Synthesis of Optically and Redox Active Polyenaminones from Diamines and α,α’-Bis[(dimethylamino)methylidene]cyclohexanediones
Abstract
:1. Introduction
2. Experimental
2.1. Materials and General Methods
2.1.1. Materials
2.1.2. General Methods
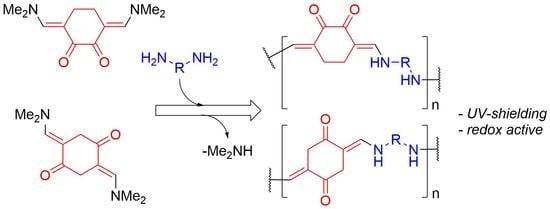
2.2. General Procedure for the Synthesis of Compounds 2a and 2b
2.2.1. (3E,6E)-3,6-bis[(dimethylamino)methylidene]cyclohexane-1,2-dione (2a)
2.2.2. (2Z,5E)-2,5-bis((dimethylamino)methylidene)cyclohexane-1,4-dione (2b)
2.3. General Procedure for the Synthesis of Compounds 4aa–4af and 4ba–4bf
2.3.1. Poly{(E)-[3-({[2-(λ2-azaneyl)ethyl]amino}methylene)-6-(λ3-methylene)cyclohexane-1,2-dione]} (4aa)
2.3.2. Poly{(E)-[3-({[3-(λ2-azaneyl)propyl]amino}methylene)-6-(λ3-methylene)cyclohexane-1,2-dione]} (4ab)
2.3.3. Poly{(E)-[3-({[4-(λ2-azaneyl)butyl]amino}methylene)-6-(λ3-methylene)cyclohexane-1,2-dione]} (4ac)
2.3.4. Poly{(E)-[3-({[2-(λ2-azaneyl)phenyl]amino}methylene)-6-(λ3-methylene)cyclohexane-1,2-dione]} (4ad)
2.3.5. Poly{(E)-[3-({[3-(λ2-azaneyl)phenyl]amino}methylene)-6-(λ3-methylene)cyclohexane-1,2-dione]} (4ae)
2.3.6. Poly{(E)-[4-({[4-(λ2-azaneyl)phenyl]amino}methylene)-6-(λ3-methylene)cyclohexane-1,2-dione]} (4af)
2.3.7. Poly{(E)-[3-({[2-(λ2-azaneyl)ethyl]amino}methylene)-5-(λ3-methylene)cyclohexane-1,4-dione]} (4ba)
2.3.8. Poly{(E)-[3-({[3-(λ2-azaneyl)propyl]amino}methylene)-5-(λ3-methylene)-cyclohexane-1,4-dione]} (4bb)
2.3.9. Poly{(E)-[3-({[4-(λ2-azaneyl)butyl]amino}methylene)-5-(λ3-methylene)cyclohexane-1,4-dione]} (4bc)
2.3.10. Poly{(E)-[3-({[2-(λ2-azaneyl)phenyl]amino}methylene)-5-(λ3-methylene)cyclohexane-1,4-dione]} (4bd)
2.3.11. Poly{(E)-[3-({[3-(λ2-azaneyl)phenyl]amino}methylene)-5-(λ3-methylene)cyclohexane-1,4-dione]} (4be)
2.3.12. Poly{(E)-[3-({[4-(λ2-azaneyl)phenyl]amino}methylene)-5-(λ3-methylene)cyclohexane-1,4-dione]} (4bf)
3. Results
3.1. Synthesis
3.2. Characterisation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kricheldorf, H.R.; Nuyken, O.; Swift, G. Handbook of Polymers Synthesis, 2nd ed.; Marcel Dekker: New York, NY, USA, 2005; pp. 1–945. [Google Scholar]
- Braun, D.; Cherdron, H.; Rehahn, M.; Ritter, H.; Voit, B. Polymer Synthesis: Theory and Practice. Fundamentals, Methods, Experiments, 5th ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–402. [Google Scholar]
- Su, W.-F. Principles of Polymer Design and Synthesis; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–306. [Google Scholar]
- Koltzenburg, S.; Maskos, M.; Nuyken, O. Polymer Chemistry; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–584. [Google Scholar]
- Edelman, P.G.; Mathisen, R.J.; Huang, S.J. Poly(amide-enamines). In Advances in Polymer Synthesis; Culbertson, B.M., McGrath, J.E., Eds.; Plenum Press: New York, NY, USA, 1985; pp. 275–290. [Google Scholar]
- Kimura, S. Preparation of Polyquinolones. Makromol. Chem. 1968, 117, 203–209. [Google Scholar] [CrossRef]
- Higashi, F.; Tai, A.; Adachi, K. The Reaction Between Diethyl Succinylsuccinate (1,4-Diethoxycarbonyl-2,5-dihydroxy-l,4-cyclohexadiene) and Amines and Its Application to Polymer Synthesis. J. Polym. Sci. A-1 1970, 8, 2563–2577. [Google Scholar] [CrossRef]
- Moore, J.A.; Kochanowski, J.E. Poly(amine esters) Derived from Diethyl 1,4-Cyclohexanedione-2,5-dicarboxylate. Macromolecules 1975, 8, 121–127. [Google Scholar] [CrossRef]
