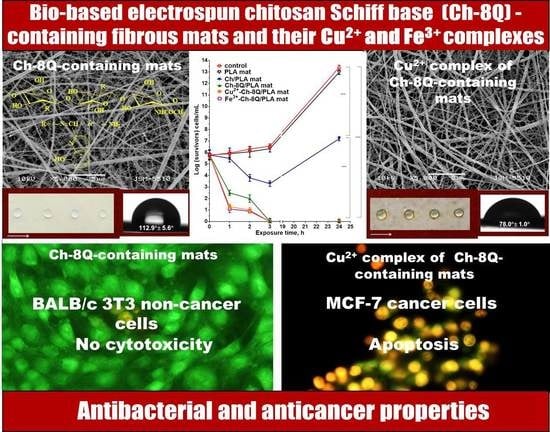
Bio-Based Electrospun Fibers from Chitosan Schiff Base and Polylactide and Their Cu2+ and Fe3+ Complexes: Preparation and Antibacterial and Anticancer Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Schiff Base Derivative from Ch and 8QCHO
2.3. Preparation of Ch-8Q/PLA Fibrous Materials by Electrospinning
2.4. Preparation of the Cu2+(Fe3+) Complexes of Ch-8Q/PLA Mats, of Ch-8Q, of 8QCHO and of Jeff-8Q
2.5. Characterization
2.6. Assessment of the Antibacterial Activity
2.7. MTT Cytotoxicity Assay
2.8. Fluorescent Microscopic Imaging
2.8.1. Double Staining with AO and EtBr
2.8.2. DAPI Staining
2.9. Statistical Analysis
3. Results and Discussion
3.1. Morphology
3.2. ATR-FTIR Spectra of Fibrous Materials
3.3. Thermal Behavior of the Fibrous Mats
3.4. Water Contact Angle Measurements
3.5. XPS Analysis
3.6. EPR Spectroscopy Analysis of Cu2+ and Fe3+ Complexes of the Fibrous Materials
3.7. Evaluation of the Antibacterial Activity
3.8. Cytotoxicity of Fibrous Mats against HeLa and MCF-7 Cells and BALB/c 3T3 Fibroblasts
3.9. Analysis of Cell Death by Staining Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fadil, F.; Affandi, N.D.N.; Misnon, M.I.; Bonnia, N.N.; Harun, A.M.; Alam, M.K. Review on electrospun nanofiber-applied products. Polymers 2021, 13, 2087. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.S.; Tian, Y.; Zhang, C.; Ye, Z.; Roy, K.; Chinnappan, A.; Ramakrishna, S.; Liu, W.; Ghosh, R. A review on electrospun nanofibers based advanced applications: From health care to energy devices. Polymers 2021, 13, 3746. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Gough, C.; Deng, Q.; Gu, Z.; Wang, F.; Hu, X. Recent advances in electrospun sustainable composites for biomedical, environmental, energy, and packaging applications. Int. J. Mol. Sci. 2020, 21, 4019. [Google Scholar] [CrossRef] [PubMed]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for drug delivery applications: A review. J. Control. Release 2021, 334, 463–484. [Google Scholar] [CrossRef]
- Torres-Martínez, E.J.; Bravo, J.M.C.; Medina, A.S.; González, G.L.P.; Gómez, L.J.V. A summary of electrospun nanofibers as drug delivery system: Drugs loaded and biopolymers used as matrices. Curr. Drug Deliv. 2018, 15, 1360–1374. [Google Scholar] [CrossRef]
- Balaji, A.; Vellayappan, M.V.; John, A.A.; Subramanian, A.P.; Jaganathan, S.K.; Supriyanto, E.; Razak, S.I.A. An insight on electrospun-nanofibers-inspired modern drug delivery system in the treatment of deadly cancers. RSC Adv. 2015, 5, 57984–58004. [Google Scholar] [CrossRef]
- Li, J.; Cai, C.; Li, J.; Sun, T.; Wang, L.; Wu, H.; Yu, G. Chitosan-based nanomaterials for drug delivery. Molecules 2018, 23, 2661. [Google Scholar] [CrossRef] [Green Version]
- Al-Jbour, N.D.; Beg, M.D.; Gimbun, J.; Moshiul Alam, A.K.M. An overview of chitosan nanofibers and their applications in the drug delivery process. Curr. Drug Deliv. 2019, 6, 272–294. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef]
- Chang, X.X.; Mubarak, N.M.; Mazari, S.A.; Jatoi, A.S.; Ahmad, A.; Khalid, M.; Walvekar, R.; Abdullah, E.C.; Karri, R.R.; Siddiqui, M.T.H.; et al. A review on the properties and applications of chitosan, cellulose and deep eutectic solvent in green chemistry. J. Ind. Eng. Chem. 2021, 104, 362–380. [Google Scholar] [CrossRef]
