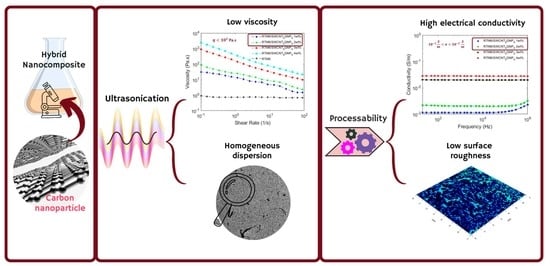
Hybrid Carbon Nanocomposites Made of Aerospace-Grade Epoxy Showing Synergistic Effects in Electrical Properties and High Processability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Processing of Nanocomposites
2.3. Characterization Methods
3. Results and Discussion
3.1. Dispersion and Electrical Properties of RTM6/Carbon Nanocomposites
3.2. Rheological Properties of RTM6/carbon Mixtures
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, S.; Zheng, Z.; Huang, Z.; Dong, S.; Luo, P.; Zhang, Z.; Wang, Y. Cu matrix composites reinforced with aligned carbon nanotubes: Mechanical, electrical and thermal properties. Mater. Sci. Eng. A 2016, 675, 82–91. [Google Scholar] [CrossRef]
- Samani, M.K.; Khosravian, N.; Chen, G.C.K.; Shakerzadeh, M.; Baillargeat, D.; Tay, B.K. Thermal conductivity of individual multiwalled carbon nanotubes. Int. J. Therm. Sci. 2012, 62, 40–43. [Google Scholar] [CrossRef]
- Yang, D.J.; Wang, S.G.; Zhang, Q.; Sellin, P.J.; Chen, G. Thermal and electrical transport in multi-walled carbon nanotubes. Phys. Lett. A 2004, 329, 207–213. [Google Scholar] [CrossRef]
- Yu, M.-F.; Files, B.S.; Arepalli, S.; Ruoff, R.S. Tensile Loading of Ropes of Single Wall Carbon Nanotubes and their Mechanical Properties. Phys. Rev. Lett. 2000, 84, 5552–5555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yazdani, H.; Hatami, K.; Eftekhari, M. Mechanical properties of single-walled carbon nanotubes: A comprehensive molecular dynamics study. Mater. Res. Express 2017, 4, 055015. [Google Scholar] [CrossRef]
- Nurazzi, N.M.; Sabaruddin, F.A.; Harussani, M.M.; Kamarudin, S.H.; Rayung, M.; Asyraf, M.R.M.; Aisyah, H.A.; Norrrahim, M.N.F.; Ilyas, R.A.; Abdullah, N.; et al. Mechanical Performance and Applications of CNTs Reinforced Polymer Composites—A Review. Nanomaterials 2021, 11, 2186. [Google Scholar] [CrossRef]
- Biercuk, M.; Llaguno, M.C.; Radosavljevic, M.; Hyun, J.; Johnson, A.T.; Fischer, J.E. Carbon nanotube composites for thermal management. Appl. Phys. Lett. 2002, 80, 2767–2769. [Google Scholar] [CrossRef]
- Coleman, J.N.; Khan, U.; Blau, W.J.; Gun’ko, Y.K. Small but strong: A review of the mechanical properties of carbon nanotube–polymer composites. Carbon 2006, 44, 1624–1652. [Google Scholar] [CrossRef]
- Gibson, R.F. A review of recent research on mechanics of multifunctional composite materials and structures. Compos. Struct. 2010, 92, 2793–2810. [Google Scholar] [CrossRef]
- Spitalsky, Z.; Tasis, D.; Papagelis, K.; Galiotis, C. Carbon nanotube–polymer composites: Chemistry, processing, mechanical and electrical properties. Prog. Polym. Sci. 2010, 35, 357–401. [Google Scholar] [CrossRef]
- Clausi, M.; Santonicola, M.G.; Schirone, L.; Laurenzi, S. Analysis of ultraviolet exposure effects on the surface properties of epoxy/graphene nanocomposite films on Mylar substrate. Acta Astronaut. 2017, 134, 307–313. [Google Scholar] [CrossRef]
- Siochi, E.J.; Harrison, J.S. Structural nanocomposites for aerospace applications. MRS Bull. 2015, 40, 829–835. [Google Scholar] [CrossRef] [Green Version]
