Even the nominal particle size of the nanosilica particles in the aqueous dispersion is 12 nm, the spherical nanosilica particles appear agglomerated in clusters of about 150 nm (
Figure 3). The agglomeration of the nanosilica particles can be ascribed to the functionalization with acrylic moieties rather than to the interactions between the silanol groups on the surface of the nanosilica particles [
38]. In fact, the chemical composition of the nanosilica powder obtained by IR spectroscopy (
Figure 4) consists of typical bands of silica (Si-O bending at 798 cm
−1, Si-O-Si stretching at 1106 and 962 cm
−1) and another band at 1621 cm
−1 due to C=C stretching [
39]. Furthermore, the chemical composition of the nanosilica powder surface obtained from XPS experiments consists of 56.3 at.% oxygen, 40.2 at.% silicon, 2.5 at.% carbon, 0.9 at.% sodium and traces of chlorine (
Figure S2 of Supplementary Materials). Therefore, the nanosilica powder surface is functionalized with carbon- and oxygen-containing species. In addition, according to the curve fitting of the C1s high-resolution XPS spectrum (
Figure S3 of Supplementary Materials), these species are acrylic moieties because of the presence of 4 at.% C-O species at a binding energy of 287.0 eV and 16 at.% –O=C-OH species at a binding energy of 289.0 eV [
40].
3.1. Characterization of the Waterborne Polyurethane Dispersions (PUDs) without and with Different Amounts of Nanosilica
The dispersion of the nanosilica particles in the waterborne polyurethane was assessed by TEM micrographs (
Figure 5). The phase separation in the PUD without nanosilica can be noticed in the TEM micrographs, in which the hard (dark zones) and soft (light zones) domains are distinguished [
42]. The addition of nanosilica changes the degree of phase separation in the waterborne polyurethane to a greater extent by increasing the amount. The addition of only 0.1 wt.% nanosilica produces a good dispersion of the particles in the waterborne polyurethane matrix—the most particles are isolated and a few bundles of 3–4 particles are distinguished (
Figure 5). Similarly, the addition of 0.5 wt.% nanosilica also allows a good dispersion of the nanosilica particles, but most of the nanosilica particles form bundles of 9–12 particles. However, the addition of 3 wt.% nanosilica produces agglomerates of nanosilica particles of about 150 nm in length and, in some minor zones, smaller clusters of nanosilica particles can be distinguished (
Figure 5). Therefore, the physical mixing at a low stirring rate when 0.1–0.5 wt.% nanosilica is added seems sufficient for breaking the nanosilica agglomerates in the aqueous dispersion and a change in the degree of phase separation in the waterborne polyurethane is produced. However, the nanosilica bundles in the aqueous dispersion containing 3 wt.% nanosilica are not disagglomerated completely when a low stirring rate is applied, and separated domains of nanosilica particles and polyurethane can be distinguished.
Some properties of the waterborne polyurethane dispersions (PUDs) with and without different amounts of nanosilica are shown in
Table 1. No sedimentation of the nanosilica particles in the PUDs was noticed over time. The solid content of the dispersions was 49–50 wt.% and decreased slightly by adding nanosilica because of the lower content of nanosilica in the aqueous dispersion (28.9 wt.%) than in the PUD (50 wt.%). The pH values of the PUDs are basic and increase gradually by increasing the amount of nanosilica because the pH of the nanosilica dispersion (9.2) is more basic than the one of the PUD without nanosilica (7.9). On the other hand, the surface tension of the PUDs increases by adding nanosilica and by increasing its amount because the surface tension of the nanosilica dispersion (63 mN/m) is higher than the one of the PUD without nanosilica (49 mN/m). Therefore, the higher the nanosilica content in the PUD, the higher the pH and the surface tension.
The magnitude of the zeta (Z) potential indicates the degree of electrostatic repulsion between adjacent, similarly charged particles in a dispersion. The Z-potential values of the PUDs are negative because the waterborne polyurethane is anionic and the nanosilica particles in the aqueous dispersion are negatively charged. The Z-potential values of the PUDs become more negative by adding nanosilica because of the more negative Z-potential value of the nanosilica dispersion (−87 mV) with respect to the one of the PUD without nanosilica (−57 mV). The more negative Z-potential value means a higher concentration of negative charges and higher resistance to the aggregation of the particles. On the other hand, the Z-potential values of the PUDs are similar irrespective of their nanosilica content (−63 to −65 mV). This indicates that the degree of nanosilica agglomeration does not affect their net negative charge.