- Paneva, D.; Ignatova, M.; Manolova, N.; Rashkov, I. Novel chitosan–containing micro- and nanofibrous materials by electrospinning: Preparation and biomedical application. In Nanofibers: Fabrication, Performance, and Applications; Chang, W.N., Ed.; Nova Science Publishers: New York, NY, USA, 2009; pp. 73–151. [Google Scholar]
- Liu, X.F.; Guan, Y.L.; Yang, D.Z.; Li, Z.; De Yao, K. Antibacterial action of chitosan and carboxymethylated chitosan. J. Appl. Polym. Sci. 2000, 79, 1324–1335. [Google Scholar]
- Qin, C.; Du, Y.; Xiao, L.; Li, Z.; Gao, X. Enzymic preparation of water-soluble chitosan and their antitumor activity. Int. J. Biol. Macromol. 2002, 31, 111–117. [Google Scholar] [CrossRef]
- Martínez-Camacho, A.P.; Cortez-Rocha, M.O.; Castillo-Ortega, M.M.; Burgos-Hernández, A.; Ezquerra-Brauer, J.M.; Plascencia-Jatomea, M. Antimicrobial activity of chitosan nanofibers obtained by electrospinning. Polym. Int. 2011, 60, 1663–1669. [Google Scholar] [CrossRef]
- Ohkawa, K.; Cha, D.; Kim, H.; Nishida, A.; Yamamoto, H. Electrospinning of chitosan. Macromol. Rapid Commun. 2004, 25, 1600–1605. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Ocio, M.J.; Lagaron, J.M. Development of active antimicrobial fiber-based chitosan polysaccharide nanostructures using electrospinning. Eng. Life Sci. 2008, 8, 303–314. [Google Scholar] [CrossRef]
- Correia, D.M.; Amparo Gámiz-González, M.; Botelho, G.; Vidaurre, A.; Gomez Ribelles, J.L.; Lanceros-Mendez, S.; Sencadas, V. Effect of neutralization and cross-linking on the thermal degradation of chitosan electrospun membranes. J. Therm. Anal. Calorim. 2014, 117, 123–130. [Google Scholar] [CrossRef] [Green Version]
- Su, H.; Liu, K.; Karydis, A.; Abebe, D.G.; Wu, C.; Anderson, K.M.; Ghadri, N.; Adatrow, P.; Fujiwara, T.; Bumgardner, J.D. In vitro and in vivo evaluations of a novel post-electrospinning treatment to improve the fibrous structure of chitosan membranes for guided bone regeneration. J. Biomed. Mater. Res. 2017, 12, 015003. [Google Scholar] [CrossRef]
- Ohkawa, K.; Minato, K.-I.; Kumagai, G.; Hayashi, S.; Yamamoto, H. Chitosan nanofiber. Biomacromolecules 2006, 9, 3291–3294. [Google Scholar] [CrossRef]
- Sangsanoh, P.; Supaphol, P. Stability improvement of electrospun chitosan nanofibrous membranes in neutral or weak basic aqueous solutions. Biomacromolecules 2006, 7, 2710–2727. [Google Scholar] [CrossRef]
- Nirmala, R.; Il, B.W.; Navamathavan, R.; El-Newehy, M.H.; Kim, H.Y. Preparation and characterizations of anisotropic chitosan nanofibers via electrospinning. Macromol. Res. 2011, 19, 345–350. [Google Scholar] [CrossRef]
- De Vrieze, S.; Westbroek, P.; Van Camp, T.; Van Langenhove, L. Electrospinning of chitosan nanofibrous structures: Feasibility study. J. Mater. Sci. 2007, 42, 8029–8034. [Google Scholar] [CrossRef]
- Geng, X.; Kwon, O.H.; Jang, J. Electrospinning of chitosan dissolved in concentrated acetic acid solution. Biomaterials 2005, 26, 5427–5432. [Google Scholar] [CrossRef] [PubMed]
- Spasova, M.; Manolova, N.; Paneva, D.; Rashkov, I. Preparation of chitosan-containing nanofibres by electrospinning of chitosan/poly(ethylene oxide) blend solutions. e-Polymers 2004, 4, 056. [Google Scholar] [CrossRef]
- Duan, B.; Dong, C.; Yuan, X.; Yao, K. Electrospinning of chitosan solutions in acetic acid with poly (ethylene oxide). J. Biomater. Sci. Polym. Ed. 2004, 15, 797–811. [Google Scholar] [CrossRef]
