Effect of the Addition of Nano-Silica and Poly(ε-caprolactone) on the Mechanical and Thermal Properties of Poly(lactic acid) Blends and Possible Application in Embossing Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PLA/PCL Blends
2.3. Characterization Methods
3. Results
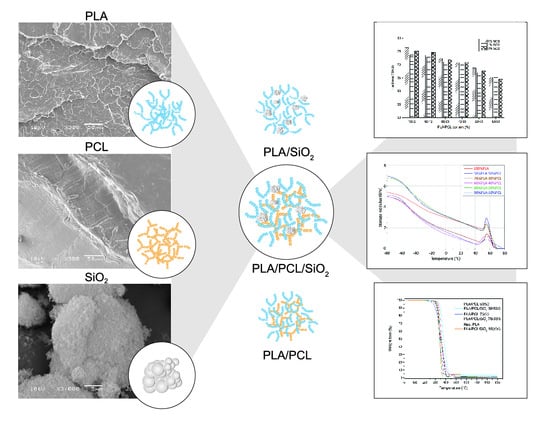
3.1. Morphology of Samples
3.2. Mechanical Properties
3.3. Thermal Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, J.H.; Murphy, R.J.; Narayan, R.; Davies, G.B.H. Biodegradable and Compostable Alternatives to Conventional Plastics. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2127–2139. [Google Scholar] [CrossRef] [Green Version]
- Tokiwa, Y.; Calabia, B.; Ugwu, C.; Aiba, S. Biodegradability of Plastics. Int. J. Mol. Sci. 2009, 10, 3722–3742. [Google Scholar] [CrossRef] [Green Version]
- Bari, E.; Morrell, J.J.; Sistani, A. Durability and Life Prediction in Biocomposites, Fibre-Reinforced Composites and Hybrid Composites. Elsevier: Amsterdam, The Netherlands, 2019; pp. 15–26. [Google Scholar]
- Imre, B.; Pukánszky, B. Compatibilization in Bio–Based and Biodegradable Polymer Blends. Eur. Polym. J. 2013, 49, 1215–1233. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.–S. Improving Polylactide/Starch Biocomposites by Grafting Polylactide with Acrylic Acid Characterization and Biodegradability Assessment. Macromol. Biosci. 2005, 5, 352–361. [Google Scholar] [CrossRef]
- Su, S.; Kopitzky, R.; Tolga, S.; Kabasci, S. Polylactide (PLA) and Its Blends with Poly(Butylene Succinate) (PBS): A Brief Review. Polymers 2019, 11, 1193. [Google Scholar] [CrossRef] [Green Version]
- Przybysz–Romatowska, M.; Haponiuk, J.; Formela, K. Poly(ε-caprolactone)/Poly(lactic acid) Blends Compatibilized by Peroxide Initiators: Comparison of Two Strategies. Polymers 2020, 12, 228. [Google Scholar] [CrossRef] [Green Version]
- Armentano, I.; Fortunati, E.; Burgos, N.; Dominici, F.; Luzi, F.; Fiori, S.; Jimenez, A.; Yoon, K.; Ahn, J.; Kang, S.; et al. Processing and Characterization of Plasticized PLA/PHB Blends for Biodegradable Multiphase Systems. Express Polym. Lett. 2015, 9, 583–596. [Google Scholar] [CrossRef]
- Akos, N.I.; Wahit, M.U.; Mohamed, R.; Yussuf, A.A. Preparation, Characterization, and Mechanical Properties of Poly(ε-caprolactone)/Polylactic Acid Blend Composites. Polym. Compos. 2013, 34, 763–768. [Google Scholar] [CrossRef]
- Yussuf, A.A.; Massoumi, I.; Hassan, A. Comparison of Polylactic Acid/Kenaf and Polylactic Acid/Rise Husk Composites: The Influence of the Natural Fibers on the Mechanical, Thermal and Biodegradability Properties. J. Polym. Environ. 2010, 18, 422–429. [Google Scholar] [CrossRef]
