Studying the Physical and Chemical Properties of Polydimethylsiloxane Matrix Reinforced by Nanostructured TiO2 Supported on Mesoporous Silica
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. TiO2/MCM/PDMS Preparation
2.3. Characterization Methods
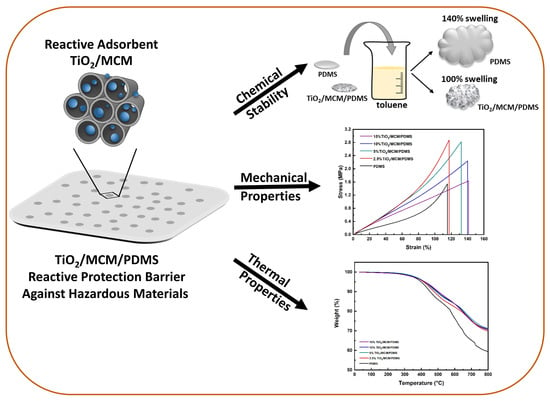
3. Results and Discussion
3.1. Chemical Structure and Composition
3.2. Mechanical Properties of the TiO2/MCM/PDMS Composite Materials
3.3. Thermal Properties
3.4. Chemical Stability
3.5. Surface Hydrophilicity/Hydrophobicity
3.6. Optical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waldbaur, A.; Rapp, H.; Länge, K.; Rapp, B.E. Let There Be Chip—Towards Rapid Prototyping of Microfluidic Devices: One-Step Manufacturing Processes. Anal. Methods 2011, 3, 2681–2716. [Google Scholar] [CrossRef]
- Ariati, R.; Sales, F.; Souza, A.; Lima, R.A.; Ribeiro, J. Polydimethylsiloxane Composites Characterization and Its Applications: A Review. Polymers 2021, 13, 4258. [Google Scholar] [CrossRef]
- Mukhopadhyay, R. When PDMS Isn’t the Best. Anal. Chem. 2007, 79, 3248–3253. [Google Scholar] [CrossRef]
- Eduok, U.; Faye, O.; Szpunar, J. Recent Developments and Applications of Protective Silicone Coatings: A Review of PDMS Functional Materials. Prog. Org. Coat. 2017, 111, 124–163. [Google Scholar] [CrossRef]
- RajM, K.; Chakraborty, S. PDMS Microfluidics: A Mini Review. J. Appl. Polym. Sci. 2020, 137, 48958. [Google Scholar] [CrossRef]
- Johnston, I.D.; Tracey, M.C.; Davis, J.B.; Tan, C.K.L. Micro Throttle Pump Employing Displacement Amplification in an Elastomeric Substrate. J. Micromechanics Microengineering 2005, 15, 1831–1839. [Google Scholar] [CrossRef]
- Wu, X.; Kim, S.H.; Ji, C.H.; Allen, M.G. A Solid Hydraulically Amplified Piezoelectric Microvalve. J. Micromechanics Microengineering 2011, 21, 095003. [Google Scholar] [CrossRef] [Green Version]
- Scholes, C.A.; Stevens, G.W.; Kentish, S.E. Membrane Gas Separation Applications in Natural Gas Processing. Fuel 2012, 96, 15–28. [Google Scholar] [CrossRef]
- Victor, A.; Ribeiro, J.; Araújo, F.S. Study of PDMS Characterization and Its Applications in Biomedicine: A Review. J. Mech. Eng. Biomech. 2019, 4, 1–9. [Google Scholar] [CrossRef]
- Ramji, R.; Khan, N.T.; Muñoz-Rojas, A.; Miller-Jensen, K. “Pop-Slide” Patterning: Rapid Fabrication of Microstructured PDMS Gasket Slides for Biological Applications. RSC Adv. 2015, 5, 66294–66300. [Google Scholar] [CrossRef]
- Rotter, H.; Osovsky, R.; Hafif, N.; Pevzner, A.; Nir, I. Nanostructured TiO2/MCM-41-Functionalized PDMS as a Reactive Protective Barrier against Chemical Warfare Agents via Adsorption and Catalyzed Degradation. Ind. Eng. Chem. Res. 2022, 61, 10860–10869. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, A.; Han, S.S.; Park, S.-S. RTV Silicone Rubber Composites Reinforced with Carbon Nanotubes, Titanium-Di-Oxide and Their Hybrid: Mechanical and Piezoelectric Actuation Performance. Nano Mater. Sci. 2021, 3, 233–240. [Google Scholar] [CrossRef]
