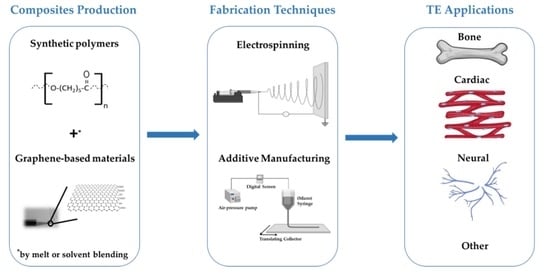
Fabrication of Polymer/Graphene Biocomposites for Tissue Engineering
Abstract
:1. Introduction
2. Polymers
2.1. Poly(ε-Caprolactone)
2.1.1. Synthesis
2.1.2. Biodegradation
2.1.3. Biocompatibility
2.2. Poly(lactic acid)
2.2.1. Synthesis
2.2.2. Biodegradation
2.2.3. Biocompatibility
3. Graphene-Based Materials
3.1. Production
3.2. Physicochemical Properties
3.3. Biodegradation
3.4. Biocompatibility
4. Polymer/GBM Composites
4.1. Electrospinning of Polymer/GBM Composites
4.2. Processing of Polymer/GBM Composites by Additive Manufacturing
5. Overview and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weiser, T.G.; Haynes, A.B.; Molina, G.; Lipsitz, S.R.; Esquivel, M.M.; Uribe-Leitz, T.; Fu, R.; Azad, T.; Chao, T.E.; Berry, W.R.; et al. Size and distribution of the global volume of surgery in 2012. Bull. World Health Organ. 2016, 94, 201F–209F. [Google Scholar] [CrossRef] [PubMed]
- Khan, Y.; Yaszemski, M.J.; Mikos, A.G.; Laurencin, C.T. Tissue Engineering of Bone: Material and Matrix Considerations. JBJS 2008, 90, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Seifu, D.G.; Purnama, A.; Mequanint, K.; Mantovani, D. Small-diameter vascular tissue engineering. Nat. Rev. Cardiol. 2013, 10, 410. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Geetha Bai, R.; Muthoosamy, K.; Manickam, S.; Hilal-Alnaqbi, A. Graphene-based 3D scaffolds in tissue engineering: Fabrication, applications, and future scope in liver tissue engineering. Int. J. Nanomed. 2019, 14, 5753–5783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uhrich, K.E.; Abdelhamid, D. Biodegradable and Bioerodible Polymers for Medical Applications; Elsevier: Amsterdam, The Netherlands, 2016; pp. 63–83. [Google Scholar] [CrossRef]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical applications of biodegradable polymers. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koleske, J.V.; Lundberg, R.D. Lactone polymers. I. Glass transition temperature of poly-ε-caprolactone by means on compatible polymer mixtures. J. Polym. Sci. Part A-2 Polym. Phys. 1969, 7, 795–807. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, L.; Yang, L.; Zhu, F.; Ding, M.; Lin, F.; Wang, Z.; Li, Y. “Click” chemistry in polymeric scaffolds: Bioactive materials for tissue engineering. J. Control. Release 2018, 273, 160–179. [Google Scholar] [CrossRef]
- Tang, L.-C.; Zhao, L.; Guan, L.-Z. 7 Graphene/Polymer Composite Materials: Processing, Properties and Applications. Adv. Compos. Mater. Prop. Appl. 2017, 349–419. [Google Scholar] [CrossRef]
- Henriques, P.C.; Borges, I.; Pinto, A.M.; Magalhães, F.D.; Gonçalves, I.C. Fabrication and antimicrobial performance of surfaces integrating graphene-based materials. Carbon 2018, 132, 709–732. [Google Scholar] [CrossRef]
- Ponnamma, D.; Yin, Y.; Salim, N.; Parameswaranpillai, J.; Thomas, S.; Hameed, N. Recent progress and multifunctional applications of 3D printed graphene nanocomposites. Compos. Part B Eng. 2021, 204, 108493. [Google Scholar] [CrossRef]
- Mesquita-Guimarães, J.; Henriques, B.; Silva, F.S.; Souza, J.C.M.; Novaes De Oliveira, A.P.; Hotza, D.; Do Nascimento, R.M.; Fredel, M.C. Nanostructured Biocompatible Ceramics and Glass-Ceramics; Elsevier: Amsterdam, The Netherlands, 2018; pp. 97–118. [Google Scholar] [CrossRef]
- Li, Y.; Bou-Akl, T. Electrospinning in Tissue Engineering; InTech: London, UK, 2016. [Google Scholar] [CrossRef] [Green Version]
- Mota, C.; Puppi, D.; Chiellini, F.; Chiellini, E. Additive manufacturing techniques for the production of tissue engineering constructs. J. Tissue Eng. Regen. Med. 2015, 9, 174–190. [Google Scholar] [CrossRef] [PubMed]
- Brennan, C.M.; Eichholz, K.F.; Hoey, D.A. The effect of pore size within fibrous scaffolds fabricated using melt electrowriting on human bone marrow stem cell osteogenesis. Biomed. Mater. 2019, 14, 065016. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen–glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbasian, M.; Massoumi, B.; Mohammad-Rezaei, R.; Samadian, H.; Jaymand, M. Scaffolding polymeric biomaterials: Are naturally occurring biological macromolecules more appropriate for tissue engineering? Int. J. Biol. Macromol. 2019, 134, 673–694. [Google Scholar] [CrossRef] [PubMed]