- Mahović Poljaček, S.; Priselac, D.; Stanković Elesini, U.; Leskovšek, M.; Leskovac, M. Preparation, Properties, and Laser Processing of Poly(ε-caprolactone)/Poly(lactic acid) Blends with Addition of Natural Fibers as a Potential for Printing Plates Application. Polym. Eng. Sci. 2021, 61, 2295–2310. [Google Scholar] [CrossRef]
- Priselac, D.; Mahović Poljaček, S.; Tomašegović, T.; Leskovac, M. Blends Based on Poly(ε-caprolactone) with Addition of Poly(lactic acid) and Coconut Fibers: Thermal Analysis, Ageing Behavior and Application for Embossing Process. Polymers 2022, 14, 1792. [Google Scholar] [CrossRef] [PubMed]
- Izdebska, J.; Thomas, S. Printing on Polymers: Fundamentals and Applications, 1st ed.; Elsevier: Boston, MA, USA, 2016; ISBN 9780323374682. [Google Scholar] [CrossRef]
- Chang, R.; Rohindra, D.; Lata, R.; Kuboyama, K.; Ougizawa, T. Development of Poly(ε-caprolactone)/Pine Resin Blends: Study of Thermal, Mechanical, and Antimicrobial Properties. Polym. Eng. Sci. 2019, 59, 32–41. [Google Scholar] [CrossRef]
- Banerjee, R.; Ray, S.S. An Overview of the Recent Advances in Polylactide-based Sustainable Nanocomposites. Polym. Eng. Sci. 2021, 61, 617–649. [Google Scholar] [CrossRef]
- Wachirahuttapong, S.; Thongpin, C.; Sombatsompop, N. Effect of PCL and Compatibility Contents on the Morphology, Crystallization and Mechanical Properties of PLA/PCL Blends. Energy Procedia 2016, 89, 198–206. [Google Scholar] [CrossRef] [Green Version]
- Matta, A.K.; Rao, R.U.; Suman, K.N.S.; Rambabu, V. Preparation and Characterization of Biodegradable PLA/PCL Polymeric Blends. Procedia Mater. Sci. 2014, 6, 1266–1270. [Google Scholar] [CrossRef] [Green Version]
- Iglesias–Montes, M.L.; Soccio, M.; Siracusa, V.; Gazzano, M.; Lotti, N.; Cyras, V.P.; Manfredi, L.B. Chitin Nanocomposite Based on Plasticized Poly(lactic acid)/Poly(3–Hydroxybutyrate) (PLA/PHB) Blends as Fully Biodegradable Packaging Materials. Polymers 2022, 14, 3177. [Google Scholar] [CrossRef]
- Åkerlund, E.; Diez–Escudero, A.; Grzeszczak, A.; Persson, C. The Effect of PCL Addition on 3D–Printable PLA/HA Composite Filaments for the Treatment of Bone Defects. Polymers 2022, 14, 3305. [Google Scholar] [CrossRef]
- Leonés, A.; Peponi, L.; Fiori, S.; Lieblich, M. Effect of the Addition of MgO Nanoparticles on the Thermally–Activated Shape Memory Behavior of Plasticized PLA Electrospun Fibers. Polymers 2022, 14, 2657. [Google Scholar] [CrossRef]
