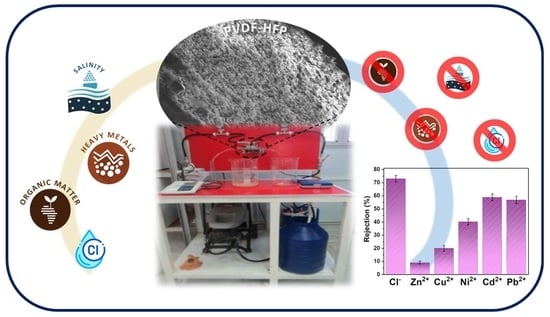
Wastewater Treatment of Real Effluents by Microfiltration Using Poly(vinylidene fluoride–hexafluoropropylene) Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. PVDF-HFP Membrane Preparation
2.3. Membrane Characterization
2.4. Wastewater Characterization
2.5. Filtration System
3. Results and Discussion
3.1. Membrane Characterization
3.2. Membrane Filtration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Salazar, H.; Martins, P.M.; Batista, D.; Shejale, K.P.; Sharma, R.K.; Krishnapriya, R.; Ferdov, S.; Botelho, G.; Fidalgo-Marijuan, A.; Cássio, F.; et al. Comparative Performance and Ecotoxicity Assessment of Y2(CO3)3, ZnO/TiO2, and Fe3O4 Nanoparticles for Arsenic Removal from Water. Environ. Sci. Water Res. Technol. 2022, 8, 1719–1730. [Google Scholar] [CrossRef]
- Prüss-Ustün, A.; Wolf, J.; Bartram, J.; Clasen, T.; Cumming, O.; Freeman, M.C.; Gordon, B.; Hunter, P.R.; Medlicott, K.; Johnston, R. Burden of Disease from Inadequate Water, Sanitation and Hygiene for Selected Adverse Health Outcomes: An Updated Analysis with a Focus on Low- and Middle-Income Countries. Int. J. Hyg. Environ. Health 2019, 222, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Pang, X.; Yue, Z.; Zhou, Y.; Duan, H.; Shen, W.; Li, J.; Liu, Y.; Cheng, Q. Sulfonamides Removed from Simulated Livestock and Poultry Breeding Wastewater Using an In-Situ Electro-Fenton Process Powered by Photovoltaic Energy. Chem. Eng. J. 2020, 397, 125466. [Google Scholar] [CrossRef]
- Martins, P.M.; Santos, B.; Salazar, H.; Carabineiro, S.A.C.; Botelho, G.; Tavares, C.J.; Lanceros-Mendez, S. Multifunctional Hybrid Membranes for Photocatalytic and Adsorptive Removal of Water Contaminants of Emerging Concern. Chemosphere 2022, 293, 133548. [Google Scholar] [CrossRef] [PubMed]
- Rao, T.; Ma, X.; Yang, Q.; Cheng, S.; Ren, G.; Wu, Z.; Sirés, I. Upgrading the Peroxi-Coagulation Treatment of Complex Water Matrices Using a Magnetically Assembled MZVI/DSA Anode: Insights into the Importance of ClO Radical. Chemosphere 2022, 303, 134948. [Google Scholar] [CrossRef]
- Lee, J.; Wang, J.; Oh, Y.; Jeong, S. Highly Efficient Microplastics Removal from Water Using In-Situ Ferrate Coagulation: Performance Evaluation by Micro-Fourier-Transformed Infrared Spectroscopy and Coagulation Mechanism. Chem. Eng. J. 2023, 451, 138556. [Google Scholar] [CrossRef]
- Monajjemi, M.; Mollaamin, F.; Küçük, Ö. Removing Non-Biodegradable Toxic Ions via Bio-Oxidation Analysis in Anzali Lagoon: A Usage of Seaweed in Biodiversity. Biointerface Res. Appl. Chem. 2023, 13, 282. [Google Scholar] [CrossRef]
- Soo, P.L.; Bashir, M.J.K.; Wong, L.-P. Recent Advancements in the Treatment of Palm Oil Mill Effluent (POME) Using Anaerobic Biofilm Reactors: Challenges and Future Perspectives. J. Environ. Manag. 2022, 320, 115750. [Google Scholar] [CrossRef]
