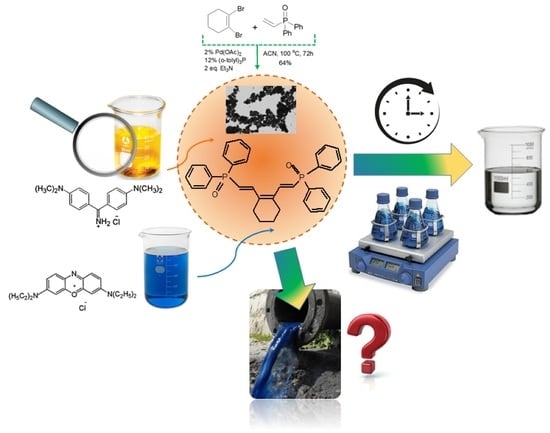
Synthesis, Characterization and Application of a New Functionalized Polymeric Sorbent Based on Alkenylphoshine Oxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of CyP(Ph)4
- 1-chlorocyclohexene 2
- b.
- 1,2-dibromocyclohexene 4
- c.
- 1,2-bis ((E)-2-diphenylphospinoylethenyl)-cyclohex-1-ene 6
2.3. Synthesis of Polymeric Sorbent
2.4. Characteristics of Polymeric Sorbent
2.5. Batch Adsorption Experiments
3. Results
3.1. Visualization of Sorbent
3.2. ATR-FT-IR Analysis
3.3. DSC Analysis
3.4. Determination of pHPZC of CyP(Ph)4-DVB
3.5. Evaluation of Adsorption Capacity of CyP(Ph)4-DVB
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xia, Q.; Qin, A.; Tang, B.Z. Recent advances in chiral AIE polymers. J. Nanopart. Res. 2023, 25, 17. [Google Scholar] [CrossRef]
- Chyr, G.; DeSimone, J.M. Review of high-performance sustainable polymers in additive manufacturing. Green Chem. 2023, 25, 453–466. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, S.; Luo, J.; Zhao, Y.; He, J.; Liu, C.; Chen, Z.; Yu, X. Research progress of antistatic-reinforced polymer materials: A review. Polym. Adv. Technol. 2023, 34, 1393–1404. [Google Scholar] [CrossRef]
- Makvandi, P.; Iftekhar, S.; Pizzetti, F.; Zarepour, A.; Zare, E.N.; Ashrafizadeh, M.; Agarwal, T.; Padil, V.V.T.; Mohammadinejad, R.; Sillanpaa, M.; et al. Functionalization of polymers and nanomaterials for water treatment, food packaging, textile and biomedical applications: A review. Environ. Chem. Lett. 2020, 19, 583–611. [Google Scholar] [CrossRef]
- Mane, S. Functional polymers: A review. Can. Chem. Trans. 2016, 4, 316–327. [Google Scholar]
- Monge, S.; David, G. Phosphorus-Based Polymers: From Synthesis to Applications; Burlington House: London, UK, 2014. [Google Scholar]
- Hamed, F.; Biji, P. A novel polymer containing phosphorus–nitrogen ligands for stabilization of palladium nanoparticles: An efficient and recyclable catalyst for Suzuki and Sonogashira reactions in neat water. Dalton Trans. 2015, 44, 14293–14303. [Google Scholar] [CrossRef]
- Hiranphinyophata, S.; Iwasaki, Y. Controlled biointerfaces with biomimetic phosphorus-containing polymers. Sci. Technol. Adv. Mater. 2021, 22, 301–316. [Google Scholar] [CrossRef]
- Akhmedov, V.M.; Maharramov, A.M.; Azizov, A.A.; Alosmanov, R.M.; Bunyad-Zadeh, I.A.; Aliyeva, S.B. Equilibrium, kinetic, and thermodynamic studies on the sorption of some heavy metal ions by the phosphorus-containing polymer sorbent. Russ. Chem. Bull. Int. Ed. 2019, 68, 514–520. [Google Scholar] [CrossRef]
- Allcock, H.R. A Perspective of polyphosphazene research. J. Inorg. Organomet. Polym. Mat. 2006, 16, 277–294. [Google Scholar] [CrossRef]
- Chen, D.-P.; Wang, J. Synthesis and characterization of block copolymer of polyphosphoester and poly(ε-caprolactone). Macromolecules 2006, 39, 473–475. [Google Scholar] [CrossRef]
- Kloda, M.; Ondrušová, S.; Lang, K.; Demel, J. Phosphinic acids as building units in materials chemistry. Coordin. Chem. Rev. 2021, 433, 213748. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.-Z. Aryl polyphosphonates: Useful halogen-free flame retardants for polymers. Materials 2010, 3, 4746–4760. [Google Scholar] [CrossRef] [Green Version]
- Wehbi, M.; Mehdi, A.; Negrell, C.; David, G.; Alaaeddine, A.; Ameduri, B. Phosphorus-containing fluoropolymers: State of the art and applications. ACS Appl. Mater. Interfaces 2020, 12, 38–59. [Google Scholar] [CrossRef] [PubMed]