- Navarro–Baena, I.; Sessini, V.; Dominici, F.; Torre, L.; Kenny, J.M.; Peponi, L. Design of Biodegradable Blends Based on PLA and PCL: From Morphological, Thermal and Mechanical Studies to Shape Memory Behavior. Polym. Degrad. Stab. 2016, 132, 97–108. [Google Scholar] [CrossRef]
- López–Rodríguez, N.; López–Arraiza, A.; Meaurio, E.; Sarasua, J.R. Crystallization, Morphology, and Mechanical Behavior of Polylactide/Poly(ε-caprolactone) Blends. Polym. Eng. Sci. 2006, 46, 1299–1308. [Google Scholar] [CrossRef]
- Bian, X.; Zhang, B.; Sun, B.; Sun, Z.; Xiang, S.; Li, G.; Chen, X. Preparation of High Toughness and High Transparency Polylactide Blends Resin Based on Multiarmed Polycaprolactone– Block –Poly(l–Lactide). Polym. Eng. Sci. 2016, 56, 1125–1137. [Google Scholar] [CrossRef]
- Pilic, B.; Radusin, T.; Ristic, I.; Silvestre, C.; Lazic, V.; Balos, S.; Duraccio, D. Hydrophobic Silica Nanoparticles as Reinforcing Filler for Poly(lactic acid) Polymer Matrix. Hem. Ind. 2016, 70, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Fukushima, K.; Tabuani, D.; Abbate, C.; Arena, M.; Rizzarelli, P. Preparation, Characterization and Biodegradation of Biopolymer Nanocomposites Based on Fumed Silica. Eur. Polym. J. 2011, 47, 139–152. [Google Scholar] [CrossRef]
- Lv, H.; Song, S.; Sun, S.; Ren, L.; Zhang, H. Enhanced Properties of Poly(lactic acid) with Silica Nanoparticles. Polym. Adv. Technol. 2016, 27, 1156–1163. [Google Scholar] [CrossRef]
- Montes–Zavala, I.; Pérez–González, M.J.; Castrejón–González, E.O.; Santamaría–Razo, D.A.; Almendárez–Camarillo, A.; Pérez, E.; Gonzalez–Calderon, J.A. Thermal and Mechanical Properties of Poly(lactic acid) Filled with Modified Silicon Dioxide: Importance of the Surface Area. Polym. Bull. 2022, 79, 1409–1435. [Google Scholar] [CrossRef]
- Vrsaljko, D.; Macut, D.; Kovačević, V. Potential Role of Nanofillers as Compatibilizers in Immiscible PLA/LDPE Blends. J. Appl. Polym. Sci. 2015, 132, 41414. [Google Scholar] [CrossRef]
- Hutmacher, D.; Hürzeler, M.B.; Schliephake, H. A Review of Material Properties of Biodegradable and Bioresorbable Polymers and Devices for GTR and GBR Applications. Int. J. Oral Maxillofac. Implants 1996, 11, 667–678. [Google Scholar]
- Wu, C.–S. Physical Properties and Biodegradability of Maleated–Polycaprolactone/Starch Composite. Polym. Degrad. Stab. 2003, 80, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Kulinski, Z.; Piorkowska, E. Crystallization, Structure and Properties of Plasticized Poly(l–Lactide). Polymers 2005, 46, 10290–10300. [Google Scholar] [CrossRef]