- Khan, M.A.; Ngo, H.H.; Guo, W.; Liu, Y.; Chang, S.W.; Nguyen, D.D.; Nghiem, L.D.; Liang, H. Can Membrane Bioreactor Be a Smart Option for Water Treatment? Bioresour. Technol. Rep. 2018, 4, 80–87. [Google Scholar] [CrossRef]
- Wang, S.; Ma, L.; Wang, R.; Jin, C.; Zhao, Y.; Tan, X.; Zhang, Y.; Liu, M.; Yao, C.; Wei, H.; et al. Fe3C@C/C for Catalytic Ozonation of Silicon-Containing Wastewater: Dual Improvement of Silicon Resistance and Catalytic Effect. J. Mater. Sci. Technol. 2023, 136, 65–77. [Google Scholar] [CrossRef]
- Martins, P.M.; Salazar, H.; Aoudjit, L.; Gonçalves, R.; Zioui, D.; Fidalgo-Marijuan, A.; Costa, C.M.; Ferdov, S.; Lanceros-Mendez, S. Crystal Morphology Control of Synthetic Giniite for Enhanced Photo-Fenton Activity against the Emerging Pollutant Metronidazole. Chemosphere 2021, 262, 128300. [Google Scholar] [CrossRef]
- Hassan, F.; Mushtaq, R.; Saghar, S.; Younas, U.; Pervaiz, M.; muteb Aljuwayid, A.; Habila, M.A.; Sillanpaa, M. Fabrication of Graphene-Oxide and Zeolite Loaded Polyvinylidene Fluoride Reverse Osmosis Membrane for Saltwater Remediation. Chemosphere 2022, 307, 136012. [Google Scholar] [CrossRef]
- Tang, Y.; Lin, Y.; Ma, W.; Wang, X. A Review on Microporous Polyvinylidene Fluoride Membranes Fabricated via Thermally Induced Phase Separation for MF/UF Application. J. Membr. Sci. 2021, 639, 119759. [Google Scholar] [CrossRef]
- Ong, M.D.; Vasquez, I.; Alvarez, B.; Cho, D.R.; Williams, M.B.; Vincent, D.; Ali, M.A.; Aich, N.; Pinto, A.H.; Choudhury, M.R. Modification of Cellulose Acetate Microfiltration Membranes Using Graphene Oxide–Polyethyleneimine for Enhanced Dye Rejection. Membranes 2023, 13, 143. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, Y.; Banat, F.; Yousef, A.F.; Bahamon, D.; Vega, L.F.; Hasan, S.W. Surface Modification of Anti-Fouling Novel Cellulose/Graphene Oxide (GO) Nanosheets (NS) Microfiltration Membranes for Seawater Desalination Applications. J. Chem. Technol. Biotechnol. 2020, 95, 1915–1925. [Google Scholar] [CrossRef]
- Salazar, H.; Martins, P.M.; Fernandes, M.M.; Costa, P.; Ferdov, S.; Botelho, G.; Lanceros-Mendez, S. Reusable Nanocomposite-Filters for Arsenite and Arsenate Dual Real Effluents Remediation in an up-Scaled Membrane Reactor. J. Hazard. Mater. 2022, 440, 129756. [Google Scholar] [CrossRef]
- Hailemariam, L.M.; Johnson, A.; Roy, A.; Olanrewaju, K.; Reyntjens, K. Membranes for Produced Water Treatment. In Encyclopedia of Membrane Science and Technology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 1–35. ISBN 9781118522318. [Google Scholar]
- Queirós, J.M.; Salazar, H.; Valverde, A.; Botelho, G.; Fernández de Luis, R.; Teixeira, J.; Martins, P.M.; Lanceros-Mendez, S. Reusable Composite Membranes for Highly Efficient Chromium Removal from Real Water Matrixes. Chemosphere 2022, 307, 135922. [Google Scholar] [CrossRef]
- Zhu, C.; Niu, Q.; Liu, D.; Wu, J.; Hao, Y.; Jiang, B. Integrating Divided Electrolysis-Microfiltration Process for Energy-Efficient Phosphorus Recovery in the Form of Calcium Phosphate. Sep. Purif. Technol. 2022, 301, 121922. [Google Scholar] [CrossRef]
