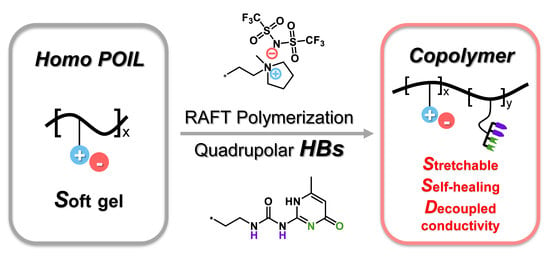
Synthesis and Characterization of Quadrupolar-Hydrogen-Bonded Polymeric Ionic Liquids for Potential Self-Healing Electrolytes
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Synthesis of Monomers and Polymers
3.2. Thermal Characterization via Thermogravimetric Analysis (TGA) and Differential Scanning Calorimetry (DSC)
3.3. Conductivity via Broadband Dielectric Spectroscopy (BDS) and Zero Shear Viscosity via Rheology
3.4. Mechanical and Self-Healing Characterization via Tensile Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Placke, T.; Kloepsch, R.; Dühnen, S.; Winter, M. Lithium ion, lithium metal, and alternative rechargeable battery technologies: The odyssey for high energy density. J. Solid State Electrochem. 2017, 21, 1939–1964. [Google Scholar] [CrossRef]
- Lu, D.; Shao, Y.; Lozano, T.; Bennett, W.D.; Graff, G.L.; Polzin, B.; Zhang, J.; Engelhard, M.H.; Saenz, N.T.; Henderson, W.A.; et al. Failure Mechanism for Fast-Charged Lithium Metal Batteries with Liquid Electrolytes. Adv. Energy Mater. 2015, 5, 1400993. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Pei, A.; Yan, K.; Sun, Y.; Wu, C.-L.; Joubert, L.-M.; Chin, R.; Koh, A.L.; Yu, Y.; et al. Atomic structure of sensitive battery materials and interfaces revealed by cryo–electron microscopy. Science 2017, 358, 506–510. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, L.; Yu, Y.; Sun, J. Progress of enhancing the safety of lithium ion battery from the electrolyte aspect. Nano Energy 2019, 55, 93–114. [Google Scholar] [CrossRef]
- Zhang, D.-Z.; Ren, Y.-Y.; Hu, Y.; Li, L.; Yan, F. Ionic Liquid/Poly (ionic liquid)-based Semi-solid State Electrolytes for Lithium-ion Batteries. Chin. J. Polym. Sci. 2020, 38, 506–513. [Google Scholar] [CrossRef]
- Li, H.; Xu, Z.; Yang, J.; Wang, J.; Hirano, S.-I. Polymer electrolytes for rechargeable lithium metal batteries. Sustain. Energy Fuels 2020, 4, 5469–5487. [Google Scholar] [CrossRef]
- Wang, J.; Li, S.; Zhao, Q.; Song, C.; Xue, Z. Structure Code for Advanced Polymer Electrolyte in Lithium-Ion Batteries. Adv. Funct. Mater. 2021, 31, 2008208. [Google Scholar] [CrossRef]
- Meng, N.; Lian, F.; Cui, G. Macromolecular Design of Lithium Conductive Polymer as Electrolyte for Solid-State Lithium Batteries. Small 2021, 17, e2005762. [Google Scholar] [CrossRef]
- Huang, X.; Nakagawa, S.; Houjou, H.; Yoshie, N. Insights into the Role of Hydrogen Bonds on the Mechanical Properties of Polymer Networks. Macromolecules 2021, 54, 4070–4080. [Google Scholar] [CrossRef]
- Song, K.; Zhu, W.; Li, X.; Yu, Z. A novel mechanical robust, self-healing and shape memory hydrogel based on PVA reinforced by cellulose nanocrystal. Mater. Lett. 2020, 260, 126884. [Google Scholar] [CrossRef]
- Lugger, S.J.D.; Houben, S.J.A.; Foelen, Y.; Debije, M.G.; Schenning, A.P.H.J.; Mulder, D.J. Hydrogen-Bonded Supramolecular Liquid Crystal Polymers: Smart Materials with Stimuli-Responsive, Self-Healing, and Recyclable Properties. Chem. Rev. 2022, 122, 4946–4975. [Google Scholar] [CrossRef]
- Chen, S.; Mahmood, N.; Beiner, M.; Binder, W.H. Self-Healing Materials from V- and H-Shaped Supramolecular Architectures. Angew. Chem. Int. Ed. 2015, 54, 10188–10192. [Google Scholar] [CrossRef]