- Patel, A.R.; Mankoč, B.; Bin Sintang, M.D.; Lesaffer, A.; Dewettinck, K. Fumed Silica–Based Organogels and ‘Aqueous–Organic’ Bigels. RSC Adv. 2015, 5, 9703–9708. [Google Scholar] [CrossRef]
- Awad, S.A.; Jawaid, M.; Fouad, H.; Saba, N.; Dhakal, H.N.; Alothman, O.Y.; Khalaf, E.M. A Comparative Assessment of Chemical, Mechanical, and Thermal Characteristics of Treated Oil Palm/Pineapple Fiber/Bio Phenolic Composites. Polym. Compos. 2022, 43, 2115–2128. [Google Scholar] [CrossRef]
- Dadras Chomachayi, M.; Jalali–arani, A.; Beltrán, F.R.; de la Orden, M.U.; Urreaga, J.M. Biodegradable Nanocomposites Developed from PLA/PCL Blends and Silk Fibroin Nanoparticles: Study on the Microstructure, Thermal Behavior, Crystallinity and Performance. J. Polym. Environ. 2020, 28, 1252–1264. [Google Scholar] [CrossRef]
- Priselac, D.; Tomašegović, T.; Mahović Poljaček, S.; Cigula, T.; Leskovac, M. Thermal, Surface and Mechanical Properties of PCL/PLA Composites with Coconut Fibres as an Alternative Material to Photopolymer Printing Plates. Teh. Glas. 2017, 11, 111–116. [Google Scholar]
- Abdelwahab, M.A.; Flynn, A.; Chiou, B.–S.; Imam, S.; Orts, W.; Chiellini, E. Thermal, Mechanical and Morphological Characterization of Plasticized PLA–PHB Blends. Polym. Degrad. Stab. 2012, 97, 1822–1828. [Google Scholar] [CrossRef]
- Delgado–Aguilar, M.; Puig, R.; Sazdovski, I.; Fullana–i–Palmer, P. Polylactic Acid/Polycaprolactone Blends: On the Path to Circular Economy, Substituting Single–Use Commodity Plastic Products. Materials 2020, 13, 2655. [Google Scholar] [CrossRef] [PubMed]
- Antunes, A.; Luyt, A.S.; Popelka, A.; Mahmoud, A.; Aljarod, O.; Hassan, M.K.; Kasak, P. Influence of Accelerated Weathering on the Physical and Structural Properties of Poly(Lactic–Acid)/Poly(3–Hydroxybutyrate–Co–3–Hydroxyvalerate) (PLA/PHBV) Blends. Express Polym. Lett. 2021, 15, 687–707. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The Return of a Forgotten Polymer—Polycaprolactone in the 21st Century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef] [Green Version]
- Luyt, A.S.; Antunes, A.; Popelka, A.; Mahmoud, A.; Hassan, M.K.; Kasak, P. Effect of Poly(Ε-caprolactone) and Titanium (IV) Dioxide Content on the UV and Hydrolytic Degradation of Poly(lactic acid)/Poly(Ε-caprolactone) Blends. J. Appl. Polym. Sci. 2021, 138, 51266. [Google Scholar] [CrossRef]