- Moradi, G.; Rahimi, M.; Zinadini, S.; Shamsipur, M.; Babajani, N. Natural Deep Eutectic Solvent Modified Nanofiltration Membranes with Superior Antifouling Properties for Pharmaceutical Wastewater Treatment. Chem. Eng. J. 2022, 448, 137704. [Google Scholar] [CrossRef]
- Zambianchi, M.; Khaliha, S.; Bianchi, A.; Tunioli, F.; Kovtun, A.; Navacchia, M.L.; Salatino, A.; Xia, Z.; Briñas, E.; Vázquez, E.; et al. Graphene Oxide-Polysulfone Hollow Fibers Membranes with Synergic Ultrafiltration and Adsorption for Enhanced Drinking Water Treatment. J. Membr. Sci. 2022, 658, 120707. [Google Scholar] [CrossRef]
- Salazar, H.; Martins, P.M.; Valverde, A.; de Luis, R.; Vilas-Vilela, J.L.; Ferdov, S.; Botelho, G.; Lanceros-Mendez, S. Reusable Nanocomposite Membranes for Highly Efficient Arsenite and Arsenate Dual Removal from Water. Adv. Mater. Interfaces 2022, 9, 2101419. [Google Scholar] [CrossRef]
- Lalia, B.S.; Kochkodan, V.; Hashaikeh, R.; Hilal, N. A Review on Membrane Fabrication: Structure, Properties and Performance Relationship. Desalination 2013, 326, 77–95. [Google Scholar] [CrossRef]
- Ribeiro, C.; Costa, C.M.; Correia, D.M.; Nunes-Pereira, J.; Oliveira, J.; Martins, P.; Gonçalves, R.; Cardoso, V.F.; Lanceros-Méndez, S. Electroactive Poly(Vinylidene Fluoride)-Based Structures for Advanced Applications. Nat. Protoc. 2018, 13, 681–704. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.K.; In, J.; Lee, S. Standard Deviation and Standard Error of the Mean. Korean J. Anesthesiol. 2015, 68, 220–223. [Google Scholar] [CrossRef]
- Polyakov, Y.S.; Zydney, A.L. Ultrafiltration Membrane Performance: Effects of Pore Blockage/Constriction. J. Membr. Sci. 2013, 434, 106–120. [Google Scholar] [CrossRef]
- Aoudjit, L.; Salazar, H.; Zioui, D.; Sebti, A.; Martins, P.M.; Lanceros-Mendez, S. Reusable Ag@TiO2-Based Photocatalytic Nanocomposite Membranes for Solar Degradation of Contaminants of Emerging Concern. Polymers 2021, 13, 3718. [Google Scholar] [CrossRef]
- Leng, X.; Yang, M.; Li, C.; Arifeen, W.U.; Ko, T.J. High-Performance Separator for Lithium-Ion Battery Based on Dual-Hybridizing of Materials and Processes. Chem. Eng. J. 2022, 433, 133773. [Google Scholar] [CrossRef]
- Li, Y.; Huang, S.; Zhou, S.; Fane, A.G.; Zhang, Y.; Zhao, S. Enhancing Water Permeability and Fouling Resistance of Polyvinylidene Fluoride Membranes with Carboxylated Nanodiamonds. J. Membr. Sci. 2018, 556, 154–163. [Google Scholar] [CrossRef]
- Goel, J.; Kadirvelu, K.; Rajagopal, C. Competitive Sorption of Cu(II), Pb(II) and Hg(II) Ions from Aqueous Solution Using Coconut Shell-Based Activated Carbon. Adsorpt. Sci. Technol. 2004, 22, 257–273. [Google Scholar] [CrossRef]
- Zakmout, A.; Sadi, F.; Portugal, C.A.M.; Crespo, J.G.; Velizarov, S. Tannery Effluent Treatment by Nanofiltration, Reverse Osmosis and Chitosan Modified Membranes. Membranes 2020, 10, 378. [Google Scholar] [CrossRef]