- Chiulan, I.; Frone, A.N.; Brandabur, C.; Panaitescu, D.M. Recent Advances in 3D Printing of Aliphatic Polyesters. Bioengineering 2018, 5, 2. [Google Scholar] [CrossRef] [Green Version]
- Azimi, B.; Nourpanah, P.; Rabiee, M.; Arbab, S. Poly (ε-caprolactone) Fiber: An Overview. J. Eng. Fibers Fabr. 2014, 9, 155892501400900. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef] [Green Version]
- Laranjeira, M.; Domingues, R.M.A.; Costa-Almeida, R.; Reis, R.L.; Gomes, M.E. 3D Mimicry of Native-Tissue-Fiber Architecture Guides Tendon-Derived Cells and Adipose Stem Cells into Artificial Tendon Constructs. Small 2017, 13, 1700689. [Google Scholar] [CrossRef]
- Labet, M.; Thielemans, W. Synthesis of polycaprolactone: A review. Chem. Soc. Rev. 2009, 38, 3484–3504. [Google Scholar] [CrossRef] [PubMed]
- Albertsson, A.-C.; Varma, I.K. Recent Developments in Ring Opening Polymerization of Lactones for Biomedical Applications. Biomacromolecules 2003, 4, 1466–1486. [Google Scholar] [CrossRef] [PubMed]
- Atta, S.; Cohen, J.; Kohn, J.; Gormley, A.J. Ring opening polymerization of ε-caprolactone through water. Polym. Chem. 2021, 12, 159–164. [Google Scholar] [CrossRef]
- Guarino, V.; Gentile, G.; Sorrentino, L.; Ambrosio, L. Polycaprolactone: Synthesis, Properties, and Applications. Encycl. Polym. Sci. Technol. 2017, 1–36. [Google Scholar] [CrossRef]
- Mondal, D.; Griffith, M.; Venkatraman, S.S. Polycaprolactone-based biomaterials for tissue engineering and drug delivery: Current scenario and challenges. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 255–265. [Google Scholar] [CrossRef]
- Siddiqui, N.; Asawa, S.; Birru, B.; Baadhe, R.; Rao, S. PCL-Based Composite Scaffold Matrices for Tissue Engineering Applications. Mol. Biotechnol. 2018, 60, 506–532. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [Green Version]
- Joshi, P.; Madras, G. Degradation of polycaprolactone in supercritical fluids. Polym. Degrad. Stab. 2008, 93, 1901–1908. [Google Scholar] [CrossRef]
- Manoukian, O.S.; Sardashti, N.; Stedman, T.; Gailiunas, K.; Ojha, A.; Penalosa, A.; Mancuso, C.; Hobert, M.; Kumbar, S.G. Biomaterials for Tissue Engineering and Regenerative Medicine. In Encyclopedia of Biomedical Engineering; Narayan, R., Ed.; Elsevier: Oxford, UK, 2019; pp. 462–482. [Google Scholar] [CrossRef]
- Samavedi, S.; Olsen Horton, C.; Guelcher, S.A.; Goldstein, A.S.; Whittington, A.R. Fabrication of a model continuously graded co-electrospun mesh for regeneration of the ligament–bone interface. Acta Biomater. 2011, 7, 4131–4138. [Google Scholar] [CrossRef]
- Serrano, M.C.; Pagani, R.; Vallet-Regí, M.; Peña, J.; Rámila, A.; Izquierdo, I.; Portolés, M.T. In vitro biocompatibility assessment of poly(ε-caprolactone) films using L929 mouse fibroblasts. Biomaterials 2004, 25, 5603–5611. [Google Scholar] [CrossRef]
- Corden, T.J.; Jones, I.A.; Rudd, C.D.; Christian, P.; Downes, S.; McDougall, K.E. Physical and biocompatibility properties of poly-ε-caprolactone produced using in situ polymerisation: A novel manufacturing technique for long-fibre composite materials. Biomaterials 2000, 21, 713–724. [Google Scholar] [CrossRef]
- Lopes, M.S.; Jardini, A.L.; Filho, R.M. Poly (Lactic Acid) Production for Tissue Engineering Applications. Procedia Eng. 2012, 42, 1402–1413. [Google Scholar] [CrossRef] [Green Version]
- Zhong, H.; Chan, G.; Hu, Y.; Hu, H.; Ouyang, D. A Comprehensive Map of FDA-Approved Pharmaceutical Products. Pharmaceutics 2018, 10, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jimenez, A.; Peltzer, M.; Ruseckaite, R. Poly(lactic acid) Science and Technology. Processing, Properties, Additives and Applications; Royal Society of Chemistry: Cambridge, UK, 2014. [Google Scholar]
- Ramot, Y.; Haim-Zada, M.; Domb, A.J.; Nyska, A. Biocompatibility and safety of PLA and its copolymers. Adv. Drug Deliv. Rev. 2016, 107, 153–162. [Google Scholar] [CrossRef]
- Singhvi, M.; Gokhale, D. Biomass to biodegradable polymer (PLA). RSC Adv. 2013, 3, 13558–13568. [Google Scholar] [CrossRef]
- Lasprilla, A.J.R.; Martinez, G.A.R.; Lunelli, B.H.; Jardini, A.L.; Filho, R.M. Poly-lactic acid synthesis for application in biomedical devices—A review. Biotechnol. Adv. 2012, 30, 321–328. [Google Scholar] [CrossRef]
- Casalini, T.; Rossi, F.; Castrovinci, A.; Perale, G. A Perspective on Polylactic Acid-Based Polymers Use for Nanoparticles Synthesis and Applications. Front. Bioeng. Biotechnol. 2019, 7, 259. [Google Scholar] [CrossRef]