- Ueda, M.; Kino, K.; Hirono, T.; Imai, Y. Synthesis of Polyenamines by Vinylogous Nucleophilic Substitution Polymerisation of 2,2’-Disubstituted Bis(4-ethoxymethylene-5-Oxazolone) with Diamines. J. Polym. Sci. Polym. Chem. Ed. 1976, 14, 931–938. [Google Scholar] [CrossRef]
- Ueda, M.; Otaira, K.; Imai, Y. Synthesis of Polyenamines by Vinylogous Nucleophilic Substitution Polymerisation of 1,6-Diethoxy-1,5-hexadiene-3,4-dione with Diamines. J. Polym. Sci. Polym. Chem. Ed. 1978, 16, 2809–2815. [Google Scholar] [CrossRef]
- Ueda, M.; Funayama, M.; Imai, Y. Synthesis of Polyenamines with Pendant Hydroxyl Groups by Ring-Opening Polyaddition of 5,5′-Oxalylbis(3,4-dihydro-2H-pyran) with Diamines. Polym. J. 1979, 11, 491–495. [Google Scholar] [CrossRef] [Green Version]
- Christensen, P.R.; Schuermann, A.M.; Loeffler, K.E.; Helms, B.A. Closed-loop recycling of plastics enabled by dynamic covalent diketoenamine bonds. Nat. Chem. 2019, 11, 442–448. [Google Scholar] [CrossRef]
- Huang, S.J.; Pavlisko, J.; Hong, E. Poly(enamine-amides) and poly(enamine-ketones). Polym. Prepr. (ACS Div. Polym. Chem.) 1978, 19, 57–62. [Google Scholar]
- Edelmann, P.G.; Huang, S.J. Poly(amide-enamine-ethers): Synthesis and properties. Polym. Mater. Sci. Eng. 1984, 51, 244–248. [Google Scholar]
- Deng, H.; Hu, R.; Leung, A.C.S.; Zhao, E.; Lam, J.W.Y.; Tang, B.Z. Construction of regio- and stereoregular poly(enaminone)s by multicomponent tandem polymerisations of diynes, diaroyl chloride and primary amines. Polym. Chem. 2015, 6, 4436–4446. [Google Scholar] [CrossRef] [Green Version]
- Deng, H.; He, Z.; Lam, J.W.Y.; Tang, B.Z. Regio- and stereoselective construction of stimuli-responsive macromolecules by a sequential coupling-hydroamination polymerisation route. Polym. Chem. 2015, 6, 8297–8305. [Google Scholar] [CrossRef] [Green Version]
- He, B.; Zhen, S.; Wu, Y.; Hu, R.; Zhao, Z.; Qin, A.; Tang, B.Z. Cu(I)-Catalyzed amino-yne click polymerisation. Polym. Chem. 2016, 7, 7375–7382. [Google Scholar] [CrossRef]
- Imai, Y.; Sakai, N.; Sasaki, J.; Ueda, M. Synthesis of Polyenamines by Vinylogous Nucleophilic Substitution Polymerisation of 1,1’-(3,4-Dioxo-1,5-hexadienylene)di-2-pyrrolidone with Diamines. Makromol. Chem. 1979, 180, 1797–1800. [Google Scholar] [CrossRef]
- Tomažin, U.; Alič, B.; Kristl, A.; Ručigaj, A.; Grošelj, U.; Požgan, F.; Krajnc, M.; Štefane, B.; Šebenik, U.; Svete, J. Synthesis of polyenaminones by acid-catalysed amino–enaminone ‘click’ polymerisation. Eur. Polym. J. 2018, 108, 603–616. [Google Scholar] [CrossRef]
- Zupanc, A.; Kotnik, T.; Štanfel, U.; Brodnik Žugelj, H.; Kristl, A.; Ručigaj, A.; Matoh, L.; Pahovnik, D.; Grošelj, U.; Opatz, T.; et al. Chemical recycling of polyenaminones by transamination reaction via amino–enaminone polymerisation/depolymerisation. Eur. Polym. J. 2019, 121, 109282. [Google Scholar] [CrossRef]
- Goldstein, I.S. (Ed.) Organic Chemicals From Biomass; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Boca Raton, FL, USA, 1981; reissued 2018; pp. 1–310. [Google Scholar]
- Alina, R.L.; Balu, M. (Eds.) Producing Fuels and Fine Chemicals from Biomass Using Nanomaterials; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Boca Raton, FL, USA, 2014; pp. 1–313. [Google Scholar]
- Kuila, A.; Sharma, V. (Eds.) Lignocellulosic Biomass Production and Industrial Applications; CRC Press: Hoboken, NJ, USA; John Wiley & Sons, Inc.: Hoboken, NJ, USA; Scrivener Publishing LLC: Hoboken, NJ, USA, 2017; pp. 1–283. [Google Scholar]