- Caminade, A.-M.; Majoral, J.-P. Bifunctional phosphorus dendrimers and their properties. Molecules 2016, 21, 538. [Google Scholar] [CrossRef] [Green Version]
- Alosmanov, R.; Wolski, K.; Matuschek, G.; Magerramov, A.; Azizov, A.; Zimmermann, R.; Aliyev, E.; Zapotoczny, S. Effect of functional groups on the thermal degradation of phosphorus- and phosphorus/nitrogen-containing functional polymers. J. Therm. Anal. Calorim. 2017, 130, 799–812. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Wang, J.; Ji, Q.; Shultz, A.R.; Ward, T.C.; McGrath, J.E. Miscibility and morphologies of poly(arylene ether phenyl phosphine oxide/sulfone) copolymer/vinyl ester resin mixtures and their cured networks. J. Polym. Sci. B 2000, 38, 2409–2421. [Google Scholar] [CrossRef]
- Podkościelna, B.; Bartnicki, A.; Gawdzik, B. Porous microspheres, copolymers of bis [4-(2-hydroxy-3-methacryloyloxypropoxy)phenyl]sulfide, and divinylbenzene as stationary phase for HPLC. J. App. Polym. Sci. 2009, 111, 1257–1267. [Google Scholar] [CrossRef]
- Podkościelna, B.; Kołodyńska, D. A new type of cation-exchange polymeric microspheres with pendant methylenethiol groups. Polym. Adv. Technol. 2013, 24, 866–872. [Google Scholar] [CrossRef]
- Wawrzkiewicz, M.; Podkościelna, B.; Podkościelny, P. Application of functionalized DVB-co-GMA polymeric microspheres in the enhanced sorption process of hazardous dyes from dyeing baths. Molecules 2020, 25, 5247. [Google Scholar] [CrossRef]
- Rabinowitz, R.; Marcus, R.; Pellon, J. Synthesis, polymerization, and copolymerization of diphenyl-p-styrylphosphine, phosphine oxide, and phosphine sulfide. J. Polym. Sci. A 1964, 2, 1241. [Google Scholar] [CrossRef]
- Ebdon, J.R.; Price, D.; Hunt, B.J.; Joseph, P.; Gao, F.; Milnes, G.J.; Cunliffe, L.K. Flame retardance in some polystyrenes and poly(methyl methacrylate)s with covalently bound phosphorus-containing groups: Initial screening experiments and some laser pyrolysis mechanistic studies. Polym. Degrad. Stab. 2000, 69, 267–277. [Google Scholar] [CrossRef]
- Trochimczuk, A.; Czerwińska, S. In(III) and Ga(III) sorption by polymeric resins with substituted phenylphosphinic acid ligands. React. Funct. Polym. 2005, 63, 215–220. [Google Scholar] [CrossRef]
- Zhang, A.; Wei, Y.; Kumagai, M.; Koma, Y.; Koyama, T. Resistant behavior of a novel silica-based octyl(phenyl)-N,N-diisobutyl carbamoylmethylphoshine oxide neutral extraction resin against nitric acid, temperature and γ-radiation. Radiat. Phys. Chem. 2005, 72, 455–463. [Google Scholar] [CrossRef]
- Zhang, A.; Kuraoka, E.; Hoshi, H.; Kumagai, M. Synthesis of two novel macroporous silica-based impregnated polymeric composites and their application in highly active liquid waste partitioning by extraction chromatography. J. Chromatogr. A 2004, 1061, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Rahaman, M.S.; Yeum, J.H. Phosphine-functionalized electrospun poly(vinyl alcohol)/silica nanofibers as highly effective adsorbent for removal of aqueous manganese and nickel ions. Colloids Surf. A 2015, 484, 9–18. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Li, J.; Liu, W.; Cheng, G.; Ke, H. Phosphine-based covalent organic framework for highly efficient iodine capture. Micropor. Mesopor. Mater. 2021, 325, 111351. [Google Scholar] [CrossRef]
- Naini, N.; Sid Kalal, H.; Almasian, M.R.; Niknafs, D.; Taghiof, M.; Hoveidi, H. Phosphine-functionalized Fe3O4/SiO2/composites as efficient magnetic nanoadsorbents for the removal of palladium ions from aqueous solution: Kinetic, thermodynamic and isotherm studies. Mater. Chem. Phys. 2022, 287, 126242. [Google Scholar] [CrossRef]