- Li, G.; Zhao, M.; Xu, F.; Yang, B.; Li, X.; Meng, X.; Teng, L.; Sun, F.; Li, Y. Synthesis and Biological Application of Polylactic Acid. Molecules 2020, 25, 5023. [Google Scholar] [CrossRef]
- Abdelwahab, M.A.; Flynn, A.; Chiou, B.-S.; Imam, S.; Orts, W.; Chiellini, E. Thermal, mechanical and morphological characterization of plasticized PLA–PHB blends. Polym. Degrad. Stab. 2012, 97, 1822–1828. [Google Scholar] [CrossRef]
- Zaaba, N.F.; Jaafar, M. A review on degradation mechanisms of polylactic acid: Hydrolytic, photodegradative, microbial, and enzymatic degradation. Polym. Eng. Sci. 2020, 60, 2061–2075. [Google Scholar] [CrossRef]
- Mehta, R.; Kumar, V.; Bhunia, H.; Upadhyay, S.N. Synthesis of Poly(Lactic Acid): A Review. J. Macromol. Sci. Part C 2005, 45, 325–349. [Google Scholar] [CrossRef]
- Garlotta, D.J. A literature review of poly (lactic acid). Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Avérous, L.; Pollet, E. Biodegradable Polymers; Springer: London, UK, 2012; pp. 13–39. [Google Scholar] [CrossRef]
- Elmowafy, E.M.; Tiboni, M.; Soliman, M.E. Biocompatibility, biodegradation and biomedical applications of poly(lactic acid)/poly(lactic-co-glycolic acid) micro and nanoparticles. J. Pharm. Investig. 2019, 49, 347–380. [Google Scholar] [CrossRef]
- Vieira, A.C.; Vieira, J.C.; Ferra, J.M.; Magalhães, F.D.; Guedes, R.M.; Marques, A.T. Mechanical study of PLA–PCL fibers during in vitro degradation. J. Mech. Behav. Biomed. Mater. 2011, 4, 451–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schakenraad, J.M.; Hardonk, M.J.; Feijen, J.; Molenaar, I.; Nieuwenhuis, P. Enzymatic activity toward poly(L-lactic acid) implants. J. Biomed. Mater. Res. 1990, 24, 529–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correia Pinto, V.; Costa-Almeida, R.; Rodrigues, I.; Guardão, L.; Soares, R.; Miranda Guedes, R. Exploring the in vitro and in vivo compatibility of PLA, PLA/GNP and PLA/CNT-COOH biodegradable nanocomposites: Prospects for tendon and ligament applications. J. Biomed. Mater. Res. Part A 2017, 105, 2182–2190. [Google Scholar] [CrossRef]
- Da Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Roitman, J.; Schroeder, A. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. 2018, 340, 9–14. [Google Scholar] [CrossRef]
- Parks, A.C.; Sung, K.; Wu, B.M. A three-dimensional in vitro model to quantify inflammatory response to biomaterials. Acta Biomater. 2014, 10, 4742–4749. [Google Scholar] [CrossRef]
- Bos, R.R.M.; Rozema, F.B.; Boering, G.; Nijenhius, A.J.; Pennings, A.J.; Verwey, A.B.; Nieuwenhuis, P.; Jansen, H.W.B. Degradation of and tissue reaction to biodegradable poly(L-lactide) for use as internal fixation of fractures: A study in rats. Biomaterials 1991, 12, 32–36. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and Prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [Green Version]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. In Nanoscience and Technology: A Collection of Reviews from Nature Journals; World Scientific: Singapore, 2010; pp. 11–19. [Google Scholar]
- Ren, W.; Cheng, H.-M. The global growth of graphene. Nat. Nanotechnol. 2014, 9, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Ruoff, R.S.; Bielawski, C.W. From Conception to Realization: An Historial Account of Graphene and Some Perspectives for Its Future. Angew. Chem. Int. Ed. 2010, 49, 9336–9344. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.S.; Coleman, K.S. Graphene synthesis: Relationship to applications. Nanoscale 2013, 5, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Pinto, A.M.; Cabral, J.; Tanaka, D.A.P.; Mendes, A.M.; Magalhães, F.D. Effect of incorporation of graphene oxide and graphene nanoplatelets on mechanical and gas permeability properties of poly(lactic acid) films. Polym. Int. 2013, 62, 33–40. [Google Scholar] [CrossRef]
- Chua, C.K.; Pumera, M. Chemical reduction of graphene oxide: A synthetic chemistry viewpoint. Chem. Soc. Rev. 2014, 43, 291–312. [Google Scholar] [CrossRef]
- Chen, W.; Yan, L.; Bangal, P. Preparation of graphene by the rapid and mild thermal reduction of graphene oxide induced by microwaves. Carbon 2010, 48, 1146–1152. [Google Scholar] [CrossRef]
