Low-Temperature Curable Negative-Tone Photosensitive Polyimides: Structure and Properties
Abstract
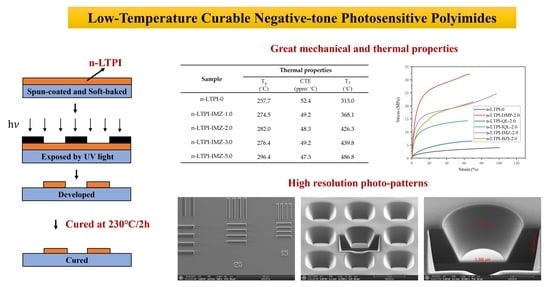
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of pc-PAE Resin
2.3. Preparation of Low-Temperature Curable Photosensitive Polyimides (n-LTPIs)
2.4. Measurements
2.5. Imidization Degree
2.6. Photo-Patterning of n-LTPIs
3. Results and Discussion
3.1. Synthesis and Characterization
3.2. Photo-Patterning Performance
3.3. Low Temperature Imidization
3.4. Mechanical Properties
3.5. Thermal and Electrical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ahne, H.R.; Leuschner, R.; Rubner, R. Recent advances in photosensitive polyimides. Polym. Adv. Technol. 2010, 4, 217–233. [Google Scholar] [CrossRef]
- Seino, H.; Haba, O.; Ueda, M.; Mochizuki, A. Photosensitive polyimide-precursor based on polyisoimide: Dimensionally stable polyimide with a low dielectric constant. Polymer 1999, 40, 551–558. [Google Scholar] [CrossRef]
- Higashihara, T.; Saito, Y.; Mizoguchi, K.; Ueda, M. Recent progress in negative-working photosensitive and thermally stable polymers. React. Funct. Polym. 2013, 73, 303–315. [Google Scholar] [CrossRef]
- Fukukawa, K.-I.; Ueda, M. Recent Progress of Photosensitive Polyimides. Polym. J. 2008, 40, 281–296. [Google Scholar] [CrossRef]
- Rubner, R. Photoreactive polymers for electronics. Adv. Mater. 1990, 2, 452–457. [Google Scholar] [CrossRef]
- Yoda, N.; Hiramoto, H. New Photosensitive High Temperature Polymers for Electronic Applications. J. Macromol. Sci. Part A Chem. 1984, 21, 1641–1663. [Google Scholar] [CrossRef]
- Hou, H.Q.; Jiang, J.G.; Ding, M.X. Ester-type precursor of polyimide and photosensitivity. Eur. Polym. J. 1999, 35, 1993–2000. [Google Scholar] [CrossRef]
- Volksen, W.; Yoon, D.Y.; Hedrick, J.L.; Hofer, D. Chemistry and Characterization Of Polyimides Derived from Poly(Amic Alkyl Esters). MRS Online Proc. Libr. 2011, 227, 23. [Google Scholar] [CrossRef]
- Windrich, F.; Malanin, M.; Eichhorn, K.J.; Voit, B.; Lang, K.D. Low-Temperature Photosensitive Polyimide Processing for Use in 3D Integration Technologies. MRS Online Proc. Libr. 2014, 1692, Mrss14-1692-cc06-02. [Google Scholar] [CrossRef]
- Jin, X.Z.; Ishii, H. A novel positive-type photosensitive polyimide based on soluble block copolyimide showing low dielectric constant with a low-temperature curing process. J. Appl. Polym. Sci. 2010, 100, 4240–4246. [Google Scholar] [CrossRef]
- Yuba, T.; Suwa, M.; Fujita, Y.; Tomikawa, M.; Ohbayashi, G. A Novel Positive Working Photosensitive Polyimide for Wafer-level CSP Packages. J. Photopolym. Sci. Technol. 2002, 15, 201–203. [Google Scholar] [CrossRef]
- Metz, S.; Jiguet, S.; Bertsch, A.; Renaud, P. Polyimide and SU-8 microfluidic devices manufactured by heat-depolymerizable sacrificial material technique. Lab Chip 2004, 4, 114–120. [Google Scholar] [CrossRef]
- Stoffel, N.C.; Kramer, E.J.; Volksen, W.; Russell, T.P. Solvent and isomer effects on the imidization of pyromellitic dianhydride-oxydianiline-based poly(amic ethyl ester)s. Polymer 1993, 34, 4524–4530. [Google Scholar] [CrossRef]
- Echigo, Y.; Iwaya, Y.; Tomioka, I.; Yamada, H. Solvent Effects in Thermal Curing of Poly(4,4′-oxybis(phenylenepyromellitamic acid)). Macromolecules 1995, 28, 4861–4865. [Google Scholar] [CrossRef]