- Wu, C.-L.; Lin, H.-C.; Hsu, J.-S.; Yip, M.-C.; Fang, W. Static and Dynamic Mechanical Properties of Polydimethylsiloxane/Carbon Nanotube Nanocomposites. Thin Solid Film. 2009, 517, 4895–4901. [Google Scholar] [CrossRef]
- Burns, G.T.; Taylor, R.B.; Xu, Y.; Zangvil, A.; Zank, G.A. High-Temperature Chemistry of the Conversion of Siloxanes to Silicon Carbide. Chem. Mater. 1992, 4, 1313–1323. [Google Scholar] [CrossRef]
- Zhao, W.; Li, T.; Li, Y.; O’Brien, D.J.; Terrones, M.; Wei, B.; Suhr, J.; Lucas Lu, X. Mechanical Properties of Nanocomposites Reinforced by Carbon Nanotube Sponges. J. Mater. 2018, 4, 157–164. [Google Scholar] [CrossRef]
- Vlassov, S.; Oras, S.; Timusk, M.; Zadin, V.; Tiirats, T.; Sosnin, I.M.; Lõhmus, R.; Linarts, A.; Kyritsakis, A.; Dorogin, L.M. Thermal, Mechanical, and Acoustic Properties of Polydimethylsiloxane Filled with Hollow Glass Microspheres. Materials 2022, 15, 1652. [Google Scholar] [CrossRef]
- Tavares, M.T.S.; Santos, A.S.F.; Santos, I.M.G.; Silva, M.R.S.; Bomio, M.R.D.; Longo, E.; Paskocimas, C.A.; Motta, F.V. TiO2/PDMS Nanocomposites for Use on Self-Cleaning Surfaces. Surf. Coat. Technol. 2014, 239, 16–19. [Google Scholar] [CrossRef] [Green Version]
- Dalod, A.R.M.; Grendal, O.G.; Blichfeld, A.B.; Furtula, V.; Pérez, J.; Henriksen, L.; Grande, T.; Einarsrud, M.A. Structure and Optical Properties of Titania-PDMS Hybrid Nanocomposites Prepared by In Situ Non-Aqueous Synthesis. Nanomaterials 2017, 7, 460. [Google Scholar] [CrossRef] [Green Version]
- Lamberti, A. Microfluidic Photocatalytic Device Exploiting PDMS/TiO2 Nanocomposite. Appl. Surf. Sci. 2015, 335, 50–54. [Google Scholar] [CrossRef]
- Silva, V.P.; Paschoalino, M.P.; Gonçalves, M.C.; Felisberti, M.I.; Jardim, W.F.; Yoshida, I.V.P. Silicone Rubbers Filled with TiO2: Characterization and Photocatalytic Activity. Mater. Chem. Phys. 2009, 113, 395–400. [Google Scholar] [CrossRef]
- Chen, D.; Liu, Y.; Zhang, H.; Zhou, Y.; Huang, C.; Xiong, C. Influence of Polyhedral Oligomeric Silsesquioxanes (POSS) on Thermal and Mechanical Properties of Polydimethylsiloxane (PDMS) Composites Filled with Fumed Silica. J. Inorg. Organomet. Polym. Mater. 2013, 23, 1375–1382. [Google Scholar] [CrossRef]
- Suzuki, N.; Kiba, S.; Kamachi, Y.; Miyamoto, N.; Yamauchi, Y. Unusual Reinforcement of Silicone Rubber Compounds Containing Mesoporous Silica Particles as Inorganic Fillers. Phys. Chem. Chem. Phys. 2012, 14, 3400. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Kiba, S.; Kamachi, Y.; Miyamoto, N.; Yamauchi, Y. Mesoporous Silica as Smart Inorganic Filler: Preparation of Robust Silicone Rubber with Low Thermal Expansion Property. J. Mater. Chem. 2011, 21, 5338. [Google Scholar] [CrossRef]