- Smith Jr, J.G.; Delozier, D.M.; Connell, J.W.; Watson, K.A. Carbon nanotube-conductive additive-space durable polymer nanocomposite films for electrostatic charge dissipation. Polymer 2004, 45, 6133–6142. [Google Scholar] [CrossRef] [Green Version]
- Laurenzi, S.; de Zanet, G.; Santonicola, M.G. Numerical investigation of radiation shielding properties of polyethylene-based nanocomposite materials in different space environments. Acta Astronaut. 2020, 170, 530–538. [Google Scholar] [CrossRef]
- Toto, E.; Santonicola, M.G.; Mancini, M.C.; Laurenzi, S. Ultraviolet-sensing surfaces based on hybrid nanocomposites for radiation monitoring systems. In Proceedings of the 2017 IEEE International Workshop on Metrology for AeroSpace (MetroAeroSpace), Padua, Italy, 21–23 June 2017; pp. 369–373. [Google Scholar] [CrossRef]
- Toto, E.; Botti, S.; Laurenzi, S.; Gabriella Santonicola, M. UV-induced modification of PEDOT:PSS-based nanocomposite films investigated by Raman microscopy mapping. Appl. Surf. Sci. 2020, 513, 145839. [Google Scholar] [CrossRef]
- Toto, E.; Laurenzi, S.; Santonicola, M.G. Recent Trends in Graphene/Polymer Nanocomposites for Sensing Devices: Synthesis and Applications in Environmental and Human Health Monitoring. Polymers 2022, 14, 1030. [Google Scholar] [CrossRef]
- Ma, P.-C.; Siddiqui, N.A.; Marom, G.; Kim, J.-K. Dispersion and functionalization of carbon nanotubes for polymer-based nanocomposites: A review. Compos. Part A Appl. Sci. Manuf. 2010, 41, 1345–1367. [Google Scholar] [CrossRef]
- Wan, Y.-J.; Tang, L.-C.; Yan, D.; Zhao, L.; Li, Y.-B.; Wu, L.-B.; Jiang, J.-X.; Lai, G.-Q. Improved dispersion and interface in the graphene/epoxy composites via a facile surfactant-assisted process. Compos. Sci. Technol. 2013, 82, 60–68. [Google Scholar] [CrossRef]
- Song, Y.S.; Youn, J.R. Influence of dispersion states of carbon nanotubes on physical properties of epoxy nanocomposites. Carbon 2005, 43, 1378–1385. [Google Scholar] [CrossRef]
- Laurenzi, S.; Botti, S.; Rufoloni, A.; Santonicola, M.G. Mapping the residual strain of carbon nanotubes in DWCNT/epoxy nanocomposites after tensile load using Raman microscopy. Compos. Commun. 2020, 21, 100424. [Google Scholar] [CrossRef]
- Gojny, F.H.; Wichmann, M.H.G.; Fiedler, B.; Kinloch, I.A.; Bauhofer, W.; Windle, A.H.; Schulte, K. Evaluation and identification of electrical and thermal conduction mechanisms in carbon nanotube/epoxy composites. Polymer 2006, 47, 2036–2045. [Google Scholar] [CrossRef]
- Wu, S.; Ladani, R.B.; Zhang, J.; Bafekrpour, E.; Ghorbani, K.; Mouritz, A.P.; Kinloch, A.J.; Wang, C.H. Aligning multilayer graphene flakes with an external electric field to improve multifunctional properties of epoxy nanocomposites. Carbon 2015, 94, 607–618. [Google Scholar] [CrossRef] [Green Version]
- Rubel, R.I.; Ali, M.H.; Jafor, M.A.; Alam, M.M. Carbon nanotubes agglomeration in reinforced composites: A review. AIMS Mater. Sci. 2019, 6, 756–780. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, J.; Qiu, L.; Li, D. Bioinspired Effective Prevention of Restacking in Multilayered Graphene Films: Towards the Next Generation of High-Performance Supercapacitors. Adv. Mater. 2011, 23, 2833–2838. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, N.G.; Rana, S.; Cho, J.W.; Li, L.; Chan, S.H. Polymer nanocomposites based on functionalized carbon nanotubes. Prog. Polym. Sci. 2010, 35, 837–867. [Google Scholar] [CrossRef]