- Ignatova, M.; Manolova, N.; Markova, N.; Rashkov, I. Electrospun non-woven nanofibrous hybrid mats based on chitosan and PLA for wound-dressing applications. Macromol. Biosci. 2009, 9, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Maleki, H.; Azimi, B.; Ismaeilimoghadam, S.; Danti, S. Poly(lactic acid)-based electrospun fibrous structures for biomedical applications. Appl. Sci. 2022, 12, 3192. [Google Scholar] [CrossRef]
- Toncheva, A.; Spasova, M.; Paneva, D.; Manolova, N.; Rashkov, I. Polylactide (PLA)-based electrospun fibrous materials containing ionic drugs as wound dressing materials: A review. Int. J. Polym. Mater. Polym. Biomater. 2014, 63, 657–671. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, J.; Gao, W.; Liang, H.; Wang, H.; Li, J. Preparation of chitosan/PLA blend micro/nanofibers by electrospinning. Mater. Lett. 2009, 63, 658–660. [Google Scholar] [CrossRef]
- Siqueira, N.M.; Garcia, K.C.; Bussamara, R.; Both, M.H.; Vainstein, R.; Soares, M.D. Poly(lactic acid)/chitosan fiber mats: Investigation of effects of the support on lipase immobilization. Int. J. Biol. Macromol. 2015, 72, 998–1004. [Google Scholar] [CrossRef]
- Tighzert, W.; Habi, A.; Ajji, A.; Sadoun, T.; Boukraa-Oulad Daoud, F. Fabrication and characterization of nanofibers based on poly(lactic acid)/chitosan blends by electrospinning and their functionalization with phospholipase A1. Fibers Polym. 2017, 18, 514–524. [Google Scholar] [CrossRef]
- Jung, K.-H.; Huh, M.-W.; Meng, W.; Yuan, J.; Hyun, S.H.; Bae, J.-S.; Hudson, S.M.; Kang, I.-K. Preparation and antibacterial activity of PET/chitosan nanofibrous mats using an electrospinning technique. J. Appl. Polym. Sci. 2007, 105, 2816–2823. [Google Scholar] [CrossRef]
- Sadeghi, D.; Karbasi, S.; Razavi, S.; Mohammadi, S.; Shokrgozar, M.A.; Bonakdar, S. Electrospun poly(hydroxybutyrate)/chitosan blend fibrous scaffolds for cartilage tissue engineering. J. Appl. Polym. Sci. 2016, 133, 44171. [Google Scholar] [CrossRef]
- Dinan, B.; Bhattarai, N.; Li, Z.; Zhang, M. Characterization of chitosan based hybrid nanofiber scaffolds for tissue engineering. J. Undergrad. Res. Bioeng. 2007, 7, 33–37. [Google Scholar]
- Ignatova, M.G.; Manolova, N.E.; Toshkova, R.A.; Rashkov, I.B.; Gardeva, E.G.; Yossifova, L.S.; Alexandrov, M.T. Electrospun nanofibrous mats containing quaternized chitosan and polylactide with in vitro antitumor activity against HeLa cells. Biomacromolecules 2010, 11, 1633–1645. [Google Scholar] [CrossRef]
- Ignatova, M.; Yossifova, L.; Gardeva, E.; Manolova, N.; Toshkova, R.; Rashkov, I.; Alexandrov, M. Antiproliferative activity of nanofibers containing quaternized chitosan and/or doxorubicin against MCF-7 human breast carcinoma cell line by apoptosis. J. Bioact. Compat. Polym. 2011, 26, 539–551. [Google Scholar] [CrossRef]
- Toshkova, R.; Manolova, N.; Gardeva, E.; Ignatova, M.; Yossifova, L.; Rashkov, I.; Alexandrov, M. Antitumor activity of quaternized chitosan-based electrospun implants against Graffi myeloid tumor. Int. J. Pharm. 2010, 400, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Luxami, V.; Paul, K. Insights of 8-hydroxyquinolines: A novel target in medicinal chemistry. Bioorg. Chem. 2021, 108, 104633. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Xu, H.; Chen, W.; Zhan, P.; Liu, X. 8-Hydroxyquinoline: A privileged structure with a broad-ranging pharmacological potential. MedChemComm 2014, 6, 61–74. [Google Scholar] [CrossRef]