- Suzuki, N.; Kamachi, Y.; Takai, K.; Kiba, S.; Sakka, Y.; Miyamoto, N.; Yamauchi, Y. Effective Use of Mesoporous Silica Filler: Comparative Study on Thermal Stability and Transparency of Silicone Rubbers Loaded with Various Kinds of Silica Particles. Eur. J. Inorg. Chem. 2014, 2014, 2773–2778. [Google Scholar] [CrossRef]
- Liu, J.; Zong, G.; He, L.; Zhang, Y.; Liu, C.; Wang, L. Effects of Fumed and Mesoporous Silica Nanoparticles on the Properties of Sylgard 184 Polydimethylsiloxane. Micromachines 2015, 6, 855–864. [Google Scholar] [CrossRef] [Green Version]
- Cui, X.; Zhu, G.; Pan, Y.; Shao, Q.; Zhao, C.; Dong, M.; Zhang, Y.; Guo, Z. Polydimethylsiloxane-Titania Nanocomposite Coating: Fabrication and Corrosion Resistance. Polymer 2018, 138, 203–210. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, H.; Liu, Q.; Wang, S.; Yan, S. Effect of 3-Mercaptopropyltriethoxysilane Modified Illite on the Reinforcement of SBR. Materials 2022, 15, 3459. [Google Scholar] [CrossRef]
- Efimenko, K.; Wallace, W.E.; Genzer, J. Surface Modification of Sylgard-184 Poly (Dimethyl Siloxane) Networks by Ultraviolet and Ultraviolet/Ozone Treatment. J. Colloid Interface Sci. 2002, 254, 306–315. [Google Scholar] [CrossRef]
- Téllez, L.; Rubio, J.; Rubio, F.; Morales, E.; Oteo, J.L. FT-IR Study of the Hydrolysis and Polymerization of Tetraethyl Orthosilicate and Polydimethyl Siloxane in the Presence of Tetrabutyl Orthotitanate. Spectrosc. Lett. 2004, 37, 11–31. [Google Scholar] [CrossRef]
- Gupta, N.S.; Lee, K.S.; Labouriau, A. Tuning Thermal and Mechanical Properties of Polydimethylsiloxane with Carbon Fibers. Polymers 2021, 13, 1141. [Google Scholar] [CrossRef]
- Stafie, N.; Stamatialis, D.F.; Wessling, M. Effect of PDMS Cross-Linking Degree on the Permeation Performance of PAN/PDMS Composite Nanofiltration Membranes. Sep. Purif. Technol. 2005, 45, 220–231. [Google Scholar] [CrossRef]
- Berean, K.; Ou, J.Z.; Nour, M.; Latham, K.; McSweeney, C.; Paull, D.; Halim, A.; Kentish, S.; Doherty, C.M.; Hill, A.J.; et al. The Effect of Crosslinking Temperature on the Permeability of PDMS Membranes: Evidence of Extraordinary CO2 and CH4 Gas Permeation. Sep. Purif. Technol. 2014, 122, 96–104. [Google Scholar] [CrossRef]
- Nour, M.; Berean, K.; Griffin, M.J.; Matthews, G.I.; Bhaskaran, M.; Sriram, S.; Kalantar-zadeh, K. Nanocomposite Carbon-PDMS Membranes for Gas Separation. Sens. Actuators B Chem. 2012, 161, 982–988. [Google Scholar] [CrossRef] [Green Version]
- Konku-Asase, Y.; Yaya, A.; Kan-Dapaah, K. Curing Temperature Effects on the Tensile Properties and Hardness of γ−Fe2O3 Reinforced PDMS Nanocomposites. Adv. Mater. Sci. Eng. 2020, 2020, 1–11. [Google Scholar] [CrossRef]
- Beghi, M.; Chiurlo, P.; Costa, L.; Palladino, M.; Pirini, M.F. Structural Investigation of the Silica-Titania Gel/Glass Transition. J. Non Cryst. Solids 1992, 145, 175–179. [Google Scholar] [CrossRef]