- Wang, K.; Wang, W.; Wang, H.; Liu, L.; Xu, Z.; Fu, H.; Zhao, L.; Zhang, X.; Chen, L.; Zhao, Y. 3D graphene foams/epoxy composites with double-sided binder polyaniline interlayers for maintaining excellent electrical conductivities and mechanical properties. Compos. Part A Appl. Sci. Manuf. 2018, 110, 246–257. [Google Scholar] [CrossRef]
- Teng, K.; Ni, Y.; Wang, W.; Wang, H.; Xu, Z.; Chen, L.; Kuang, L.; Ma, M.; Fu, H.; Li, J. Adjustable micro-structure, higher-level mechanical behavior and conductivities of preformed graphene architecture/epoxy composites via RTM route. Compos. Part A Appl. Sci. Manuf. 2017, 94, 178–188. [Google Scholar] [CrossRef]
- Tian, L.; Meziani, M.J.; Lu, F.; Kong, C.Y.; Cao, L.; Thorne, T.J.; Sun, Y.-P. Graphene Oxides for Homogeneous Dispersion of Carbon Nanotubes. ACS Appl. Mater. Interfaces 2010, 2, 3217–3222. [Google Scholar] [CrossRef]
- Zhang, C.; Ren, L.; Wang, X.; Liu, T. Graphene Oxide-Assisted Dispersion of Pristine Multiwalled Carbon Nanotubes in Aqueous Media. J. Phys. Chem. C 2010, 114, 11435–11440. [Google Scholar] [CrossRef]
- Martin-Gallego, M.; Bernal, M.M.; Hernandez, M.; Verdejo, R.; Lopez-Manchado, M.A. Comparison of filler percolation and mechanical properties in graphene and carbon nanotubes filled epoxy nanocomposites. Eur. Polym. J. 2013, 49, 1347–1353. [Google Scholar] [CrossRef] [Green Version]
- Schulz, S.C.; Faiella, G.; Buschhorn, S.T.; Prado, L.A.S.A.; Giordano, M.; Schulte, K.; Bauhofer, W. Combined electrical and rheological properties of shear induced multiwall carbon nanotube agglomerates in epoxy suspensions. Eur. Polym. J. 2011, 47, 2069–2077. [Google Scholar] [CrossRef]
- Reia da Costa, E.F.; Skordos, A.A.; Partridge, I.K.; Rezai, A. RTM processing and electrical performance of carbon nanotube modified epoxy/fibre composites. Compos. Part A Appl. Sci. Manuf. 2012, 43, 593–602. [Google Scholar] [CrossRef] [Green Version]
- Ma, A.W.K.; Chinesta, F.; Mackley, M.R. The rheology and modeling of chemically treated carbon nanotubes suspensions. J. Rheol. 2009, 53, 547–573. [Google Scholar] [CrossRef] [Green Version]
- Chapartegui, M.; Markaide, N.; Florez, S.; Elizetxea, C.; Fernandez, M.; Santamaría, A. Specific rheological and electrical features of carbon nanotube dispersions in an epoxy matrix. Compos. Sci. Technol. 2010, 70, 879–884. [Google Scholar] [CrossRef]
- Clausi, M.; Santonicola, M.G.; Laurenzi, S. Steady-shear rheological properties of graphene-reinforced epoxy resin for manufacturing of aerospace composite films. AIP Conf. Proc. 2016, 1736, 020024. [Google Scholar] [CrossRef]
- Platzer, N. Non-Newtonian Flow and Heat Transfer. J. Appl. Polym. Sci. 1967, 11, 1822–1823. [Google Scholar] [CrossRef]
- Banerjee, K.; Li, H.; Srivastava, N. Current Status and Future Perspectives of Carbon Nanotube Interconnects. In Proceedings of the 2008 8th IEEE Conference on Nanotechnology, Arlington, TX, USA, 18–21 August 2008; pp. 432–436. [Google Scholar] [CrossRef]
