified Polypropylene Fabric. Polymers 2023, 15, 1686. https://doi.org/10.3390/polym15071686
Janů L, Dvořáková E, Polášková K, Buchtelová M, Ryšánek P, Chlup Z, Kruml T, Galmiz O, Nečas D, Zajíčková L. Enhanced Adhesion of Electrospun Polycaprolactone Nanofibers to Plasma-Modified Polypropylene Fabric. Polymers. 2023; 15(7):1686. https://doi.org/10.3390/polym15071686
Chicago/Turabian StyleJanů, Lucie, Eva Dvořáková, Kateřina Polášková, Martina Buchtelová, Petr Ryšánek, Zdeněk Chlup, Tomáš Kruml, Oleksandr Galmiz, David Nečas, and Lenka Zajíčková. 2023. "Enhanced Adhesion of Electrospun Polycaprolactone Nanofibers to Plasma-Modified Polypropylene Fabric" Polymers 15, no. 7: 1686. https://doi.org/10.3390/polym15071686