- Cai, D.; Neyer, A.; Kuckuk, R.; Heise, H.M. Raman, Mid-Infrared, near-Infrared and Ultraviolet–Visible Spectroscopy of PDMS Silicone Rubber for Characterization of Polymer Optical Waveguide Materials. J. Mol. Struct. 2010, 976, 274–281. [Google Scholar] [CrossRef]
- Bae, S.C.; Lee, H.; Lin, Z.; Granick, S. Chemical Imaging in a Surface Forces Apparatus: Confocal Raman Spectroscopy of Confined Poly(Dimethylsiloxane). Langmuir 2005, 21, 5685–5688. [Google Scholar] [CrossRef]
- Hadjiivanov, K.I.; Panayotov, D.A.; Mihaylov, M.Y.; Ivanova, E.Z.; Chakarova, K.K.; Andonova, S.M.; Drenchev, N.L. Power of Infrared and Raman Spectroscopies to Characterize Metal-Organic Frameworks and Investigate Their Interaction with Guest Molecules. Chem. Rev. 2021, 121, 1286–1424. [Google Scholar] [CrossRef]
- Kusabiraki, K. Infrared Spectra of Vitreous Silica and Sodium Silicates Containing Titanium. J. Non Cryst. Solids 1986, 79, 208–212. [Google Scholar] [CrossRef]
- Ma, W.; Lu, Z.; Zhang, M. Investigation of Structural Transformations in Nanophase Titanium Dioxide by Raman Spectroscopy. Appl. Phys. A Mater. Sci. Process. 1998, 66, 621–627. [Google Scholar] [CrossRef]
- Scannell, G.; Barra, S.; Huang, L. Structure and Properties of Na2O-TiO2-SiO2 Glasses: Role of Na and Ti on Modifying the Silica Network. J. Non Cryst. Solids 2016, 448, 52–61. [Google Scholar] [CrossRef] [Green Version]
- Johnston, I.D.; McCluskey, D.K.; Tan, C.K.L.; Tracey, M.C. Mechanical Characterization of Bulk Sylgard 184 for Microfluidics and Microengineering. J. Micromechanics Microengineering 2014, 24. [Google Scholar] [CrossRef]
- Anoop, V.; Sankaraiah, S.; Chakraborty, S.; Mary, N.L. Enhanced Mechanical, Thermal and Adhesion Properties of Addition Cured Polydimethylsiloxane Nanocomposite Adhesives. Int. J. Adhes. Adhes. 2022, 117, 103177. [Google Scholar] [CrossRef]
- Bosq, N.; Guigo, N.; Persello, J.; Sbirrazzuoli, N. Melt and Glass Crystallization of PDMS and PDMS Silica Nanocomposites. Phys. Chem. Chem. Phys. 2014, 16, 7830–7840. [Google Scholar] [CrossRef]
- Fragiadakis, D.; Pissis, P. Glass Transition and Segmental Dynamics in Poly (Dimethylsiloxane)/Silica Nanocomposites Studied by Various Techniques. J. Non Cryst. Solids 2007, 353, 4344–4352. [Google Scholar] [CrossRef]
- Klonos, P.; Panagopoulou, A.; Bokobza, L.; Kyritsis, A.; Peoglos, V.; Pissis, P. Comparative Studies on Effects of Silica and Titania Nanoparticles on Crystallization and Complex Segmental Dynamics in Poly (Dimethylsiloxane). Polymer 2010, 51, 5490–5499. [Google Scholar] [CrossRef]
- Bershtein, V.A.; Egorova, L.M.; Yakushev, P.N.; Pissis, P.; Sysel, P.; Brozova, L. Molecular Dynamics in Nanostructured Polyimide-Silica Hybrid Materials and Their Thermal Stability. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 1056–1069. [Google Scholar] [CrossRef]
- Corporation., D.C. Information about Dow Corning Silicone Encapsulants. Available online: https://krayden.com/pdf/dow_silicone_encapsulant.pdf (accessed on 24 December 2022).