- Alosmanov, R.M. Adsorption of arsenazo III dye by phosphorus-containing polymer sorbent. J. Serb. Chem. Soc. 2016, 81, 907–921. [Google Scholar] [CrossRef]
- Davidescu, C.-M.; Ardelean, R.; Popa, A. New polymeric adsorbent materials used for removal of phenolic derivatives from wastewaters. Pure Appl. Chem. 2019, 91, 443–458. [Google Scholar] [CrossRef]
- Garba, A.; Tahir, A.; Yusuf, A.K. Adsorption of methylene blue using activated carbon made from watermelon rinds. J. Sustain. Mater. Process. Manag. 2021, 1, 38–43. [Google Scholar] [CrossRef]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Das, J.; Dangar, T.K.; Panigrahy, M. Bioremediation of Heavy Metals: A substantive potential for clean earth. J. Sustain. Mater. Process. Manag. 2022, 2, 80–89. [Google Scholar] [CrossRef]
- Hessel, C.; Allegre, C.; Maisseu, M.; Charbit, F.; Moulin, P. Guidelines and legislation for dye house effluents. J. Environ. Manag. 2007, 83, 171–180. [Google Scholar] [CrossRef]
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresource Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Alderete, B.L.; da Silva, J.; Godoi, R.; Rabaioli da Silva, F.; Taffarel, S.R.; Pisoni da Silva, L.; Hilario Garcia, A.L.; Mitteregger Júnior, H.; Neubauer de Amorim, H.L.; Picada, J.N. Evaluation of toxicity and mutagenicity of a synthetic effluent containing azo dye after advanced oxidation process treatment. Chemosphere 2020, 263, 128291. [Google Scholar] [CrossRef] [PubMed]
- Holme, I. Ecological aspects of color chemistry. In Developments in the Chemistry and Technology of Organic Dyes; Griffiths, J., Ed.; Society for Chemical Industry: Oxford, UK, 1984; pp. 111–128. [Google Scholar]
- Dotto, G.L.; Santos, J.M.N.; Rodrigues, I.L.; Rosa, R.; Pavan, F.A.; Lima, E.C. Adsorption of Methylene Blue by ultrasonic surface modified chitin. J. Colloid Interface Sci. 2015, 446, 133–140. [Google Scholar] [CrossRef]
- Axenov, K.V.; Moemming, C.M.; Kehr, G.; Frohlich, R.; Erker, G. Structure and dynamic features of an intramolecular frustrated Lewis pair. Eur. J. Chem. 2010, 16, 14069–14073. [Google Scholar] [CrossRef] [PubMed]
- Frynas, S.; Łastawiecka, E.; Kozioł, A.E.; Flis, A.; Pietrusiewicz, K.M. [4 + 2] Cycloaddition of vinylphosphine oxides to α-oxy-o-xylylene as a route to phosphorylated naphthyl and biaryl scaffolds. J. Org. Chem. 2019, 84, 1818–1833. [Google Scholar] [CrossRef]
- Gawdzik, B.; Podkościelna, B.; Bartnicki, A. Synthesis, structure, and properties of new methacrylic derivatives of naphthalene-2,3-diol. J. App. Poly. Sci. 2006, 102, 1886–1895. [Google Scholar] [CrossRef]
- Podkościelna, B.; Gawdzik, B. Influence of diluent compositions on the porous structure of methacrylate derivatives of aromatic diols and divinylbenzene. App. Surf. Sci. 2010, 256, 2462–2467. [Google Scholar] [CrossRef]
- Podkościelna, B. Synthesis, modification, and porous properties of new glycidyl methacrylate copolymers. J. Appl. Polym. Sci. 2011, 120, 3020–3026. [Google Scholar] [CrossRef]
- Wan Ngah, W.S.; Hanafiah, M.A.K.M. Adsorption of copper on rubber (Hevea brasiliensis) leaf powder: Kinetic, equilibrium and thermodynamic studies. Biochem. Eng. J. 2008, 39, 521–530. [Google Scholar] [CrossRef]
- Available online: https://www.dupont.com/content/dam/dupont/amer/us/en/water-solutions/public/documents/en/IER-AmberLite-XAD4-PDS-45-D00781-en.pdf (accessed on 13 March 2023).