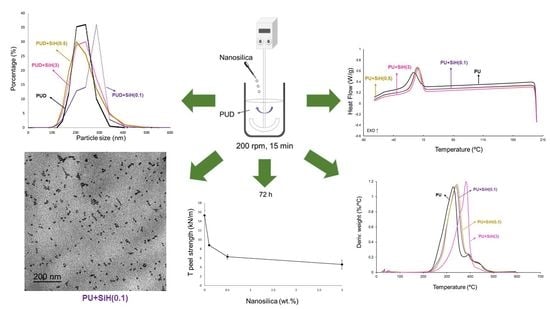
Figure 6 shows the particle size distributions of the PUDs. The PUD without nanosilica shows a relatively narrow particle-size distribution, 67% made by particles of 217 nm, 22% particles of 25 nm and 11% particles of 297 nm. The addition of 0.1 wt.% nanosilica noticeably changes the particle-size distribution (
Figure 6) because a decrease in the percentage of particles of 212 nm and an increase in the one of 298 nm particles is noticed (
Table S1 of the Supplementary Materials). Considering that most of the nanosilica particles in the aqueous dispersion have mean particle sizes of 140 nm and 768 nm (
Table S1 of the Supplementary Materials), the addition of 0.1 wt.% nanosilica to the PUD by using a mild physical mixing causes a good dispersion of the nanosilica particles (
Figure 5). However, the addition of 0.5 and 3 wt.% nanosilica causes a moderate broadening of the particle size distribution of the PUD to lower and higher particle sizes, more noticeably by adding 3 wt.% nanosilica (
Figure 6), the broadening is likely due to the existence of nanosilica agglomerates (
Figure 5). Thus, the addition of 0.5 and 3 wt.% nanosilica decreases the percentage of particles of 202–209 nm and increases the ones of 265–266 nm. Their percentages are almost 50% (
Table S1 of the Supplementary Materials). Thus, depending on the amount of nanosilica, the particle size distributions of the PUDs change differently.
Figure 7 shows the variation of the Brookfield viscosity of the PUDs as a function of the shear rate. Because the Brookfield viscosity of the nanosilica dispersion (11 mPa.s) is significantly lower than that of the PUD (140 nm), the addition of the nanosilica dispersion decreases the Brookfield viscosities of the PUDs. Whereas the Brookfield viscosities of the PUD without and with 0.1 wt.% nanosilica are similar, a decrease is produced by increasing the amount of nanosilica (
Table 2). On the other hand, whereas the aqueous nanosilica dispersion shows a Newtonian rheological behavior, the PUDs show shear thinning, i.e., the viscosity decreases by increasing the shear rate (
Figure 7). The extent of shear thinning can be quantified by the ratio of the Brookfield viscosities at 1 and 20 s
−1 (pseudoplastic index). The pseudoplastic index and the extent of shear thinning of the PUDs decrease by adding nanosilica, more markedly by adding 3 wt.% nanosilica. Because the shear thinning in the waterborne polyurethane dispersion is caused by the reversible destruction of the interactions between the polyurethane particles, less shear thinning indicates the intercalation of the nanosilica particles between the polyurethane particles reducing their interactions, more efficiently by increasing the amount of nanosilica. Interestingly, the decrease in the viscosity of PUD+SiH(3) is not related to the extent of agglomeration/dispersion of the nanosilica particles.
3.2. Characterization of the Solid Waterborne Polyurethanes (PUs) without and with Different Amounts of Nanosilica
Upon evaporation of the water in the PUDs, solid-waterborne-polyurethane–nanosilica (PU+nanosilica) materials were obtained, and their structural, viscoelastic and mechanical properties were assessed.