- Prachayasittikul, V.; Prachayasittikul, V.; Prachayasittikul, S.; Ruchirawat, S. 8-Hydroxyquinolines: A review of their metal chelating properties and medicinal applications. Drug Des. Dev. Ther. 2013, 7, 1157–1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mincheva, R.; Manolova, N.; Paneva, D.; Rashkov, I. Preparation of polyelectrolyte-containing nanofibers by electrospinning in the presence of a non-ionogenic water-soluble polymer. J. Bioact. Compat. Polym. 2005, 20, 419–435. [Google Scholar] [CrossRef]
- Spasova, M.; Manolova, N.; Rashkov, I.; Naydenov, M. Electrospun 5-chloro-8-hydroxyquinoline-loaded cellulose acetate/polyethylene glycol antifungal membranes against Esca. Polymers 2019, 11, 1617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spasova, M.; Manolova, N.; Rashkov, I.; Tsekova, P.; Georgieva, A.; Toshkova, R.; Markova, N. Cellulose acetate-based electrospun materials with a variety of biological potentials: Antibacterial, antifungal and anticancer. Polymers 2021, 13, 1631. [Google Scholar] [CrossRef] [PubMed]
- Ignatova, M.; Manolova, N.; Rashkov, I.; Markova, N.; Kukeva, R.; Stoyanova, R.; Georgieva, A.; Toshkova, R. 8-Hydroxyquinoline-5-sulfonic acid-containing poly(vinyl alcohol)/chitosan electrospun materials and their Cu2+ and Fe3+ complexes: Preparation, antibacterial, antifungal and antitumor activities. Polymers 2021, 13, 2690. [Google Scholar] [CrossRef] [PubMed]
- Spasova, M.; Manolova, N.; Markova, N.; Rashkov, I. Superhydrophobic PVDF and PVDF-HFP nanofibrous mats with antibacterial and anti-biofouling properties. Appl. Surf. Sci. 2016, 363, 363–371. [Google Scholar] [CrossRef]
- Toncheva, A.; Mincheva, R.; Kancheva, M.; Manolova, N.; Rashkov, I.; Dubois, P.; Markova, N. Antibacterial PLA/PEG electrospun fibers: Comparative study between grafting and blending PEG. Eur. Polym. J. 2016, 75, 223–233. [Google Scholar] [CrossRef]
- Stoyanova, N.; Paneva, D.; Mincheva, R.; Toncheva, A.; Manolova, N.; Dubois, P.; Rashkov, I. Poly (l-lactide) and poly (butylene succinate) immiscible blends: From electrospinning to biologically active materials. Mater. Sci. Eng. C 2014, 41, 119–126. [Google Scholar] [CrossRef]
- Ignatova, M.; Stoilova, O.; Manolova, N.; Markova, N.; Rashkov, I. Electrospun mats from styrene/maleic anhydride copolymers: Modification with amines and assessment of antimicrobial activity. Macromol. Biosci. 2010, 10, 944–954. [Google Scholar] [CrossRef]
- Ignatova, M.; Stoyanova, N.; Manolova, N.; Rashkov, I.; Kukeva, R.; Stoyanova, R.; Toshkova, R.; Georgieva, A. Electrospun materials from polylactide and Schiff base derivative of Jeffamine ED® and 8-hydroxyquinoline-2-carboxaldehyde and its complex with Cu2+: Preparation, antioxidant and antitumor activities. Mater. Sci. Eng. C 2020, 116, 111185. [Google Scholar] [CrossRef]
- Barilli, A.; Atzeri, C.; Bassanetti, I.; Ingoglia, F.; Dall’Asta, V.; Bussolati, O.; Maffini, M.; Mucchino, C.; Marchiò, L. Oxidative stress induced by copper and iron complexes with 8-hydroxyquinoline derivatives causes paraptotic death of HeLa cancer cells. Mol. Pharm. 2014, 11, 1151–1163. [Google Scholar] [CrossRef]
- Mossmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Tsai, S.C.; Lu, C.C.; Lee, C.Y.; Lin, Y.C.; Chung, J.G.; Kuo, S.C.; Amagaya, S.; Chen, F.N.; Chen, F.N.; Chen, M.Y.; et al. AKT serine/threonine protein kinase modulates bufalin-triggered intrinsic pathway of apoptosis in CAL 27 humanoral cancer cells. Int. J. Oncol. 2012, 41, 1683–1692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koombhongse, S.; Liu, W.; Reneker, D.H. Flat polymer ribbons and other shapes by electrospinning. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 2598–2606. [Google Scholar] [CrossRef]