- Fortelny, I.; Ujcic, A.; Fambri, L.; Slouf, M. Phase Structure, Compatibility, and Toughness of PLA/PCL Blends: A Review. Front. Mater. 2019, 6, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Dorigato, A.; Sebastiani, M.; Pegoretti, A.; Fambri, L. Effect of Silica Nanoparticles on the Mechanical Performances of Poly(lactic acid). J. Polym. Environ. 2012, 20, 713–725. [Google Scholar] [CrossRef]
- Fragiadakis, D.; Pissis, P.; Bokobza, L. Glass Transition and Molecular Dynamics in Poly(Dimethylsiloxane)/Silica Nanocomposites. Polymers 2005, 46, 6001–6008. [Google Scholar] [CrossRef]
- Patrício, T.; Bártolo, P. Thermal Stability of PCL/PLA Blends Produced by Physical Blending Process. Procedia Eng. 2013, 59, 292–297. [Google Scholar] [CrossRef]
- Ferri, J.M.; Fenollar, O.; Jorda–Vilaplana, A.; García–Sanoguera, D.; Balart, R. Effect of Miscibility on Mechanical and Thermal Properties of Poly(lactic acid)/ Polycaprolactone Blends. Polym. Int. 2016, 65, 453–463. [Google Scholar] [CrossRef]
- Bulatović, V.O.; Mandić, V.; Kučić Grgić, D.; Ivančić, A. Biodegradable Polymer Blends Based on Thermoplastic Starch. J. Polym. Environ. 2021, 29, 492–508. [Google Scholar] [CrossRef]
- Spina, R. Performance Analysis of Colored PLA Products with a Fused Filament Fabrication Process. Polymers 2019, 11, 1984. [Google Scholar] [CrossRef] [Green Version]
- Madhavan Nampoothiri, K.; Nair, N.R.; John, R.P. An Overview of the Recent Developments in Polylactide (PLA) Research. Bioresour. Technol. 2010, 101, 8493–8501. [Google Scholar] [CrossRef]







| PLA/PCL (wt%) | SiO2 (wt%) | E (MPa) | SD | σ (MPa) | SD | εb (%) | SD | W (Nm) | SD |
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1590.4 | 125.1 | 39.5 | 2.9 | 2.78 | 0.18 | 0.55 | 0.10 | |
| 100/0 | 1 | 1619.2 | 61.7 | 50.5 | 4.6 | 4.50 | 0.34 | 1.31 | 0.15 |
| 3 | 1741.6 | 59.7 | 50.5 | 11.6 | 3.74 | 1.32 | 1.02 | 0.52 | |
| 0 | 1513.2 | 89.3 | 45.2 | 2.5 | 5.85 | 3.48 | 1.13 | 0.30 | |
| 90/10 | 1 | 1583.0 | 203.2 | 38.2 | 2.1 | 3.14 | 0.64 | 0.65 | 0.17 |
| 3 | 1584.2 | 108.6 | 37.4 | 8.0 | 4.46 | 2.46 | 1.19 | 0.89 | |
| 0 | 1219.3 | 128.4 | 35.9 | 5.2 | 4.83 | 1.30 | 1.08 | 0.43 | |
| 80/20 | 1 | 1379.5 | 84.3 | 31.0 | 4.6 | 2.59 | 0.17 | 0.40 | 0.17 |
| 3 | 1335.1 | 80.7 | 32.4 | 1.2 | 7.37 | 0.67 | 1.28 | 0.23 | |
| 0 | 1019.9 | 172.5 | 31.2 | 3.0 | 5.37 | 1.77 | 0.91 | 0.37 | |
| 70/30 | 1 | 1109.5 | 94.9 | 24.6 | 7.3 | 2.67 | 0.99 | 0.62 | 0.36 |
| 3 | 1122.7 | 121.9 | 29.6 | 2.6 | 3.88 | 0.83 | 0.62 | 0.21 | |
| 0 | 972.2 | 82.8 | 27.9 | 3.6 | 6.30 | 2.53 | 1.02 | 0.42 | |
| 60/40 | 1 | 1161.1 | 67.6 | 27.3 | 0.8 | 6.66 | 1.43 | 1.09 | 0.14 |
| 3 | 1200.7 | 114.7 | 27.4 | 1.1 | 4.40 | 1.76 | 0.66 | 0.26 | |