- Jaiswal, K.K.; Sudhakar, S.; Ramaswamy, A.P. ‘Graphene’–World’s Thinnest Material for Revolutionizing Applications. Everymans Sci. 2018, 53, 219–223. [Google Scholar]
- Bera, B. A review on polymer, graphene and carbon nanotube: Properties, synthesis and applications. Imp. J. Interdiscip. Res. IJIR 2017, 3, 61–70. [Google Scholar]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Memarian, F.; Fereidoon, A.; Darvish Ganji, M. Graphene Young’s modulus: Molecular mechanics and DFT treatments. Superlattices Microstruct. 2015, 85, 348–356. [Google Scholar] [CrossRef]
- Sang, M.; Shin, J.; Kim, K.; Yu, K. Electronic and Thermal Properties of Graphene and Recent Advances in Graphene Based Electronics Applications. Nanomaterials 2019, 9, 374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, C.; Zhang, J.; Chen, X.; Weng, G.J. Calculating the Electrical Conductivity of Graphene Nanoplatelet Polymer Composites by a Monte Carlo Method. Nanomaterials 2020, 10, 1129. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Liu, X.; Zhang, W.; Zeng, Z.; Liu, Z.; Zhang, C.; Liu, Y.; Shao, B.; Liang, Q.; Tang, W.; et al. Advances in the application, toxicity and degradation of carbon nanomaterials in environment: A review. Environ. Int. 2020, 134, 105298. [Google Scholar] [CrossRef] [PubMed]
- Kurapati, R.; Mukherjee, S.P.; Martín, C.; Bepete, G.; Vázquez, E.; Pénicaud, A.; Fadeel, B.; Bianco, A. Degradation of Single-Layer and Few-Layer Graphene by Neutrophil Myeloperoxidase. Angew. Chem. Int. Ed. 2018, 57, 11722–11727. [Google Scholar] [CrossRef] [PubMed]
- Kurapati, R.; Russier, J.; Squillaci, M.A.; Treossi, E.; Ménard-Moyon, C.; Del Rio-Castillo, A.E.; Vazquez, E.; Samorì, P.; Palermo, V.; Bianco, A. Dispersibility-Dependent Biodegradation of Graphene Oxide by Myeloperoxidase. Small 2015, 11, 3985–3994. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.P.; Gliga, A.R.; Lazzaretto, B.; Brandner, B.; Fielden, M.; Vogt, C.; Newman, L.; Rodrigues, A.F.; Shao, W.; Fournier, P.M.; et al. Graphene oxide is degraded by neutrophils and the degradation products are non-genotoxic. Nanoscale 2018, 10, 1180–1188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotchey, G.P.; Allen, B.L.; Vedala, H.; Yanamala, N.; Kapralov, A.A.; Tyurina, Y.Y.; Klein-Seetharaman, J.; Kagan, V.E.; Star, A. The Enzymatic Oxidation of Graphene Oxide. ACS Nano 2011, 5, 2098–2108. [Google Scholar] [CrossRef]
- Bullock, C.J.; Bussy, C. Biocompatibility Considerations in the Design of Graphene Biomedical Materials. Adv. Mater. Interfaces 2019, 6, 1900229. [Google Scholar] [CrossRef]
- Pinto, A.M.; Gonçalves, I.C.; Magalhães, F.D. Graphene-based materials biocompatibility: A review. Colloids Surf. B Biointerfaces 2013, 111, 188–202. [Google Scholar] [CrossRef]
- Fadeel, B.; Bussy, C.; Merino, S.; Vázquez, E.; Flahaut, E.; Mouchet, F.; Evariste, L.; Gauthier, L.; Koivisto, A.J.; Vogel, U.; et al. Safety Assessment of Graphene-Based Materials: Focus on Human Health and the Environment. ACS Nano 2018, 12, 10582–10620. [Google Scholar] [CrossRef] [PubMed]
- Jasim, D.A.; Murphy, S.; Newman, L.; Mironov, A.; Prestat, E.; McCaffrey, J.; Ménard-Moyon, C.; Rodrigues, A.F.; Bianco, A.; Haigh, S.; et al. The Effects of Extensive Glomerular Filtration of Thin Graphene Oxide Sheets on Kidney Physiology. ACS Nano 2016, 10, 10753–10767. [Google Scholar] [CrossRef] [PubMed]
- Bussy, C.; Ali-Boucetta, H.; Kostarelos, K. Safety Considerations for Graphene: Lessons Learnt from Carbon Nanotubes. Acc. Chem. Res. 2013, 46, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Sayyar, S.; Murray, E.; Thompson, B.C.; Gambhir, S.; Officer, D.L.; Wallace, G.G. Covalently linked biocompatible graphene/polycaprolactone composites for tissue engineering. Carbon 2013, 52, 296–304. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, C.; Gonçalves, I.; Magalhães, F.; Pinto, A. Poly(lactic acid) Composites Containing Carbon-Based Nanomaterials: A Review. Polymers 2017, 9, 269. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Deng, X.-Y.; Du, A.-K.; Zhao, T.-H.; Zeng, J.-B. Poly(sodium 4-styrenesulfonate) modified graphene for reinforced biodegradable poly(ε-caprolactone) nanocomposites. RSC Adv. 2015, 5, 73146–73154. [Google Scholar] [CrossRef]