- Chatterjee, S.; Nafezarefi, F.; Tai, N.H.; Schlagenhauf, L.; Nüesch, F.A.; Chu, B.T.T. Size and synergy effects of nanofiller hybrids including graphene nanoplatelets and carbon nanotubes in mechanical properties of epoxy composites. Carbon 2012, 50, 5380–5386. [Google Scholar] [CrossRef]
- Yang, S.-Y.; Lin, W.-N.; Huang, Y.-L.; Tien, H.-W.; Wang, J.-Y.; Ma, C.-C.M.; Li, S.-M.; Wang, Y.-S. Synergetic effects of graphene platelets and carbon nanotubes on the mechanical and thermal properties of epoxy composites. Carbon 2011, 49, 793–803. [Google Scholar] [CrossRef]
- Yue, L.; Pircheraghi, G.; Monemian, S.A.; Manas-Zloczower, I. Epoxy composites with carbon nanotubes and graphene nanoplatelets—Dispersion and synergy effects. Carbon 2014, 78, 268–278. [Google Scholar] [CrossRef]
- Biswas, S.; Fukushima, H.; Drzal, L.T. Mechanical and electrical property enhancement in exfoliated graphene nanoplatelet/liquid crystalline polymer nanocomposites. Compos. Part A Appl. Sci. Manuf. 2011, 42, 371–375. [Google Scholar] [CrossRef]
- Laurenzi, S.; Clausi, M.; Zaccardi, F.; Curt, U.; Santonicola, M.G. Spray coating process of MWCNT/epoxy nanocomposite films for aerospace applications: Effects of process parameters on surface electrical properties. Acta Astronaut. 2019, 159, 429–439. [Google Scholar] [CrossRef]
- Clausi, M.; Santonicola, M.G.; Laurenzi, S. Fabrication of carbon-based nanocomposite films by spin-coating process: An experimental and modeling study of the film thickness. Compos. Part A Appl. Sci. Manuf. 2016, 88, 86–97. [Google Scholar] [CrossRef]
- Sierra-Chi, C.A.; Aguilar-Bolados, H.; López-Manchado, M.A.; Verdejo, R.; Cauich-Rodríguez, J.V.; Avilés, F. Flexural electromechanical properties of multilayer graphene sheet/carbon nanotube/vinyl ester hybrid nanocomposites. Compos. Sci. Technol. 2020, 194, 108164. [Google Scholar] [CrossRef]
- Parsons, A.J.; Gonciaruk, A.; Zeng, X.; Thomann, F.S.; Schubel, P.; Lorrillard, J.; Johnson, M.S. Controlling mass loss from RTM6 epoxy resin under simulated vacuum infusion conditions. Polym. Test. 2022, 107, 107473. [Google Scholar] [CrossRef]
- Cebeci, H.; Villoria, R.G.d.; Hart, A.J.; Wardle, B.L. Multifunctional properties of high volume fraction aligned carbon nanotube polymer composites with controlled morphology. Compos. Sci. Technol. 2009, 69, 2649–2656. [Google Scholar] [CrossRef]
- Martone, A.; Formicola, C.; Giordano, M.; Zarrelli, M. Reinforcement efficiency of multi-walled carbon nanotube/epoxy nano composites. Compos. Sci. Technol. 2010, 70, 1154–1160. [Google Scholar] [CrossRef]
- Schilde, C.; Schlömann, M.; Overbeck, A.; Linke, S.; Kwade, A. Thermal, mechanical and electrical properties of highly loaded CNT-epoxy composites—A model for the electric conductivity. Compos. Sci. Technol. 2015, 117, 183–190. [Google Scholar] [CrossRef]
- Vertuccio, L.; Russo, S.; Raimondo, M.; Lafdi, K.; Guadagno, L. Influence of carbon nanofillers on the curing kinetics of epoxy-amine resin. RSC Adv. 2015, 5, 90437–90450. [Google Scholar] [CrossRef]