| 0 | 932.4 | 28.5 | 23.6 | 1.7 | 4.68 | 0.80 | 0.63 | 0.18 | |
| 50/50 | 1 | 929.1 | 68.1 | 26.7 | 0.7 | 8.70 | 1.75 | 1.36 | 0.18 |
| 3 | 945.6 | 78.5 | 25.8 | 0.6 | 5.28 | 1.48 | 0.79 | 0.20 |
| PLA/PCL (wt%) | SiO2 (wt%) | E’ at −80 °C (GPa) | E’ at 20 °C (GPa) |
|---|---|---|---|
| 0 | 5.374 | 2.541 | |
| 100/0 | 1 | 4.976 | 2.558 |
| 3 | 5.086 | 2.476 | |
| 0 | 6.920 | 2.561 | |
| 90/10 | 1 | 4.672 | 1.788 |
| 3 | 5.589 | 2.164 | |
| 0 | 6.736 | 2.589 | |
| 80/20 | 1 | 5.490 | 2.007 |
| 3 | 5.151 | 1.465 | |
| 0 | 4.905 | 1.312 | |
| 70/30 | 1 | 3.869 | 0.8011 |
| 3 | 5.251 | 1.119 | |
| 0 | 4.963 | 0.9030 | |
| 60/40 | 1 | 4.642 | 1.155 |
| 3 | 6.010 | 2.724 | |
| 0 | 5.016 | 1.009 | |
| 50/50 | 1 | 5.698 | 1.250 |
| 3 | 5.217 | 1.032 |
| PLA/PCL (wt%) | SiO2 (wt%) | PLA | PCL | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tg (°C) | Tcc1 (°C) | ΔHcc1 (J·g−1) | Tcc2 (°C) | ΔHcc2 (J·g−1) | Tm (°C) | ΔHm (J·g−1) | Tg (°C) | Tm (°C) | ΔHm (J·g−1) | ||
| 100/0 | 0 | 60 | 99 | 32.1 | 154 | 2.4 | 169 | −38.7 | − | − | − |
| 1 | 59 | 97 | 27.9 | 154 | 3.8 | 169 | −37.0 | − | − | − | |
| 3 | 59 | 100 | 28.2 | 156 | 2.9 | 170 | −38.3 | − | − | − | |
| 90/10 | 0 | − | 101 | 32.5 | 155 | 1.5 | 169 | −34.0 | −44 | 57 | −2.7 |
| 1 | − | 102 | 23.1 | 157 | 2.0 | 170 | −29.0 | −55 | 58 | −2.2 | |
| 3 | − | 101 | 25.6 | 157 | 1.8 | 171 | −31.4 | −71 | 55 | −1.2 | |
| 80/20 | 0 | − | 100 | 22.4 | 159 | 1.1 | 168 | −29.5 | − | 57 | −7.0 |
| 1 | − | 100 | 23.5 | 155 | 2.0 | 168 | −27.7 | −72 | 57 | −5.3 | |
| 3 | − | 105 | 24.9 | − | − | 171 | −29.4 | −69 | 56 | −3.2 | |
| 70/30 | 0 | − | 100 | 19.0 | 155 | 1.2 | 168 | −26.4 | −72 | 57 | −17.8 |
| 1 | − | 101 | 17.9 | 156 | 0.9 | 170 | −25.7 | −68 | 57 | −9.4 | |
| 3 | − | 107 | 22.2 | − | − | 171 | −25.4 | −64 | 56 | −5.1 | |
| 60/40 | 0 | − | 101 | 25.3 | 155 | 0.5 | 168 | −23.9 | −63 | 57 | −16.6 |
| 1 | − | 102 | 16.2 | 156 | 0.8 | 169 | −20.1 | −66 | 57 | −15.3 | |
| 3 | − | 104 | 18.4 | 158 | 0.4 | 171 | −21.1 | −58 | 57 | −14.1 | |
| 50/50 | 0 | − | 102 | 14.3 | 156 | 0.5 | 169 | −18.4 | −62 | 57 | −20.4 |
| 1 | − | 102 | 12.8 | 156 | 1.8 | 173 | −16.3 | −67 | 57 | −18.8 | |
| 3 | − | 102 | 14.6 | 158 | 1.3 | 172 | −19.4 | −56 | 64 | −19.3 | |
| PLA/PCL (wt%) | SiO2 (wt%) | PLA | PCL | ||
|---|---|---|---|---|---|
| Tc (°C) | ΔHc (J·g−1) | Tc (°C) | ΔHc (J·g−1) | ||
| 100/0 | 0 | 89 | 26.4 | − | − |
| 1 | 90 | 1.6 | − | − | |
| 3 | 92 | 2.3 | − | − | |
| 90/10 | 0 | 87 | 28.2 | 27 | 3.5 |
| 1 | 89 | 1.0 | 28 | 3.2 | |
| 3 | 90 | 0.4 | 23 | 3.1 | |
| 80/20 | 0 | 83 | 9.4 | 26 | 7.9 |