- Gonçalves, C.; Pinto, A.; Machado, A.V.; Moreira, J.; Gonçalves, I.C.; Magalhães, F. Biocompatible reinforcement of poly(Lactic acid) with graphene nanoplatelets. Polym. Compos. 2018, 39, E308–E320. [Google Scholar] [CrossRef] [Green Version]
- Yu, T.; Wang, G.S.; Liu, L.; Wang, P.; Wei, Z.Y.; Qi, M. Synthesis of PCL/Graphene Oxide Composites by In Situ Polymerization. Adv. Mater. Res. 2012, 518–523, 837–840. [Google Scholar] [CrossRef]
- Wang, G.-S.; Wei, Z.-Y.; Sang, L.; Chen, G.-Y.; Zhang, W.-X.; Dong, X.-F.; Qi, M. Morphology, crystallization and mechanical properties of poly(ε-caprolactone)/graphene oxide nanocomposites. Chin. J. Polym. Sci. 2013, 31, 1148–1160. [Google Scholar] [CrossRef]
- Unnithan, A.R.; Ramachandra Kurup Sasikala, A.; Pant, H. Electrospinning of Polymers for Tissue Engineering; William Andrew: Norwich, NY, USA, 2015; pp. 45–55. [Google Scholar] [CrossRef]
- Karande, T.; Agrawal, C. Function and Requirement of Synthetic Scaffolds in Tissue Engineering; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Zaszczyńska, A.; Moczulska-Heljak, M.; Gradys, A.; Sajkiewicz, P. Advances in 3D Printing for Tissue Engineering. Materials 2021, 14, 3149. [Google Scholar] [CrossRef]
- Domingues, R.M.; Chiera, S.; Gershovich, P.; Motta, A.; Reis, R.L.; Gomes, M.E. Enhancing the Biomechanical Performance of Anisotropic Nanofibrous Scaffolds in Tendon Tissue Engineering: Reinforcement with Cellulose Nanocrystals. Adv. Healthc. Mater. 2016, 5, 1364–1375. [Google Scholar] [CrossRef] [PubMed]
- Al-Dhahebi, A.M.; Gopinath, S.C.B.; Saheed, M.S.M. Graphene impregnated electrospun nanofiber sensing materials: A comprehensive overview on bridging laboratory set-up to industry. Nano Converg. 2020, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Aidun, A.; Safaei Firoozabady, A.; Moharrami, M.; Ahmadi, A.; Haghighipour, N.; Bonakdar, S.; Faghihi, S. Graphene oxide incorporated polycaprolactone/chitosan/collagen electrospun scaffold: Enhanced osteogenic properties for bone tissue engineering. Artif. Organs 2019, 43, E264–E281. [Google Scholar] [CrossRef] [PubMed]
- Marrella, A.; Tedeschi, G.; Giannoni, P.; Lagazzo, A.; Sbrana, F.; Barberis, F.; Quarto, R.; Puglisi, F.; Scaglione, S. “Green-reduced” graphene oxide induces in vitro an enhanced biomimetic mineralization of polycaprolactone electrospun meshes. Mater. Sci. Eng. C 2018, 93, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.A.; Zenovich, A.G. Cardiovascular cell therapy and endogenous repair. Diabetes Obes. Metab. 2008, 10, 5–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hitscherich, P.; Aphale, A.; Gordan, R.; Whitaker, R.; Singh, P.; Xie, L.-H.; Patra, P.; Lee, E.J. Electroactive graphene composite scaffolds for cardiac tissue engineering. J. Biomed. Mater. Res. A 2018, 106, 2923–2933. [Google Scholar] [CrossRef] [PubMed]
- Aphale, A.N.; Mahakalkar, K.; Macwan, I.G.; Mukerji, I.; Cox, P.J.; Mahapatra, M.; Singh, P.; Ajayan, P.M.; Patra, P.K. Fabrication and Experimental Analysis of Axially Oriented Nanofibers. J. Nanosci. Nanotechnol. 2016, 16, 2668–2676. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Feng, B.; Zhu, D.-Q.; Chen, Y.-W.; Ji, W.; Ji, T.-J.; Li, F. Characteristics and toxicity assessment of electrospun gelatin/PCL nanofibrous scaffold loaded with graphene in vitro and in vivo. Int. J. Nanomed. 2019, 14, 3669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, X.; Guo, H.; Zhang, W.; Fang, H.; Li, Q.; Bai, S.; Zhang, P. Reduced graphene oxide–GelMA–PCL hybrid nanofibers for peripheral nerve regeneration. J. Mater. Chem. B 2020, 8, 10593–10601. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Inoue, H.; Wu, J.C.; Yamanaka, S. Induced pluripotent stem cell technology: A decade of progress. Nat. Rev. Drug Discov. 2017, 16, 115–130. [Google Scholar] [CrossRef]
- Masaki, T.; Qu, J.; Cholewa-Waclaw, J.; Burr, K.; Raaum, R.; Rambukkana, A. Reprogramming Adult Schwann Cells to Stem Cell-like Cells by Leprosy Bacilli Promotes Dissemination of Infection. Cell 2013, 152, 51–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Mangadlao, J.D.; Wallat, J.; De Leon, A.; Pokorski, J.K.; Advincula, R.C. 3D Printing Biocompatible Polyurethane/Poly(lactic acid)/Graphene Oxide Nanocomposites: Anisotropic Properties. ACS Appl. Mater. Interfaces 2017, 9, 4015–4023. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Gao, H.; Zhu, G.; Cao, X.; Shi, X.; Wang, Y. The preparation and characterization of polycaprolactone/graphene oxide biocomposite nanofiber scaffolds and their application for directing cell behaviors. Carbon 2015, 95, 1039–1050. [Google Scholar] [CrossRef]