The ATR-IR spectrum of the PU without nanosilica (
Figure S4 of the Supplementary Materials,
Figure 8) shows the N-H stretching at 3400 cm
−1, C=O stretching of urethane at 1730 cm
−1, C-N and N-H bending at 1531 cm
−1 and COO bending of the urethane group at 736 cm
−1; all these bands correspond to the hard segments. Furthermore, different bands of the soft segments (C-H stretching at 2950, 2920 and 2870 cm
−1, C-H bending at 1464 cm
−1, C-H bending in CH
2CO group at 1420 cm
−1 and C-O-C stretching at 1238 and 1170 cm
−1) can be distinguished. The ATR-IR spectra of the PU+nanosilica materials show more intense bands at 1110 and 1140 cm
−1 due to the nanosilica, to a greater extent by increasing its amount. Some changes in the intensities of the C-H and C-O-C stretching bands of the soft segments of the polyurethane in the ATR-IR spectra are noticed, in a different manner depending on the amount of nanosilica. Thus, the addition of 0.1 wt.% nanosilica increases the C-H stretching band at 2920 cm
−1, whereas the addition of 0.5 wt.% or more nanosilica does not change the intensities of the C-H stretching bands (
Figure 8). However, while the addition of 0.1 wt.% nanosilica does not change the intensities of the C-O-C stretching bands, the addition of higher amounts of nanosilica changes the intensities of the bands at 1238 and 1170 cm
−1 of the soft segments, more noticeably in PU+SiH(3). These changes are produced by the intercalation of the nanosilica particles between the soft segments, which produces a change in the degree of phase separation [
43]. The change in the degree of phase separation can be estimated as the ratio of the intensities of the C-O-C stretching band of the soft segments at 1170 cm
−1 and the C=O band of the hard segments at 1730 cm
−1. Whereas the ratio of the intensities is 1.06 for the PU without nanosilica, lower values (0.85–0.95) are obtained in the PUs with 0.1 and 0.5 wt.% nanosilica. This confirms the phase separation due to the intercalation of the nanosilica particles between the polyurethane chains. However, similar ratios of the intensities of the C-O-C stretching and the C=O bands are obtained in the PU without and with 3 wt.% nanosilica because of the existence of nanosilica agglomerates.
The physical structures of the PU+nanosilica materials were assessed by DSC. The DSC curves of the first heating run of all PUs (
Figure S5 of Supplementary Materials) show the glass transition (T
g) of the soft segments at (−48)–(−50) °C and the melting of the soft segments. Whereas the addition of nanosilica does not change the T
g value of the soft segments, there are changes in the melting peak. While the addition of 0.1 wt.% nanosilica does not change the melting temperature and enthalpy of the PU without nanosilica, the addition of 0.5 wt.% nanosilica produces two melting peaks at 46 and 52 °C and increases the melting enthalpy. These two melting peaks should correspond to two different structures of the soft segments: one without and another with intercalated nanosilica. In contrast, the addition of 3 wt.% nanosilica displaces the melting to higher temperatures and the melting enthalpy is similar (
Table 3). Therefore, the addition of 0.5 wt.% or more nanosilica produces two different structures of the soft segments, one without and another with intercalated nanosilica.
The DSC curves of the cooling run of the PU and PU+nanosilica materials show the crystallization of the soft segments of the polyurethane (
Figure 9). Whereas in the PU without nanosilica the crystallization peak of the soft segments appears at −7 °C with a crystallization enthalpy of 30 J/g, the crystallization peak displaces to higher temperature (0–2 °C) and has higher crystallization enthalpy (33–35 J/g) in all PU+nanosilica materials, irrespective of the amount of nanosilica (
Table S2 of Supplementary Materials). This confirms the intercalation of the nanosilica particles between the soft segments of the polyurethane, which causes the formation of new crystallites.
After allowing a slow reorganization of the polyurethane chains, a second DSC heating run of the PU and PU+nanosilica materials was carried out (
Figure 10). The DSC curves of all PUs show the glass transitions of the soft (T
g1) and hard (T
g2) segments, and the melting of the soft segments. Additionally, the DSC curves of PU and PU+SiH(0.5) show a cold crystallization at −21 °C with a small enthalpy (1.5–1.6 J/g) (
Table 4). During the cold crystallization, at the transition zone between the existing crystalline structures and the amorphous regions, new ordered structures (crystallites) grow. These newly crystallites can be differentiated from the pre-existing ones by their lower melting temperatures. Whereas the DSC curves of PU and PU+SiH(0.5) are similar, they differ in PU+SiH(0.1) and PU+SiH(3), in which the cold crystallization is absent. The addition of 3 wt.% nanosilica causes similar thermal events as those caused by the PU without nanosilica, except for the higher melting enthalpy of the soft segments. However, the addition of 0.1 wt.% nanosilica mildly reduces the T
g1 value and inhibits the appearance of the cold crystallization of the polyurethane. The absence of cold crystallization in the DSC curves of PU+SiH(0.1) and PU+SiH(3) can be ascribed to the disruption of the interactions between the soft segments caused by the intercalation of the nanosilica particles; unexpectedly, the cold crystallization remains in the DSC curve of PU+SiH(0.5). On the other hand, the glass transition temperature of the hard segments is not affected by adding nanosilica (236–237 °C) because of the lack of interactions between them. In summary, the amount of nanosilica affects the segmented structure of the polyurethane differently.