- Herbst, F.; Seiffert, S.; Binder, W.H. Dynamic supramolecular poly(isobutylene)s for self-healing materials. Polym. Chem. 2012, 3, 3084–3092. [Google Scholar] [CrossRef]
- Long, T.; Li, Y.; Fang, X.; Sun, J. Salt-Mediated Polyampholyte Hydrogels with High Mechanical Strength, Excellent Self-Healing Property, and Satisfactory Electrical Conductivity. Adv. Funct. Mater. 2018, 28, 1804416. [Google Scholar] [CrossRef]
- Chen, J.; Peng, Q.; Thundat, T.; Zeng, H. Stretchable, Injectable, and Self-Healing Conductive Hydrogel Enabled by Multiple Hydrogen Bonding toward Wearable Electronics. Chem. Mater. 2019, 31, 4553–4563. [Google Scholar] [CrossRef]
- Ligthart, G.B.W.L.; Ohkawa, H.; Sijbesma, A.R.P.; Meijer, E.W. Complementary Quadruple Hydrogen Bonding in Supramolecular Copolymers. J. Am. Chem. Soc. 2005, 127, 810–811. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Su, A.; Wei, Y.; Liu, X.; Li, Y.; Guo, F.; Li, J.; Hu, Z.; Sun, J. Healable, Highly Conductive, Flexible, and Nonflammable Supramolecular Ionogel Electrolytes for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 19413–19420. [Google Scholar] [CrossRef]
- Lin, Y.; Hu, H.; Yi, P.; Sun, S.; Li, Y.; Liu, X.; Li, G. Zwitterionic hydrogels formed via quadruple hydrogen-bonds with ultra-fast room-temperature self-healing ability. Mater. Lett. 2020, 269, 127665. [Google Scholar] [CrossRef]
- Gan, H.; Zhang, Y.; Li, S.; Yu, L.; Wang, J.; Xue, Z. Self-Healing Single-Ion Conducting Polymer Electrolyte Formed via Supramolecular Networks for Lithium Metal Batteries. ACS Appl. Energy Mater. 2021, 4, 482–491. [Google Scholar] [CrossRef]
- Beijer, F.H.; Sijbesma, R.P.; Kooijman, H.; Spek, A.L.; Meijer, E.W. Strong Dimerization of Ureidopyrimidones via Quadruple Hydrogen Bonding. J. Am. Chem. Soc. 1998, 120, 6761–6769. [Google Scholar] [CrossRef]
- Guo, P.; Zhang, H.; Liu, X.; Sun, J. Counteranion-Mediated Intrinsic Healing of Poly (ionic liquid) Copolymers. ACS Appl. Mater. Interfaces 2018, 10, 2105–2113. [Google Scholar] [CrossRef]
- D’Angelo, A.J.; Panzer, M.J. Design of Stretchable and Self-Healing Gel Electrolytes via Fully Zwitterionic Polymer Networks in Solvate Ionic Liquids for Li-Based Batteries. Chem. Mater. 2019, 31, 2913–2922. [Google Scholar] [CrossRef]
- Sun, Y.; Ren, Y.-Y.; Li, Q.; Shi, R.-W.; Hu, Y.; Guo, J.-N.; Sun, Z.; Yan, F. Conductive, Stretchable, and Self-healing Ionic Gel Based on Dynamic Covalent Bonds and Electrostatic Interaction. Chin. J. Polym. Sci. 2019, 37, 1053–1059. [Google Scholar] [CrossRef]
- McGrath, L.M.; Rohan, J.F. Pyrrolidinium Containing Ionic Liquid Electrolytes for Li-Based Batteries. Molecules 2020, 25, 6002. [Google Scholar] [CrossRef] [PubMed]
- Keith, J.R.; Mogurampelly, S.; Aldukhi, F.; Wheatle, B.K.; Ganesan, V. Influence of molecular weight on ion-transport properties of polymeric ionic liquids. Phys. Chem. Chem. Phys. 2017, 19, 29134–29145. [Google Scholar] [CrossRef]
- Zhao, Q.; Evans, C.M. Effect of Molecular Weight on Viscosity Scaling and Ion Transport in Linear Polymerized Ionic Liquids. Macromolecules 2021, 54, 3395–3404. [Google Scholar] [CrossRef]
- Fan, F.; Wang, W.; Holt, A.P.; Feng, H.; Uhrig, D.; Lu, X.; Hong, T.; Wang, Y.; Kang, N.-G.; Mays, J.; et al. Effect of Molecular Weight on the Ion Transport Mechanism in Polymerized Ionic Liquids. Macromolecules 2016, 49, 4557–4570. [Google Scholar] [CrossRef]