- van Haveren, J.; Scott, E.L.; Sanders, J. In Bulk chemicals from biomass. Biofuels Bioprod. Bioref. 2008, 2, 41. [Google Scholar] [CrossRef]
- Pelckmans, M.; Renders, T.; Van de Vyver, S.; Sels, B.F. Bio-based amines through sustainable heterogeneous catalysis. Green Chem. 2017, 19, 5303. [Google Scholar] [CrossRef]
- Meng, Q.; Hou, M.; Liu, H.; Song, J.; Han, B. Synthesis of ketones from biomass-derived Feedstock. Nat. Commun. 2017, 8, 14190. [Google Scholar] [CrossRef] [Green Version]
- Han, C.; Li, H.; Shi, R.; Zhang, T.; Tong, J.; Lia, J.; Li, B. Organic quinones towards advanced electrochemical energy storage: Recent advances and challenges. J. Mater. Chem. A 2019, 7, 23378–23415. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, C.; Zhang, L.; Zhou, Y.; Yu, G. Molecular engineering of organic electroactive materials for redox flow batteries. Chem. Soc. Rev. 2018, 47, 69–103. [Google Scholar] [CrossRef]
- Stanovnik, B.; Svete, J. Synthesis of Heterocycles from Alkyl 3-(Dimethylamino)propenoates and Related Enaminones. Chem. Rev. 2004, 104, 2433–2480. [Google Scholar] [CrossRef] [PubMed]
- Develay, S.; Blackburn, O.; Thompson, A.L.; Williams, J.A.G. Cyclometalated Platinum(II) Complexes of Pyrazole-Based, N^C^N-Coordinating, Terdentate Ligands: The Contrasting Influence of Pyrazolyl and Pyridyl Rings on Luminescence. Inorg. Chem. 2008, 47, 11129–11142. [Google Scholar] [CrossRef] [PubMed]
- Al-Shiekha, M.A.; El-Dinb, A.M.S.; Hafezb, E.A.; Elnagdi, M.H. α-Enones in heterocyclic synthesis, Part I. Classical synthetic and environmentally friendly synthetic approaches to alkyl and acyl azoles and azines. J. Chem. Res. 2004, 2004, 174–179. [Google Scholar] [CrossRef]
- Yalçınkaya, E.E.; Dayan, O.; Balcan, M.; Güler, Ç. Ring-Opening Metathesis Polymerization of Bicyclo[2.2.1]hepta-2,5-diene (Norbornadiene) Initiated by New Ruthenium(II) Complex. J. Appl. Polym. Sci. 2013, 127, 1691–1692. [Google Scholar] [CrossRef]
- Gamez, P.; Steensma, R.H.; Driessen, W.L.; Reedijk, J. Copper(II) compounds of the planar-tridentate ligand 2,6-bis(pyrazol-3-yl)pyridine. Inorg. Chim. Acta 2002, 333, 51–56. [Google Scholar] [CrossRef]
- Brunner, H.; Scheck, T. Asymmetrische Katalysen, 77[1] Neue optisch aktive Pyrazolderivate für die enantioselektive Katalyse. Chem. Ber. 1992, 125, 701–709. [Google Scholar] [CrossRef]
- Prime, B.R.; Bair, H.E.; Vyazovkin, S.; Gallagher, P.K.; Riga, A. Thermogravimetric Analysis (TGA). In Thermal Analysis of Polymers: Fundamentals and Applications; Menczel, J.D., Prime, B.R., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 241–317. [Google Scholar]
- Michler, G.H. Scanning Electron Microscopy (SEM). In Electron Microscopy of Polymers; Pasch, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 87–120. [Google Scholar]
- Guo, Q. Polymer Morphology: Principles, Characterization, and Processing; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 1–445. [Google Scholar]
- Liang, Y.; Tao, Z.; Chen, J. Organic Electrode Materials for Rechargeable Lithium Batteries. Adv. Energy Mater. 2012, 2, 742–769. [Google Scholar] [CrossRef]
- Häupler, B.; Wild, A.; Schubert, U.S. Carbonyls: Powerful Organic Materials for Secondary Batteries. Adv. Energy Mater. 2015, 5, 1402034. [Google Scholar] [CrossRef]