- El-Dissouky, A.; Mohamad, G.B. Synthesis and characterization of copper(II) metal(II) binuclear complexes of N,N’-bis(8-hydroxyquinoline-7-carboxaldene)-1,3-diaminopropane. Inorg. Chim. Acta 1990, 168, 241–248. [Google Scholar] [CrossRef]
- Khandar, A.A.; Nejati, K. Synthesis and characterization of a series of copper(II) complexes with azo-linked salicylaldimine Schiff base ligands.: Crystal structure of Cu5PHAZOSALTN·CHCl3. Polyhedron 2000, 19, 607–613. [Google Scholar] [CrossRef]
- Gubendran, A.; Kesavan, M.P.; Ayyanaar, S.; Raja, J.D.; Athappan, P.; Rajesh, J. Synthesis and characterization of water-soluble copper(II), cobalt(II) and zinc(II) complexes derived from 8-hydroxyquinoline-5-sulfonic acid: DNA binding and cleavage studies. Appl. Organomet. Chem. 2017, 31, e3708. [Google Scholar] [CrossRef]
- Kim, C.H.; Khil, M.S.; Kim, H.Y.; Lee, H.U.; Jahng, K.Y. An improved hydrophilicity via electrospinning for enhanced cell attachment and proliferation. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 78, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, M.; Matsushita, T.; Ikeda, S. X-ray photoelectron spectroscopy of copper (II) complexes with donor sets of O4, N2O4, N2O2, N4, N2S2, and S4. Anal. Sci. 1993, 9, 289–291. [Google Scholar] [CrossRef] [Green Version]
- Rashid, S.; Shen, C.; Chen, X.; Li, S.; Chen, Y.; Wen, Y.; Liu, J. Enhanced catalytic ability of chitosan–Cu–Fe bimetal complex for the removal of dyes in aqueous solution. RSC Adv. 2015, 5, 90731–90741. [Google Scholar] [CrossRef]
- Pawlicka, A.; Mattos, R.I.; Tambelli, C.E.; Silva, I.D.A.; Magon, C.J.; Donoso, J.P. Magnetic resonance study of chitosan bio-membranes with proton conductivity properties. J. Membr. Sci. 2013, 429, 190–196. [Google Scholar] [CrossRef]
- Chan, S.H.; Chui, C.H.; Chan, S.W.; Kok, S.H.; Chan, D.; Tsoi, M.Y.; Leung, P.H.; Lam, A.K.; Chan, A.S.; Lam, K.H.; et al. Synthesis of 8-hydroxyquinoline derivatives as novel antitumor agents. ACS Med. Chem. Lett. 2012, 4, 170–174. [Google Scholar] [CrossRef]















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ignatova, M.; Anastasova, I.; Manolova, N.; Rashkov, I.; Markova, N.; Kukeva, R.; Stoyanova, R.; Georgieva, A.; Toshkova, R. Bio-Based Electrospun Fibers from Chitosan Schiff Base and Polylactide and Their Cu2+ and Fe3+ Complexes: Preparation and Antibacterial and Anticancer Activities. Polymers 2022, 14, 5002. https://doi.org/10.3390/polym14225002
Ignatova M, Anastasova I, Manolova N, Rashkov I, Markova N, Kukeva R, Stoyanova R, Georgieva A, Toshkova R. Bio-Based Electrospun Fibers from Chitosan Schiff Base and Polylactide and Their Cu2+ and Fe3+ Complexes: Preparation and Antibacterial and Anticancer Activities. Polymers. 2022; 14(22):5002. https://doi.org/10.3390/polym14225002
Chicago/Turabian StyleIgnatova, Milena, Ina Anastasova, Nevena Manolova, Iliya Rashkov, Nadya Markova, Rositsa Kukeva, Radostina Stoyanova, Ani Georgieva, and Reneta Toshkova. 2022. "Bio-Based Electrospun Fibers from Chitosan Schiff Base and Polylactide and Their Cu2+ and Fe3+ Complexes: Preparation and Antibacterial and Anticancer Activities" Polymers 14, no. 22: 5002. https://doi.org/10.3390/polym14225002