- Xu, H.; Mahanthappa, M.K. Ionic Conductivities of Broad Dispersity Lithium Salt-Doped Polystyrene/Poly (ethylene oxide) Triblock Polymers. Macromolecules 2021, 54, 8798–8809. [Google Scholar] [CrossRef]
- Zhou, B.; He, D.; Hu, J.; Ye, Y.; Peng, H.; Zhou, X.; Xie, X.; Xue, Z. A flexible, self-healing and highly stretchable polymer electrolyte via quadruple hydrogen bonding for lithium-ion batteries. J. Mater. Chem. A 2018, 6, 11725–11733. [Google Scholar] [CrossRef]
- Chen, X.; Yi, L.; Zou, C.; Liu, J.; Yu, J.; Zang, Z.; Tao, X.; Luo, Z.; Guo, X.; Chen, G.; et al. High-Performance Gel Polymer Electrolyte with Self-Healing Capability for Lithium-Ion Batteries. ACS Appl. Energy Mater. 2022, 5, 5267–5276. [Google Scholar] [CrossRef]
- Cheng, Q.; Ding, S.; Zheng, Y.; Wu, M.; Peng, Y.-Y.; Diaz-Dussan, D.; Shi, Z.; Liu, Y.; Zeng, H.; Cui, Z.; et al. Dual Cross-Linked Hydrogels with Injectable, Self-Healing, and Antibacterial Properties Based on the Chemical and Physical Cross-Linking. Biomacromolecules 2021, 22, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.L.; Anthamatten, M. Synthesis, swelling behavior, and viscoelastic properties of functional poly(hydroxyethyl methacrylate) with ureidopyrimidinone side-groups. Soft Matter 2013, 9, 4058–4066. [Google Scholar] [CrossRef]
- Chen, S.; Binder, W.H. Controlled copolymerization of n-butyl acrylate with semifluorinated acrylates by RAFT polymerization. Polym. Chem. 2015, 6, 448–458. [Google Scholar] [CrossRef]
- Chen, S.; Funtan, A.; Gao, F.; Cui, B.; Meister, A.; Parkin, S.S.P.; Binder, W.H. Synthesis and Morphology of Semifluorinated Polymeric Ionic Liquids. Macromolecules 2018, 51, 8620–8628. [Google Scholar] [CrossRef]
- Chen, M.; Dugger, J.; Li, X.; Wang, Y.; Kumar, R.; Meek, K.M.; Uhrig, D.W.; Browning, J.F.; Madsen, L.A.; Long, T.E.; et al. Polymerized ionic liquids: Effects of counter-anions on ion conduction and polymerization kinetics. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 1346–1357. [Google Scholar] [CrossRef]
- Rulkens, R.; Schulze, M.; Wegner, G. Rigid-rod polyelectrolytes: Synthesis of sulfonated poly(p-phenylene)s. Macromol. Rapid Commun. 1994, 15, 669–676. [Google Scholar] [CrossRef]
- Huang, F.; Hou, L.; Wu, H.; Wang, X.; Shen, H.; Cao, W.; Yang, A.W.; Cao, Y. High-Efficiency, Environment-Friendly Electroluminescent Polymers with Stable High Work Function Metal as a Cathode: Green- and Yellow-Emitting Conjugated Polyfluorene Polyelectrolytes and Their Neutral Precursors. J. Am. Chem. Soc. 2004, 126, 9845–9853. [Google Scholar] [CrossRef]
- He, H.; Zhong, M.; Adzima, B.; Luebke, D.; Nulwala, H.; Matyjaszewski, K. A Simple and Universal Gel Permeation Chromatography Technique for Precise Molecular Weight Characterization of Well-Defined Poly(ionic liquid)s. J. Am. Chem. Soc. 2013, 135, 4227–4230. [Google Scholar] [CrossRef]
- Mori, H.; Müller, A.H. New polymeric architectures with (meth) acrylic acid segments. Prog. Polym. Sci. 2003, 28, 1403–1439. [Google Scholar] [CrossRef]
- Zhu, L.; Tong, X.; Li, M.; Wang, E. Synthesis and solution properties of anionic linear-dendritic block amphiphiles. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 4282–4288. [Google Scholar] [CrossRef]