- Li, J.; Aung, H.H.; Du, B. Curing Regime-Modulating Insulation Performance of Anhydride-Cured Epoxy Resin: A Review. Molecules 2023, 28, 547. [Google Scholar] [CrossRef]
- Zaccardi, F.; Santonicola, M.G.; Laurenzi, S. Quantitative assessment of nanofiller dispersion based on grayscale image analysis: A case study on epoxy/carbon nanocomposites. Compos. Part A Appl. Sci. Manuf. 2018, 115, 302–310. [Google Scholar] [CrossRef]
- Stauffer, D.; Aharony, A. Introduction to percolation theory; Taylor & Francis: New York, NY, USA, 2018. [Google Scholar] [CrossRef]
- Gingold, D.B.; Lobb, C.J. Percolative conduction in three dimensions. Phys. Rev. B 1990, 42, 8220–8224. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Kamal, M.R. Estimation of the volume resistivity of electrically conductive composites. Polym. Compos. 1997, 18, 711–725. [Google Scholar] [CrossRef]
- Sandler, J.K.W.; Kirk, J.E.; Kinloch, I.A.; Shaffer, M.S.P.; Windle, A.H. Ultra-low electrical percolation threshold in carbon-nanotube-epoxy composites. Polymer 2003, 44, 5893–5899. [Google Scholar] [CrossRef]
- Zhang, K.; Li, G.-H.; Feng, L.-M.; Wang, N.; Guo, J.; Sun, K.; Yu, K.-X.; Zeng, J.-B.; Li, T.; Guo, Z.; et al. Ultralow percolation threshold and enhanced electromagnetic interference shielding in poly(l-lactide)/multi-walled carbon nanotube nanocomposites with electrically conductive segregated networks. J. Mater. Chem. C 2017, 5, 9359–9369. [Google Scholar] [CrossRef]







| epoxy/SWCNT | |||||
|---|---|---|---|---|---|
| 1 wt% | 2 wt% | 3 wt% | 4 wt% | ||
| K (Pa sn) | 35.58 | 215.67 | 497.58 | 1388.81 | |
| n | 0.50 | 0.12 | 0.15 | 0.15 | |
| 0.983 | 0.997 | 0.996 | 0.996 | ||
| epoxy/GNP2SWCNT8 | |||||
| 1 wt% | 2 wt% | 3 wt% | 4 wt% | ||
| K (Pa sn) | 20.91 | 176.95 | 360.31 | 851.04 | |
| n | 0.42 | 0.19 | 0.15 | 0.15 | |
| 0.991 | 0.999 | 0.999 | 0.999 | ||
| epoxy/GNP5SWCNT5 | |||||
| 1 wt% | 2 wt% | 3 wt% | 4 wt% | ||
| K (Pa sn) | 13.40 | 24.28 | 160.51 | 395.54 | |
| n | 0.60 | 0.46 | 0.25 | 0.20 | |
| 0.989 | 0.98 | 0.999 | 0.999 | ||
| epoxy/GNP8SWCNT2 | |||||
| 1 wt% | 2 wt% | 3 wt% | 4 wt% | 5 wt% | |
| K (Pa sn) | 1.49 | 3.51 | 8.69 | 20.06 | 27.18 |
| n | 0.76 | 0.59 | 0.50 | 0.50 | 0.55 |
| 0.968 | 0.976 | 0.979 | 0.998 | 0.997 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaccardi, F.; Toto, E.; Marra, F.; Santonicola, M.G.; Laurenzi, S. Hybrid Carbon Nanocomposites Made of Aerospace-Grade Epoxy Showing Synergistic Effects in Electrical Properties and High Processability. Polymers 2023, 15, 1163. https://doi.org/10.3390/polym15051163
Zaccardi F, Toto E, Marra F, Santonicola MG, Laurenzi S. Hybrid Carbon Nanocomposites Made of Aerospace-Grade Epoxy Showing Synergistic Effects in Electrical Properties and High Processability. Polymers. 2023; 15(5):1163. https://doi.org/10.3390/polym15051163
Chicago/Turabian StyleZaccardi, Federica, Elisa Toto, Fabrizio Marra, Maria Gabriella Santonicola, and Susanna Laurenzi. 2023. "Hybrid Carbon Nanocomposites Made of Aerospace-Grade Epoxy Showing Synergistic Effects in Electrical Properties and High Processability" Polymers 15, no. 5: 1163. https://doi.org/10.3390/polym15051163