- Niknam, Z.; Zali, H.; Mansouri, V.; Rezaei Tavirani, M.; Omidi, M. Morphological and Molecular Analysis of Osteoblasts Differentiated from Mesenchymal Stem Cells in Polycaprolactone/Magnesium Oxide/Graphene Oxide Scaffold. Int. J. Organ. Transpl. Med. 2019, 10, 171–182. [Google Scholar]
- Heidari, M.; Bahrami, S.H.; Ranjbar-Mohammadi, M.; Milan, P.B. Smart electrospun nanofibers containing PCL/gelatin/graphene oxide for application in nerve tissue engineering. Mater. Sci. Eng. C 2019, 103, 109768. [Google Scholar] [CrossRef] [PubMed]
- Faraji, S.; Nowroozi, N.; Nouralishahi, A.; Shabani Shayeh, J. Electrospun poly-caprolactone/graphene oxide/quercetin nanofibrous scaffold for wound dressing: Evaluation of biological and structural properties. Life Sci. 2020, 257, 118062. [Google Scholar] [CrossRef]
- Rostami, F.; Tamjid, E.; Behmanesh, M. Drug-eluting PCL/graphene oxide nanocomposite scaffolds for enhanced osteogenic differentiation of mesenchymal stem cells. Mater. Sci. Eng. C 2020, 115, 111102. [Google Scholar] [CrossRef]
- Uehara, T.M.; Paino, I.M.M.; Santos, F.A.; Scagion, V.P.; Correa, D.S.; Zucolotto, V. Fabrication of random and aligned electrospun nanofibers containing graphene oxide for skeletal muscle cells scaffold. Polym. Adv. Technol. 2020, 31, 1437–1443. [Google Scholar] [CrossRef]
- Sadeghianmaryan, A.; Karimi, Y.; Naghieh, S.; Alizadeh Sardroud, H.; Gorji, M.; Chen, X. Electrospinning of Scaffolds from the Polycaprolactone/Polyurethane Composite with Graphene Oxide for Skin Tissue Engineering. Appl. Biochem. Biotechnol. 2020, 191, 567–578. [Google Scholar] [CrossRef]
- Kang, Y.; Wang, C.; Qiao, Y.; Gu, J.; Zhang, H.; Peijs, T.; Kong, J.; Zhang, G.; Shi, X. Tissue-Engineered Trachea Consisting of Electrospun Patterned sc-PLA/GO-g-IL Fibrous Membranes with Antibacterial Property and 3D-Printed Skeletons with Elasticity. Biomacromolecules 2019, 20, 1765–1776. [Google Scholar] [CrossRef]
- Abazari, M.F.; Nasiri, N.; Nejati, F.; Kohandani, M.; Hajati-Birgani, N.; Sadeghi, S.; Piri, P.; Soleimanifar, F.; Rezaei-Tavirani, M.; Mansouri, V. Acceleration of osteogenic differentiation by sustained release of BMP2 in PLLA/graphene oxide nanofibrous scaffold. Polym. Adv. Technol. 2021, 32, 272–281. [Google Scholar] [CrossRef]
- Zhu, J.; Qi, Z.; Zheng, C.; Xue, P.; Fu, C.; Pan, S.; Yang, X. Enhanced Cell Proliferation and Osteogenesis Differentiation through a Combined Treatment of Poly-L-Lysine-Coated PLGA/Graphene Oxide Hybrid Fiber Matrices and Electrical Stimulation. J. Nanomater. 2020, 2020, 5892506. [Google Scholar] [CrossRef]
- Fu, C.; Bai, H.; Zhu, J.; Niu, Z.; Wang, Y.; Li, J.; Yang, X.; Bai, Y. Enhanced cell proliferation and osteogenic differentiation in electrospun PLGA/hydroxyapatite nanofibre scaffolds incorporated with graphene oxide. PLoS ONE 2017, 12, e0188352. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Bai, H.; Hu, Q.; Gao, T.; Bai, Y. Enhanced proliferation and osteogenic differentiation of MC3T3-E1 pre-osteoblasts on graphene oxide-impregnated PLGA–gelatin nanocomposite fibrous membranes. RSC Adv. 2017, 7, 8886–8897. [Google Scholar] [CrossRef] [Green Version]
- Shin, Y.C.; Kim, J.; Kim, S.E.; Song, S.-J.; Hong, S.W.; Oh, J.-W.; Lee, J.; Park, J.-C.; Hyon, S.-H.; Han, D.-W. RGD peptide and graphene oxide co-functionalized PLGA nanofiber scaffolds for vascular tissue engineering. Regen. Biomater. 2017, 4, 159–166. [Google Scholar] [CrossRef]
- Pan, S.; Qi, Z.; Li, Q.; Ma, Y.; Fu, C.; Zheng, S.; Kong, W.; Liu, Q.; Yang, X. Graphene oxide-PLGA hybrid nanofibres for the local delivery of IGF-1 and BDNF in spinal cord repair. Artif. Cells Nanomed. Biotechnol. 2019, 47, 651–664. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Wang, Z.; Jiang, J.; Liu, X.; Zhao, J.; Zhang, Z. Promoting tendon to bone integration using graphene oxide-doped electrospun poly(lactic-co-glycolic acid) nanofibrous membrane. Int. J. Nanomed. 2019, 14, 1835–1847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanoska-Dacikj, A.; Bogoeva-Gaceva, G.; Krumme, A.; Tarasova, E.; Scalera, C.; Stojkovski, V.; Gjorgoski, I.; Ristoski, T. Biodegradable polyurethane/graphene oxide scaffolds for soft tissue engineering: In vivo behavior assessment. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 1101–1111. [Google Scholar] [CrossRef]