- Es-Said, A.; Nafai, H.; Hamdaoui, L.E.; Bouhaouss, A.; Bchitou, R. Adsorptivity and Selectivity of Heavy Metals Cd(Ii), Cu(Ii), and Zn(Ii) toward Phosphogypsum. Desalination Water Treat. 2020, 197, 291–299. [Google Scholar] [CrossRef]
- Abba, M.U.; Man, H.C.; Azis, R.S.; Isma Idris, A.; Hazwan Hamzah, M.; Yunos, K.F.; Katibi, K.K. Novel PVDF-PVP Hollow Fiber Membrane Augmented with TiO2 Nanoparticles: Preparation, Characterization and Application for Copper Removal from Leachate. Nanomaterials 2021, 11, 399. [Google Scholar] [CrossRef] [PubMed]
- Stefanowicz, A.M.; Kapusta, P.; Zubek, S.; Stanek, M.; Woch, M.W. Soil Organic Matter Prevails over Heavy Metal Pollution and Vegetation as a Factor Shaping Soil Microbial Communities at Historical Zn–Pb Mining Sites. Chemosphere 2020, 240, 124922. [Google Scholar] [CrossRef] [PubMed]
- Engel, M.; Lezama Pacheco, J.S.; Noël, V.; Boye, K.; Fendorf, S. Organic Compounds Alter the Preference and Rates of Heavy Metal Adsorption on Ferrihydrite. Sci. Total Environ. 2021, 750, 141485. [Google Scholar] [CrossRef] [PubMed]



| Parameter | Before Treatment | After Treatment | MCL [31] |
|---|---|---|---|
| pH | 7.9 | 7.2 ± 0.2 | 6.5–8.5 |
| Conductivity (mS cm−1) | 46.7 | 23.5 ± 1.1 | 25 |
| Salinity (mg L−1) | 312 | 190 ± 6 | 250 |
| TSS * (mg L−1) | 79.8 | 30.0 ± 2.1 | 40 |
| TDS * (g L−1) | 23.0 | 18.8 ± 1.5 | 5 |
| TAC * (°F) | 450 | 110 ± 6 | - |
| TH * (°F) | 9500 | 136 ± 10 | - |
| Ca2+ (mg L−1) | 561 | 200 ± 12 | 300 |
| Cl− (g L−1) | 56.7 | 15.3 ± 1.7 | 5 |
| Zn2+ (mg L−1) | 0.33 | 0.30 ± 0.06 | 3 |
| Cu2+ (mg L−1) | 0.15 | 0.12 ± 0.03 | 0.5 |
| Ni2+ (mg L−1) | 0.92 | 0.55 ± 0.10 | 0.5 |
| Cd2+ (mg L−1) | 0.17 | 0.07 ± 0.02 | 0.2 |
| Pb2+ (mg L−1) | 1.32 | 0.57 ± 0.11 | 0.5 |
| RSD (%) | pH | Conductivity | Salinity | TSS | TDS | TAC | TH |
| 2.7 | 4.7 | 3.1 | 7.0 | 8.0 | 5.5 | 7.3 | |
| RSD (%) | Ca2+ | Cl− | Zn2+ | Cu2+ | Ni2+ | Cd2+ | Pb2+ |
| 6.0 | 11 | 20 | 25 | 18 | 29 | 19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zioui, D.; Martins, P.M.; Aoudjit, L.; Salazar, H.; Lanceros-Méndez, S. Wastewater Treatment of Real Effluents by Microfiltration Using Poly(vinylidene fluoride–hexafluoropropylene) Membranes. Polymers 2023, 15, 1143. https://doi.org/10.3390/polym15051143
Zioui D, Martins PM, Aoudjit L, Salazar H, Lanceros-Méndez S. Wastewater Treatment of Real Effluents by Microfiltration Using Poly(vinylidene fluoride–hexafluoropropylene) Membranes. Polymers. 2023; 15(5):1143. https://doi.org/10.3390/polym15051143
Chicago/Turabian StyleZioui, Djamila, Pedro Manuel Martins, Lamine Aoudjit, Hugo Salazar, and Senentxu Lanceros-Méndez. 2023. "Wastewater Treatment of Real Effluents by Microfiltration Using Poly(vinylidene fluoride–hexafluoropropylene) Membranes" Polymers 15, no. 5: 1143. https://doi.org/10.3390/polym15051143