- Shin, T.J.; Lee, B.; Youn, H.S.; Lee, K.-B.; Ree, M. Time-Resolved Synchrotron X-ray Diffraction and Infrared Spectroscopic Studies of Imidization and Structural Evolution in a Microscaled Film of PMDA-3,4′-ODA Poly(amic acid). Langmuir 2001, 17, 7842–7850. [Google Scholar] [CrossRef]
- Chen, X.J.; Yang, J.; Zhao, J. The effect of solvent to the kinetics of imidization of poly(amic acid). Polymer 2018, 143, 46–51. [Google Scholar] [CrossRef]
- Matsuoka, Y.; Yokota, K.; Ogitani, S.; Ikeda, A.; Takahashi, H.; Ai, H. Ester-type photosensitive polyimide precursor with low thermal expansion coefficient. Polym. Eng. Sci. 1992, 32, 1618–1622. [Google Scholar] [CrossRef]
- Kotera, M.; Samyul, B.; Araie, K.; Sugioka, Y.; Nishino, T.; Maji, S.; Noda, M.; Senoo, K.; Koganezawa, T.; Hirosawa, I. Microstructures of BPDA-PPD polyimide thin films with different thicknesses. Polymer 2013, 54, 2435–2439. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y.; Yin, Y.; Li, Y.; Li, J.; Sun, D.; Lu, J.; Zhang, G.; Sun, R. A comprehensive study of pyrazine-contained and low-temperature curable polyimide. Polymer 2021, 228, 123963. [Google Scholar] [CrossRef]
- Brekner, M.-J.; Feger, C. Curing studies of a polyimide precursor. J. Polym. Sci. Part A Polym. Chem. 1987, 25, 2005–2020. [Google Scholar] [CrossRef]
- Shin, T.J.; Ree, M. In Situ Infrared Spectroscopy Study on Imidization Reaction and Imidization-induced Refractive Index and Thickness Variations in Microscale Thin Films of a Poly(amic ester). Langmuir 2005, 21, 6081–6085. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.; Guerra, G.; Williams, D.J.; Karasz, F.E.; MacKnight, W.J. Catalytic activity of benzimidazole in the imidization of polyamic acids. J. Appl. Polym. Sci. 2010, 36, 243–248. [Google Scholar] [CrossRef]
- Yoon, J.-Y.; Jeong, S.; Lee, S.S.; Kim, Y.H.; Ka, J.-W.; Yi, M.H.; Jang, K.-S. Enhanced Performance of Solution-Processed Organic Thin-Film Transistors with a Low-Temperature-Annealed Alumina Interlayer between the Polyimide Gate Insulator and the Semiconductor. ACS Appl. Mater. Interfaces 2013, 5, 5149–5155. [Google Scholar] [CrossRef]
- Ahn, T.; Choi, Y.; Jung, H.M.; Yi, M. Fully aromatic polyimide gate insulators with low temperature processability for pentacene organic thin-film transistors. Org. Electron. 2009, 10, 12–17. [Google Scholar] [CrossRef]
- Huang, C.; Li, J.; Sun, D.; Xuan, R.; Sui, Y.; Li, T.; Shang, L.; Zhang, G.; Sun, R.; Wong, C.P. Comprehensive properties study of low-temperature imidized polyimide with curing accelerators. J. Mater. Chem. C 2020, 8, 14886–14894. [Google Scholar] [CrossRef]
- Sui, Y.; Li, J.; Wang, T.; Sun, D.; Huang, C.; Zhang, F.; Shan, L.; Niu, F.; Zhang, G.; Sun, R. Low temperature curing polyimides with covalent-boned 5-aminobenzimidazole. Polymer 2021, 218, 123514. [Google Scholar] [CrossRef]
- Unsal, E.; Cakmak, M. Real-Time Characterization of Physical Changes in Polyimide Film Formation: From Casting to Imidization. Macromolecules 2013, 46, 8616–8627. [Google Scholar] [CrossRef]
- Oba, M. Effect of curing accelerators on thermal imidization of polyamic acids at low temperature. J. Polym. Sci. Part A Polym. Chem. 1996, 34, 651–658. [Google Scholar] [CrossRef]
- Nguyen, L.T.T.; Nguyen, H.N.; La, T.H.T. Synthesis and characterization of a photosensitive polyimide precursor and its photocuring behavior for lithography applications. Opt. Mater. 2007, 29, 610–618. [Google Scholar] [CrossRef]