- Venkatachalam, S.; Hourlier, D. Heat Treatment of Commercial Polydimethylsiloxane PDMS Precursors: Part I. Towards Conversion of Patternable Soft Gels into Hard Ceramics. Ceram. Int. 2019, 45, 6255–6262. [Google Scholar] [CrossRef]
- Heo, B.; Fiola, M.; Yang, J.H.; Koh, A. A Low-Cost, Composite Collagen-PDMS Material for Extended Fluid Retention in the Skin-Interfaced Microfluidic Devices. Colloid Interface Sci. Commun. 2020, 38, 100301. [Google Scholar] [CrossRef]
- Gao, X.; Wachs, I.E. Titania–Silica as Catalysts: Molecular Structural Characteristics and Physico-Chemical Properties. Catal. Today 1999, 51, 233–254. [Google Scholar] [CrossRef]
- Hickman, R.; Walker, E.; Chowdhury, S. TiO2-PDMS Composite Sponge for Adsorption and Solar Mediated Photodegradation of Dye Pollutants. J. Water Process Eng. 2018, 24, 74–82. [Google Scholar] [CrossRef]









| TiO2/MCM/PDMS (wt%) | Tg (℃) | ΔCpn (J/gK) | CTE (ppm/°C) |
|---|---|---|---|
| 0 | −123.1 | 0.305 | 290 |
| 2.5 | −122.3 | 0.232 | 110 |
| 5 | −122.0 | 0.244 | 110 |
| 10 | −123.2 | 0.249 | 150 |
| TiO2/MCM/PDMS (wt%) | T5 (℃) | T10 (℃) | Tmax (℃) | Char Residue (%) |
|---|---|---|---|---|
| 0 | 399 | 453 | 628 | 59.5 |
| 2.5 | 414 | 476 | 676 | 69.9 |
| 5 | 420 | 488 | 679 | 71.2 |
| 10 | 420 | 494 | 681 | 70.6 |
| 15 | 422 | 502 | 682 | 71.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katz, S.; Lachman, N.; Hafif, N.; Rosh, L.; Pevzner, A.; Lybman, A.; Amitay-Rosen, T.; Nir, I.; Rotter, H. Studying the Physical and Chemical Properties of Polydimethylsiloxane Matrix Reinforced by Nanostructured TiO2 Supported on Mesoporous Silica. Polymers 2023, 15, 81. https://doi.org/10.3390/polym15010081
Katz S, Lachman N, Hafif N, Rosh L, Pevzner A, Lybman A, Amitay-Rosen T, Nir I, Rotter H. Studying the Physical and Chemical Properties of Polydimethylsiloxane Matrix Reinforced by Nanostructured TiO2 Supported on Mesoporous Silica. Polymers. 2023; 15(1):81. https://doi.org/10.3390/polym15010081
Chicago/Turabian StyleKatz, Sari, Noa Lachman, Nir Hafif, Lilach Rosh, Alexander Pevzner, Amir Lybman, Tal Amitay-Rosen, Ido Nir, and Hadar Rotter. 2023. "Studying the Physical and Chemical Properties of Polydimethylsiloxane Matrix Reinforced by Nanostructured TiO2 Supported on Mesoporous Silica" Polymers 15, no. 1: 81. https://doi.org/10.3390/polym15010081