- Jo, S.B.; Erdenebileg, U.; Dashnyam, K.; Jin, G.-Z.; Cha, J.-R.; El-Fiqi, A.; Knowles, J.C.; Patel, K.D.; Lee, H.-H.; Lee, J.-H. Nano-graphene oxide/polyurethane nanofibers: Mechanically flexible and myogenic stimulating matrix for skeletal tissue engineering. J. Tissue Eng. 2020, 11, 2041731419900424. [Google Scholar] [CrossRef] [Green Version]
- Karimi Alavije, S.; Kokabi, M.; Soleimani, M. Endothelial cells performance on 3D electrospun PVA/graphene nanocomposite tubular scaffolds. Polym. Bull. 2020, 78, 4797–4815. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Park, G.T.; Han, S.S. Electrospun poly(vinyl alcohol)/reduced graphene oxide nanofibrous scaffolds for skin tissue engineering. Colloids Surf. B Biointerfaces 2020, 191, 110994. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, N.; Simchi, A. On the biological performance of graphene oxide-modified chitosan/polyvinyl pyrrolidone nanocomposite membranes: In vitro and in vivo effects of graphene oxide. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Vozzi, G.; Previti, A.; De Rossi, D.; Ahluwalia, A. Microsyringe-Based Deposition of Two-Dimensional and Three-Dimensional Polymer Scaffolds with a Well-Defined Geometry for Application to Tissue Engineering. Tissue Eng. 2002, 8, 1089–1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Lei, M.; Wei, Q.; Wang, Y.; Zhang, J.; Guo, Y.; Saroia, J. 3D printing biocompatible l-Arg/GNPs/PLA nanocomposites with enhanced mechanical property and thermal stability. J. Mater. Sci. 2020, 55, 5064–5078. [Google Scholar] [CrossRef]
- Wang, W.; Junior, J.R.P.; Nalesso, P.R.L.; Musson, D.; Cornish, J.; Mendonça, F.; Caetano, G.F.; Bártolo, P. Engineered 3D printed poly(ε-caprolactone)/graphene scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2019, 100, 759–770. [Google Scholar] [CrossRef]
- Yasuda, I. Fundamental aspects of fracture treatment. J. Kyoto Med. Soc. 1953, 4, 395–406. [Google Scholar]
- Hou, Y.; Wang, W.; Bártolo, P. Novel Poly(ε-caprolactone)/Graphene Scaffolds for Bone Cancer Treatment and Bone Regeneration. 3D Print. Addit. Manuf. 2020, 7, 222–229. [Google Scholar] [CrossRef]
- Misch, C.E.; Qu, Z.; Bidez, M.W. Mechanical properties of trabecular bone in the human mandible: Implications for dental implant treatment planning and surgical placement. J. Oral Maxillofac. Surg. 1999, 57, 700–706. [Google Scholar] [CrossRef]
- Caetano, G.F.; Wang, W.; Chiang, W.-H.; Cooper, G.; Diver, C.; Blaker, J.J.; Frade, M.A.; Bártolo, P. 3D-printed poly (ε-caprolactone)/graphene scaffolds activated with P1-latex protein for bone regeneration. 3D Print. Addit. Manuf. 2018, 5, 127–137. [Google Scholar] [CrossRef] [Green Version]
- Unagolla, J.M.; Jayasuriya, A.C. Enhanced cell functions on graphene oxide incorporated 3D printed polycaprolactone scaffolds. Mater. Sci. Eng. C 2019, 102, 1–11. [Google Scholar] [CrossRef]
- Seyedsalehi, A.; Daneshmandi, L.; Barajaa, M.; Riordan, J.; Laurencin, C.T. Fabrication and characterization of mechanically competent 3D printed polycaprolactone-reduced graphene oxide scaffolds. Sci. Rep. 2020, 10, 22210. [Google Scholar] [CrossRef] [PubMed]
- Belaid, H.; Nagarajan, S.; Teyssier, C.; Barou, C.; Barés, J.; Balme, S.; Garay, H.; Huon, V.; Cornu, D.; Cavaillès, V.; et al. Development of new biocompatible 3D printed graphene oxide-based scaffolds. Mater. Sci. Eng. C 2020, 110, 110595. [Google Scholar] [CrossRef] [PubMed]
- Alam, F.; Varadarajan, K.M.; Kumar, S. 3D printed polylactic acid nanocomposite scaffolds for tissue engineering applications. Polym. Test. 2020, 81, 106203. [Google Scholar] [CrossRef]









| Property | Unit | Range | Reference |
|---|---|---|---|
| Crystallinity | % | <69 | [28] |
| Density | g/cm3 | 1.07 to 1.20 | [28] |
| Decomposition temperature | °C | 300 to 350 | [28] |
| Glass transition temperature | °C | −65 to −61 | [22,28] |
| Melting temperature | °C | 56 to 65 | [8,22,30] |
| Elongation at break | % | 20 to 1000 | [30] |
| Tensile strength | MPa | 20.7 to 42 | [30] |
| Young’s modulus | GPa | 0.21 to 0.44 | [21,22] |
| Property | Unit | Range | Reference |
|---|---|---|---|