The TGA curve of the PU without nanosilica (
Figure 11) shows two main thermal decompositions starting at 250 °C and 340 °C due to the hard and soft domains, respectively. The addition of nanosilica increases the thermal stability of the waterborne polyurethanes, irrespective of their nanosilica content, due to the existence of nanosilica–polyurethane interactions, in agreement with previous studies [
30,
37]. In fact, the temperatures at which 5 (T
5%) and 50 (T
50%) mass loss is produced are higher in all PU+nanosilica materials than in PU (
Table 5); similar higher T
5% values are obtained. However, some differences in the T
50% values are noticed because of the changes in the physical structure of the soft segments by adding different amounts of nanosilica.
The physical structures in the PU and PU+nanosilica materials can be also assessed in the derivative of the TGA curves (
Figure 12). It has been have demonstrated recently [
7,
44] that the thermal decompositions of the urethane and urea hard domains in the polyurethanes appear at 240–320 °C, and the one of the soft domains appears at 320–390 °C. All PUs show different thermal decompositions at 52–55 °C (residual water), 241–300 °C (urethane and urea hard domains), 329–384 °C (soft domains) and 436–442 °C (by-products formed during the TGA experiments [
45]) (
Table S3 of Supplementary Materials). The most important weight loss corresponds to the soft domains at 329–384 °C. The soft domains appear at 329 °C in the PU without nanosilica and the PU+nanosilica materials show higher temperatures of decomposition of the soft domains at a different temperature and with different weight loss depending on their nanosilica content. This is an indication of the intercalation of the nanosilica particles between the soft domains of the polyurethane. The addition of 0.1–0.5 wt.% nanosilica displaces the temperature of decomposition of the soft domains of the PU to 361–369 °C with 60–73% weight loss—the thermal decomposition of the soft domains in the PU without nanosilica appears at 329 °C with a weight loss of 66% (
Table S3 of Supplementary Materials). Furthermore, the addition of 0.1–0.5 wt.% nanosilica increases the temperature of decomposition of the hard domains from 241 °C to 293–300 °C because a change in the degree of phase separation is produced (
Table S3 of Supplementary Materials). The amount of soft domains with intercalated nanosilica is higher and the one of the soft domains without nanosilica is lower in PU+SiH(0.1) than in PU+SiH(0.5) because of the more efficient dispersion of the nanosilica in PU+SiH(0.1). On the other hand, the addition of 3 wt.% nanosilica displaces the main peak of the DTGA curve to a higher temperature (
Figure 12) and produces two kinds of soft domains at 356 °C and 384 °C, i.e., the temperature of the soft domains without nanosilica is higher and the one of the soft domains with intercalated nanosilica is lower (
Table S3 of Supplementary Materials). This is an indication of the worse dispersion of the nanosilica particles in the polyurethane matrix.
The structural changes caused in the polyurethane by adding nanosilica should affect their viscoelastic properties. As such, they were assessed by temperature-sweep plate–plate rheological experiments. The variation of the storage (G′) modulus of the PU and PU+nanosilica materials as a function of the temperature shows a continuous decrease in G′ by increasing the temperature (
Figure 13). The rheological curves of all PUs are somewhat similar. However, the G′ values of the PUs increase slightly by increasing the nanosilica content. All PUs show a cross-over of the storage (G′) and loss (G″) moduli (
Figure S6 of Supplementary Materials). Above the cross-over temperature (T
cross-over), the PUs are mainly elastic, and below T
cross-over, they are mainly viscous. The values of T
cross-over decrease and the moduli at the cross-over (G
cross-over) of the PUs increase by adding nanosilica, to a greater extent by increasing the nanosilica content (
Table 6). The lower T
cross-over value and the higher G
cross-over indicates higher interactions between the polyurethane chains caused by the intercalation of the nanosilica particles.
Previous studies are controversial with respect to the improvement of the mechanical properties of the waterborne polyurethanes by adding nanosilica. Whereas some literature [
30,
46] has shown improved mechanical properties of the PUs by adding silicas, other literature has evidenced the opposite trend, which was ascribed to the sedimentation of the silica particles [
34]. In this study, no sedimentation of the nanosilica particles was found, so improved mechanical properties can be expected.