- Thomas, L.C.; Chittenden, R.A. Characteristic infrared absorption frequencies of organophosphorus compounds–I. The phosphoryl (P=O) group. Spectrochim. Acta 1964, 20, 467–487. [Google Scholar] [CrossRef]
- Qin, P.; Wang, L.-A.; O’Connor, J.M.; Baldridge, K.K.; Li, Y.; Tufekci, B.; Chen, J.; Rheingold, A.L. Transition-metal catalysis of triene 6π electrocyclization: The complexation strategy realized. Angew. Chem. Int. Ed. 2020, 59, 17958–17965. [Google Scholar] [CrossRef]
- Podkościelna, B. The highly crosslinked dimethacrylic/divinylbenzene copolymers-characterization and thermal studies. J. Therm. Anal. Calor. 2011, 104, 725–730. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, J.; Li, Q.; Li, L.; Huang, Y.; Wang, Y.; Fan, G.; Zhang, L. Adsorption performance of methylene blue by KOH/FeCl3 modified biochar/alginate composite beads derived from agricultural waste. Molecules 2023, 28, 2507. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Wawrzkiewicz, M.; Onyszko, M.; Medykowska, M.; Nosal-Wiercińska, A.; Bogatyrov, V. Carbon-silica composite as adsorbent for removal of hazardous C.I. Basic Yellow 2 and C.I. Basic Blue 3 dyes. Materials 2021, 14, 3245. [Google Scholar] [CrossRef]
- Majd, M.M.; Kordzadeh-Kermani, V.; Ghalandari, V.; Askari, A.; Sillanpää, M. Adsorption isotherm models: A comprehensive and systematic review (2010–2020). Sci. Total Environ. 2022, 812, 151334. [Google Scholar] [CrossRef] [PubMed]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and interpretation of adsorption isotherms. J. Chem. 2017, 2017, 3039817. [Google Scholar] [CrossRef] [Green Version]
- Wawrzkiewicz, M.; Podkościelna, B.; Jesionowski, T.; Klapiszewski, Ł. Functionalized microspheres with co-participated lignin hybrids as a novel sorbents for toxic C.I. Basic Yellow 2 and C.I. Basic Blue 3 dyes removal from textile sewage. Ind. Crops Prod. 2022, 180, 114785. [Google Scholar] [CrossRef]
- Podkościelna, B.; Wawrzkiewicz, M.; Goliszek, M.; Lipke, A.; Chabros, A. Synthesis and characterization of new polymer sorbents based on EGDMA and cellulose. Physicochem. Probl. Miner. Process. 2022, 58, 147466. [Google Scholar] [CrossRef]
- Öztürk, A.; Malkoc, E. Adsorptive potential of cationic Basic Yellow 2 (BY2) dye onto natural untreated clay (NUC) from aqueous phase: Mass transfer analysis, kinetic and equilibrium profile. App. Surf. Sci. 2014, 299, 105–115. [Google Scholar] [CrossRef]
- Ghorai, A.; Banerjee, S. Phosphorus-containing aromatic polymers: Synthesis, structure, properties and membrane-based applications. Prog. Polym. Sci. 2023, 138, 101646. [Google Scholar] [CrossRef]










| Isotherm | Parameters | poly(DVB) | CyP(Ph)4-DVB | poly(DVB) | CyP(Ph)4-DVB |
|---|---|---|---|---|---|
| BY2 | BB3 | ||||
| Langmuir | Q0 (mg/g) | 141.7 | 93.1 | 78.3 | 136.7 |
| kL (L/mg) | 0.03 | 0.19 | 0.42 | 0.77 | |
| R2 | 0.549 | 0.653 | 0.438 | 0.880 | |
| Freundlich | kF (mg1−1/nL1/n/g) | 4.56 | 14.2 | 20.1 | 53.7 |
| 1/n | 0.91 | 0.63 | 0.64 | 0.76 | |
| R2 | 0.995 | 0.816 | 0.968 | 0.939 | |
| Temkin | A (L/mg) | 2.6 | 8.1 | 31.8 | 26.1 |
| bT (J g/mol mg) | 294.3 | 207.1 | 335.2 | 131.0 | |
| R2 | 0.767 | 0.813 | 0.652 | 0.859 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frynas, S.; Wawrzkiewicz, M. Synthesis, Characterization and Application of a New Functionalized Polymeric Sorbent Based on Alkenylphoshine Oxide. Polymers 2023, 15, 1591. https://doi.org/10.3390/polym15061591
Frynas S, Wawrzkiewicz M. Synthesis, Characterization and Application of a New Functionalized Polymeric Sorbent Based on Alkenylphoshine Oxide. Polymers. 2023; 15(6):1591. https://doi.org/10.3390/polym15061591
Chicago/Turabian StyleFrynas, Sławomir, and Monika Wawrzkiewicz. 2023. "Synthesis, Characterization and Application of a New Functionalized Polymeric Sorbent Based on Alkenylphoshine Oxide" Polymers 15, no. 6: 1591. https://doi.org/10.3390/polym15061591