| Crystallinity | % | <35 | [44] |
| Density | g/cm3 | 1.21 to 1.25 | [45] |
| Decomposition temperature | °C | 300 to 370 | [30] |
| Glass transition temperature | °C | 50 to 65 | [45,46,47] |
| Melting temperature | °C | 150 to 178 | [30,46,47] |
| Elongation at break | % | 2 to 160 | [30,46,47] |
| Tensile strength | MPa | 6.6 to 60 | [46,47,48] |
| Young’s modulus | GPa | 0.35 to 3.5 | [46,47,49] |
| Polymer | Filler (wt.%) | Other Elements | Flow Rate (mL/h) | DtC (cm) | V (kV) | Fd (nm) | Application | Outcomes | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| PCL | GO (3, 6) | Chitosan/ Collagen | 0.6 | 12 | 20 | 120 | Bone TE |
| [92] |
| PCL | GO rGO (0.25) | - | 2.0 | 12 | 10 | 430 410 | Bone TE |
| [93] |
| PCL | G (0.01, 0.5) | - | 1.5 | 15 | 17 | <1 × 103 | Cardiac TE |
| [95] |
| PCL | G (<0.5) | Gelatin | 2.0 | 12 | 15 | 600 | Cardiac TE |
| [101] |
| PCL | rGO (<1) | GelMA 1 | 2.0 | 15 | 15 | 400 | Neural TE |
| [98] |
| PCL | GO (0.1, 1) | - | 1.0 | 12 | 18 | 400 | Control cell behavior |
| [102] |
| PCL | GO (0.5) | MgO | 1.0 | 10 | 18 | 700 | Bone TE |
| [103] |
| PCL | GO (<0.4) | Gelatin | - | - | - | 135 | Neural TE |
| [104] |
| PCL | GO (0.5) | Quercetin | 0.5 | 15 | 18 | 300, 500 | Wound healing |
| [105] |
| PCL | GO (0.1) | Dexamethasone | 0.8 | 10 | 18 | 166 | Bone TE |
| [106] |
| PCL | GO | - | 0.5 | 15 | 20 | 100 | Skeletal muscle TE |
| [107] |
| PCL | GO (0.5, 4) | PU 2 | 0.3 | 15 | 9, 10 | 400, 600 | Skin TE |
| [108] |
| PLA | GO (10) | Ionic liquid | 0.5 | 20 | 15 | <1.8 × 103 | Tracheal TE |
| [109] |
| PLLA | GO (1) | BMP2 3 | 1.0 | 20 | 20 | 700 | Bone TE |
| [110] |
| PLGA 4 | GO (2) | Poly-L-Lysine | (4.2, 6.0) | 20 | 40 | <1.5 × 103 | Bone TE |
| [111] |
| PLGA | GO (2) | HA | 1.0 | 20 | 20 | <1 × 103 | Bone TE |
| [112] |
| PLGA | GO (2) | Gelatin | 1.0 | 20 | 20 | <1 × 103 | Bone TE |
| [113] |
| PLGA | GO | RGD peptide | 0.2 | 11 | 14 | 558 | Smooth muscle TE |
| [114] |
| PLGA | GO | IGF-1 + BDNF 5 | (4.2, 6) | 10 | 40 | 1 × 103 | Spinal cord injury |
| [115] |
| PLGA | GO (1) | - | - | 20 | 10 | <1.5 × 103 | Tendon to Bone Integration |
| [116] |
| PU | GO (0.5, 1) | PEG 6 | 0.4 | 11 | 18 | (322, 1 × 103) | Skin TE |
| [117] |
| PU | GO (<8) | Polycarbonate diol | 2.0 | 10 | 12, 5 | <1 × 103 | Skeletal muscle TE |
| [118] |
| PVA 7 | G (<3) | - | 0.2 | - | 15, 19 | <100 | Cardiac TE |
| [119] |
| PVA | rGO (0.1, 1) | Glucose + Glutaraldehyde | 1.6 × 10−4 | 15 | (16, 18) | 200 | Skin TE |
| [120] |
| PVP 8 | GO (<2) | Chitosan + Polyethylene | - | - | (20, 24) | 60 | Wound closure |
| [121] |
| Polymer | Filler (wt.%) | Other Elements | Blending | Fabrication Technique | T (°C) | Flow Rate (mm/s) | Fd (µm) | Application | Outcomes | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| PCL | G (5–7) | - | Melt | Extrusion | 90 | 12 | 330 | Bone TE + Cancer treatment |
| [126] |
| PCL | G (<0.8) | P1-Latex protein | Melt | Extrusion | 90 | 20 | 330 | Bone TE |
| [128] |
| PCL | G (<0.8) | - | Melt | Extrusion | 90 | 20 | 330 | Bone TE |
| [124] |
| PCL | GO (0.1, 0.5) | - | Solvent | Extrusion | 100 | 1 | 100 | Bone TE |
| [129] |
| PCL | rGO (0.5) | - | Solvent | Extrusion | 100 | 1.4 | 325 | TE |
| [130] |
| PLA | GO (5) | PU | Solvent | FDM | 210 | 20 | 400 | TE |
| [101] |
| PLA | GO (0.3) | - | Solvent | FDM | - | - | 100, 200 | Bone TE |
| [131] |
| PLA | GNP (14) | Fe2O3 | Solvent | FFF 1 | 215 | 60 | 480 | Bone TE |
| [132] |
| PLA | GNP (2) | L-arg 2 | Solvent | FDM | 180 | 50 | 400 Bone | TE |
| [123] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meneses, J.; van de Kemp, T.; Costa-Almeida, R.; Pereira, R.; Magalhães, F.D.; Castilho, M.; Pinto, A.M. Fabrication of Polymer/Graphene Biocomposites for Tissue Engineering. Polymers 2022, 14, 1038. https://doi.org/10.3390/polym14051038
Meneses J, van de Kemp T, Costa-Almeida R, Pereira R, Magalhães FD, Castilho M, Pinto AM. Fabrication of Polymer/Graphene Biocomposites for Tissue Engineering. Polymers. 2022; 14(5):1038. https://doi.org/10.3390/polym14051038
Chicago/Turabian StyleMeneses, João, Tom van de Kemp, Raquel Costa-Almeida, Rúben Pereira, Fernão D. Magalhães, Miguel Castilho, and Artur M. Pinto. 2022. "Fabrication of Polymer/Graphene Biocomposites for Tissue Engineering" Polymers 14, no. 5: 1038. https://doi.org/10.3390/polym14051038