The stress–strain curves of the PU and PU+nanosilica materials show a marked yield point followed by an ample elastic deformation region (
Figure 14); at a strain of 550% (the highest reached in the equipment), none of the PUs break, so the maximum strength was measured at a strain of 550%. Whereas the addition of 0.1 wt.% nanosilica increases moderately the yield strain and the stress at 550% of the PU, the addition of 0.5 wt.% nanosilica increases all mechanical properties. The improved mechanical properties of the PUs containing 0.1–0.5 wt.% nanosilica can be ascribed to the good dispersion of the nanosilica. However, the addition of 3 wt.% nanosilica increases the Young modulus and decreases the stress at 550% because of the existence of nanosilica agglomerates in the polyurethane.
3.3. Adhesion Properties of the Waterborne Polyurethane Dispersions (PUDs) without and with Different Amounts of Nanosilica
Because a suitable wettability of the adhesive is needed for an adequate adhesion, the water contact angles on the PU and PU+nanosilica surfaces were measured. The water contact angle on the PU surface is 68 degrees (
Table 7), which seems adequate for producing good wettability. The addition of nanosilica increases the water contact angle values, more noticeably by increasing its amount. This trend agrees with the degree of dispersion of the nanosilica particles in the polyurethane matrix (the surface tension of the nanosilica dispersion (60 mN/m) is significantly higher than that of the waterborne polyurethane (49 mN/m)). Because the wettability of the polyurethane containing 3 wt.% nanosilica surface is worse than that of the PU surface, the acrylic moieties of the nanosilica dispersion seem to migrate to the surface. As a consequence, the adhesion of the waterborne polyurethanes is not expected to increase when the nanosilica dispersion is added.
The influence of the addition of the nanosilica dispersion to the waterborne polyurethane on its adhesion properties is not clear in the existing literature. Both increased adhesion [
29,
31,
33] and decreased adhesion to different substrates [
21] of silica–waterborne polyurethanes have been shown, the increased adhesion was related to the existence of interactions between the nanosilica and the polyurethane, and to the extent of the nanosilica agglomeration.
The adhesion properties of the waterborne polyurethane dispersions without and with different amounts of nanosilica were assessed by T-peel tests of plasticized PVC/PUD/plasticized PVC joints after 15 min (immediate adhesion) and 72 h (final adhesion) of joint formation (
Figure 15). A short time (15 min) after joint formation is not sufficient for complete removal of the water in the PUDs and they are not completely cross-linked. Therefore, the T-peel strength values are somewhat low (1.8–2.5 kN/m) and the loci of failure in all joints are cohesive failures of the adhesive (
Figure 16). The amount of nanosilica in the waterborne polyurethane determines the immediate T-peel strength because the joints made with the PUD containing 0.1 wt.% and 0.5 wt.% show similar immediate T-peel strength compared to the PUD without nanosilica, but the adhesion is lower in the joint made with the PUD with 3 wt.% nanosilica. Therefore, the presence of nanosilica agglomerates in the PUD decreases the immediate T-peel strength.
When the water in the PUD is completely removed (72 h after joint formation), the full cross-linking is produced, and the T-peel strength of the plasticized PVC/PUD/plasticized PVC joints (final adhesion) increases with respect to the T-peel strength values obtained 15 min after joint formation (
Figure 15). The final T-peel strength values of the joints made with the PUDs containing nanosilica are significantly lower than the one obtained with the PUD without nanosilica. The higher the nanosilica content, the lower the final adhesion. The better the nanosilica dispersion in the PUD+nanosilica, the higher the final T-peel strength value. On the other hand, whereas the loci of failure of the joints made with the PUD without and with 0.1 wt.% nanosilica are cohesive ruptures of the PVC substrate (
Figure 16), the one in the joint made with PUD+SiH(0.5) is mixed (cohesive rupture of the PVC + Cohesion in a surface layer of PVC) (
Figure 16), and the one in the joint made with PUD+SiH(3) is cohesion in a surface layer of PVC (
Figure 16). These experimental results agree with the increase in the surface tension of the PUDs containing nanosilica and with the extent of dispersion of the nanosilica in the polyurethane. However, the decrease in the final adhesion is too important to be ascribed to those factors only, and the main reason seems to be the migration of acrylic moieties of the nanosilica particles to the interface. In fact, the more dispersed the nanosilica particles, the higher the concentration of acrylic moieties on the adhesive surface. The deleterious adhesion of the waterborne polyurethane dispersions containing nanosilica has been ascribed previously to the migration of surfactant/antiadherent moieties to the interface, a change in the loci of failure from cohesive rupture to surface cohesive failure of the substrate was evidenced [
47]. Similarly, it has been shown [
20] that the adhesion of the waterborne polyurethane dispersions containing 1–5 wt.% nanosilica made by physical mixing was lower than in the dispersion without nanosilica.