- Nokami, T.; Matsuo, T.; Inatomi, Y.; Hojo, N.; Tsukagoshi, T.; Yoshizawa, H.; Shimizu, A.; Kuramoto, H.; Komae, K.; Tsuyama, H.; et al. Polymer-Bound Pyrene-4,5,9,10-tetraone for Fast-Charge and -Discharge Lithium-Ion Batteries with High Capacity. J. Am. Chem. Soc. 2012, 134, 19694–19700. [Google Scholar] [CrossRef]
- Schon, T.B.; McAllister, B.T.; Li, P.-F.; Seferos, D.S. The rise of organic electrode materials for energy storage. Chem. Soc. Rev. 2016, 45, 6345–6404. [Google Scholar] [CrossRef] [Green Version]
- Muench, S.; Wild, A.; Friebe, C.; Häupler, B.; Janoschka, T.; Schubert, U.S. Polymer-Based Organic Batteries. Chem. Rev. 2016, 116, 9438–9484. [Google Scholar] [CrossRef] [PubMed]
- Gaberscek, M.; Dominko, R.; Jamnik, J. Is small particle size more important than carbon coating? An example study on LiFePO4 cathodes. Electrochem. Commun. 2007, 9, 2778–2783. [Google Scholar] [CrossRef]
- Cheng, F.; Liang, J.; Tao, Z.; Chen, J. Functional Materials for Rechargeable Batteries. Adv. Mater. 2011, 23, 1695–1715. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Xu, T.; Gordin, M.L.; Jiang, Y.-B.; Bae, I.-T.; Xiao, Q.; Zhan, H.; Liu, J.; Wang, D. Polymer−Graphene Nanocomposites as Ultrafast-Charge and -Discharge Cathodes for Rechargeable Lithium Batteries. Nano Lett. 2012, 12, 2205–2211. [Google Scholar] [CrossRef] [PubMed]
- Pirnat, K.; Bitenc, J.; Vizintin, A.; Krajnc, A.; Tchernychova, E. Indirect Synthesis Route toward Cross-Coupled Polymers for High Voltage Organic Positive Electrodes. Chem. Mater. 2018, 30, 5726–5732. [Google Scholar] [CrossRef]
- Yang, J.; Shi, Y.; Sun, P.; Xiong, P.; Xu, Y. Optimization of Molecular Structure and Electrode Architecture of Anthraquinone-Containing Polymer Cathode for High-Performance Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 42305–42312. [Google Scholar] [CrossRef]









| Entry | Transformation | Yield (%) 1 | Entry | Transformation | Yield (%) 1 |
|---|---|---|---|---|---|
| 1 | 2a + 3a → 4aa | 87 | 7 | 2b + 3a → 4ba | 56 |
| 2 | 2a + 3b → 4ab | 10 | 8 | 2b + 3b → 4bb | 51 |
| 3 | 2a + 3c → 4ac | 13 | 9 | 2b + 3c → 4bc | 37 |
| 4 | 2a + 3d → 4ad | 48 | 10 | 2b + 3d → 4bd | 35 |
| 5 | 2a + 3e → 4ae | 88 | 11 | 2b + 3e → 4be | 53 |
| 6 | 2a + 3f → 4af | 99 | 12 | 2b + 3f → 4bf | 81 |
| Entry | Compound | Mw (g/mol) | Mn (g/mol) | Mw/Mn |
|---|---|---|---|---|
| 1 | 4aa | 6900 | 4800 | 1.44 |
| 2 | 4af 1 | - 1 | - 1 | - 1 |
| 3 | 4ba | 14300 | 7500 | 1.91 |
| 4 | 4bc | 49700 | 9400 | 5.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Štanfel, U.; Kotnik, T.; Ričko, S.; Grošelj, U.; Štefane, B.; Pirnat, K.; Žagar, E.; Genorio, B.; Svete, J. Synthesis of Optically and Redox Active Polyenaminones from Diamines and α,α’-Bis[(dimethylamino)methylidene]cyclohexanediones. Polymers 2022, 14, 4120. https://doi.org/10.3390/polym14194120
Štanfel U, Kotnik T, Ričko S, Grošelj U, Štefane B, Pirnat K, Žagar E, Genorio B, Svete J. Synthesis of Optically and Redox Active Polyenaminones from Diamines and α,α’-Bis[(dimethylamino)methylidene]cyclohexanediones. Polymers. 2022; 14(19):4120. https://doi.org/10.3390/polym14194120
Chicago/Turabian StyleŠtanfel, Urša, Tomaž Kotnik, Sebastijan Ričko, Uroš Grošelj, Bogdan Štefane, Klemen Pirnat, Ema Žagar, Boštjan Genorio, and Jurij Svete. 2022. "Synthesis of Optically and Redox Active Polyenaminones from Diamines and α,α’-Bis[(dimethylamino)methylidene]cyclohexanediones" Polymers 14, no. 19: 4120. https://doi.org/10.3390/polym14194120