- Li, W.S.; Shen, Z.X.; Zheng, J.Z.; Tang, S.H. FT-IR Study of the Imidization Process of Photosensitive Polyimide PMDA/ODA. Appl. Spectrosc. 1998, 52, 985–989. [Google Scholar] [CrossRef]
- Fu, M.-C.; Higashihara, T.; Ueda, M. Recent progress in thermally stable and photosensitive polymers. Polym. J. 2018, 50, 57–76. [Google Scholar] [CrossRef]
- Windrich, F.; Kappert, E.J.; Malanin, M.; Eichhorn, K.-J.; Häuβler, L.; Benes, N.E.; Voit, B. In-situ imidization analysis in microscale thin films of an ester-type photosensitive polyimide for microelectronic packaging applications. Eur. Polym. J. 2016, 84, 279–291. [Google Scholar] [CrossRef]
- Zhai, Y.; Yang, Q.; Zhu, R.; Gu, Y. The study on imidization degree of polyamic acid in solution and ordering degree of its polyimide film. J. Mater. Sci. 2008, 43, 338–344. [Google Scholar] [CrossRef]











| Lithography Process | n-LTPI-0 | n-LTPI- DMP-2.0 | n-LTPI- QL-2.0 | n-LTPI- IQL-2.0 | n-LTPI- IMZ-2.0 | n-LTPI- BZI-2.0 |
|---|---|---|---|---|---|---|
| Film thickness after soft-bake/μm | 9.8 | 9.9 | 9.8 | 10.0 | 9.8 | 9.9 |
| Exposure energy/mJ | 400~600 | 600~800 | 400~600 | 300~600 | 600~800 | 300~600 |
| Film thickness after Development/μm | 8.9 | 8.2 | 9.0 | 9.0 | 8.4 | 9.0 |
| Film retention rate/% | 90.8 | 82.8 | 91.8 | 90.0 | 85.7 | 90.9 |
| Cured film thickness/μm | 4.8 | 4.9 | 5.0 | 4.9 | 4.7 | 4.4 |
| Resolution (Via)/μm | 10 | 6 | 8 | 8 | 5 | 8 |
| Sample | ID (%) | Elongation (%) | Tensile Strength (MPa) | Modulus (GPa) |
|---|---|---|---|---|
| n-LTPI-0 | 95.1 | 59.1 | 180.7 | 3.7 |
| n-LTPI-DMP-2.0 | 98.0 | 45.2 | 178.1 | 3.8 |
| n-LTPI-QL-2.0 | 98.1 | 19.2 | 113.4 | 3.9 |
| n-LTPI-IQL-2.0 | 98.3 | 21.3 | 121.2 | 4.0 |
| n-LTPI-IMZ-2.0 | 100.0 | 59.2 | 189.0 | 3.7 |
| n-LTPI-BZI-2.0 | 97.1 | 42.0 | 165.3 | 3.7 |
| Sample | ID (%) | Elongation (%) | Tensile Strength (MPa) | Modulus (GPa) |
|---|---|---|---|---|
| n-LTPI-0 | 95.1 | 59.1 | 180.7 | 3.7 |
| n-LTPI-IMZ-1.0 | 99.2 | 61.2 | 190.2 | 3.7 |
| n-LTPI-IMZ-2.0 | 100.0 | 59.2 | 189.0 | 3.7 |
| n-LTPI-IMZ-3.0 | 100.0 | 55.4 | 178.9 | 3.8 |
| n-LTPI-IMZ-5.0 | 100.0 | 36.1 | 156.8 | 3.7 |
| Sample | Thermal Properties | Electrical Properties | |||
|---|---|---|---|---|---|
| Tg (°C) | CTE (ppm/°C) | T5 (°C) | Dielectric Constant (10 GHz) | Dielectric Loss Factors (10 GHz) | |
| n-LTPI-0 | 257.7 | 52.4 | 313.0 | 2.9 | 0.0051 |
| n-LTPI-IMZ-1.0 | 274.5 | 49.2 | 368.1 | 2.8 | 0.0058 |
| n-LTPI-IMZ-2.0 | 282.0 | 48.3 | 426.3 | 2.8 | 0.0056 |
| n-LTPI-IMZ-3.0 | 276.4 | 49.2 | 439.8 | 2.9 | 0.0053 |
| n-LTPI-IMZ-5.0 | 296.4 | 47.3 | 486.8 | 2.9 | 0.0048 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, S.-n.; Yuan, L.-l.; Wang, L.-z.; Jia, B.; Ma, J.-x.; Yang, H.-x.; Yang, S.-y. Low-Temperature Curable Negative-Tone Photosensitive Polyimides: Structure and Properties. Polymers 2023, 15, 973. https://doi.org/10.3390/polym15040973
Fan S-n, Yuan L-l, Wang L-z, Jia B, Ma J-x, Yang H-x, Yang S-y. Low-Temperature Curable Negative-Tone Photosensitive Polyimides: Structure and Properties. Polymers. 2023; 15(4):973. https://doi.org/10.3390/polym15040973
Chicago/Turabian StyleFan, Sheng-nan, Li-li Yuan, Li-zhe Wang, Bin Jia, Jia-xin Ma, Hai-xia Yang, and Shi-yong Yang. 2023. "Low-Temperature Curable Negative-Tone Photosensitive Polyimides: Structure and Properties" Polymers 15, no. 4: 973. https://doi.org/10.3390/polym15040973