| 1 | 89 | 0.9 | 28 | 8.8 | |
| 3 | 93 | 0.2 | 26 | 6.5 | |
| 70/30 | 0 | / | / | 28 | 15.4 |
| 1 | 88 | 1.7 | 25 | 12.0 | |
| 3 | 93 | 0.4 | 24 | 11.8 | |
| 60/40 | 0 | 90 | 1.0 | 26 | 17.9 |
| 1 | 87 | 0.6 | 25 | 19.6 | |
| 3 | 90 | 0.6 | 25 | 16.3 | |
| 50/50 | 0 | 116 | 0.8 | 26 | 25.1 |
| 1 | 91 | 0.3 | 27 | 25.1 | |
| 3 | 90 | 1.1 | 25 | 22.3 | |
| PLA/PCL (wt%) | SiO2 (wt%) | Xc (PLA) (%) | Xc (PCL) (%) |
|---|---|---|---|
| 100/0 | 0 | 6.26 | − |
| 1 | 8.65 | − | |
| 3 | 9.58 | − | |
| 90/10 | 0 | 1.54 | 19.45 |
| 1 | 6.11 | 7.97 | |
| 3 | 6.35 | 2.94 | |
| 80/20 | 0 | 8.36 | 25.20 |
| 1 | 4.94 | 18.99 | |
| 3 | 5.32 | 11.63 | |
| 70/30 | 0 | 9.87 | 42.64 |
| 1 | 10.65 | 22.57 | |
| 3 | 4.27 | 12.18 | |
| 60/40 | 0 | 2.15 | 29.86 |
| 1 | 6.18 | 27.37 | |
| 3 | 4.28 | 25.27 | |
| 50/50 | 0 | 7.70 | 29.22 |
| 1 | 6.47 | 26.92 | |
| 3 | 9.04 | 27.70 |
| PLA/PCL (wt%) | SiO2 (wt%) | Tonset (°C) | T5% (°C) | T50% (°C) | TmaxPLA (°C) | TmaxPCL (°C) | R600 °C (%) |
|---|---|---|---|---|---|---|---|
| 0 | 312 | 316 | 345 | 349 | − | 1.3 | |
| 100/0 | 1 | 299 | 298 | 341 | 349 | − | 1.0 |
| 3 | 319 | 314 | 343 | 345 | − | 4.5 | |
| 0 | 291 | 281 | 341 | 341 | 372 | 0.7 | |
| 70/30 | 1 | 323 | 321 | 357 | 354 | 395 | 0.9 |
| 3 | 326 | 326 | 358 | 355 | 395 | 3.7 | |
| 0 | 303 | 306 | 352 | 335 | 380 | 0.5 | |
| 50/50 | 1 | 327 | 325 | 370 | 351 | 400 | 1.5 |
| 3 | 340 | 338 | 378 | 367 | 403 | 3.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahović Poljaček, S.; Priselac, D.; Tomašegović, T.; Elesini, U.S.; Leskovšek, M.; Leskovac, M. Effect of the Addition of Nano-Silica and Poly(ε-caprolactone) on the Mechanical and Thermal Properties of Poly(lactic acid) Blends and Possible Application in Embossing Process. Polymers 2022, 14, 4861. https://doi.org/10.3390/polym14224861
Mahović Poljaček S, Priselac D, Tomašegović T, Elesini US, Leskovšek M, Leskovac M. Effect of the Addition of Nano-Silica and Poly(ε-caprolactone) on the Mechanical and Thermal Properties of Poly(lactic acid) Blends and Possible Application in Embossing Process. Polymers. 2022; 14(22):4861. https://doi.org/10.3390/polym14224861
Chicago/Turabian StyleMahović Poljaček, Sanja, Dino Priselac, Tamara Tomašegović, Urška Stanković Elesini, Mirjam Leskovšek, and Mirela Leskovac. 2022. "Effect of the Addition of Nano-Silica and Poly(ε-caprolactone) on the Mechanical and Thermal Properties of Poly(lactic acid) Blends and Possible Application in Embossing Process" Polymers 14, no. 22: 4861. https://doi.org/10.3390/polym14224861