- Moad, G.; Chong, Y.; Postma, A.; Rizzardo, E.; Thang, S. Advances in RAFT polymerization: The synthesis of polymers with defined end-groups. Polymer 2005, 46, 8458–8468. [Google Scholar] [CrossRef]
- Ogihara, W.; Washiro, S.; Nakajima, H.; Ohno, H. Effect of cation structure on the electrochemical and thermal properties of ion conductive polymers obtained from polymerizable ionic liquids. Electrochimica Acta 2006, 51, 2614–2619. [Google Scholar] [CrossRef]
- A Henderson, W.; Young, V.G.; Pearson, W.; Passerini, S.; De Long, H.C.; Trulove, P.C. Thermal phase behaviour ofN-alkyl-N-methylpyrrolidinium and piperidinium bis(trifluoromethanesulfonyl)imide salts. J. Physics Condens. Matter 2006, 18, 10377–10390. [Google Scholar] [CrossRef]
- Berthier, C.; Gorecki, W.; Minier, M.; Armand, M.; Chabagno, J.; Rigaud, P. Microscopic investigation of ionic conductivity in alkali metal salts-poly (ethylene oxide) adducts. Solid State Ionics 1983, 11, 91–95. [Google Scholar] [CrossRef]
- Qing-Shan, L.; Pei-Fang, Y.; Miao, Y.; Zhi-Cheng, T.; Chang-Ping, L.; Urs, W.-B. Dynamic Viscosity and Conductivity of Ionic Liquids [Cnpy] [NTf2] (n = 2, 4, 5). Acta Physico-Chimica Sin. 2011, 27, 2762–2766. [Google Scholar] [CrossRef]
- Chang-Ping, L.; Zhuo, L.; Ben-Xue, Z.; Qing-Shan, L.; Xiao-Xia, L. Density, Viscosity and Conductivity of Protic Ionic Liquid N,N-DimethylethanolammoniumPropionate. Acta Physico-Chimica Sin. 2013, 29, 2157–2161. [Google Scholar] [CrossRef]
- Yuan, W.-L.; Yang, X.; He, L.; Xue, Y.; Qin, S.; Tao, G.-H. Viscosity, Conductivity, and Electrochemical Property of Dicyanamide Ionic Liquids. Front. Chem. 2018, 6, 59. [Google Scholar] [CrossRef]
- Dyre, J.C.; Maass, P.; Roling, B.; Sidebottom, D.L. Fundamental questions relating to ion conduction in disordered solids. Rep. Prog. Phys. 2009, 72, 046501. [Google Scholar] [CrossRef]
- Mizuno, F.; Belieres, J.-P.; Kuwata, N.; Pradel, A.; Ribes, M.; Angell, C. Highly decoupled ionic and protonic solid electrolyte systems, in relation to other relaxing systems and their energy landscapes. J. Non-Crystalline Solids 2006, 352, 5147–5155. [Google Scholar] [CrossRef]
- Ansari, Y.; Ueno, K.; Zhao, Z.; Angell, C.A. Anhydrous Superprotonic Polymer by Superacid Protonation of Cross-linked (PNCl2)n. J. Phys. Chem. C 2013, 117, 1548–1553. [Google Scholar] [CrossRef]
- Angell, C. Fast ion motion in glassy and amorphous materials. Solid State Ionics 1983, 9–10, 3–16. [Google Scholar] [CrossRef]
- Wojnarowska, Z.; Feng, H.; Fu, Y.; Cheng, S.; Carroll, B.; Kumar, R.; Novikov, V.N.; Kisliuk, A.M.; Saito, T.; Kang, N.-G.; et al. Effect of Chain Rigidity on the Decoupling of Ion Motion from Segmental Relaxation in Polymerized Ionic Liquids: Ambient and Elevated Pressure Studies. Macromolecules 2017, 50, 6710–6721. [Google Scholar] [CrossRef]





| Sample | Entry | M/CTA a | T/°C | t/h | conv. b | Mn, thc | Mn, NMR | PDI d |
|---|---|---|---|---|---|---|---|---|
| POIL- | 1 | 20:1 | 70 | 7 | 38% | 3900 | 8000 | 1.26 |
| 2 | 20:1 | 80 | 7 | 62% | 6100 | 11,000 | 1.26 | |
| 3 | 100:1 | 80 | 7 | 67% | 32,500 | 40,100 | 1.34 | |
| 4 | 200:1 | 80 | 7 | 79% | 75,800 | 82,100 | 1.36 |
| Sample | Entry | M/CTA a | fUPy b | T/°C | t/h | conv. c | FUPy, NMR d | DPIL e | DPUPy f | Mn, th g | Mn, NMR | PDI h |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CPILU- | 5 | 20:1 | 5% | 80 | 7 | 55% | 4% | 22.5 | 0.9 | 5400 | 11,800 | 1.35 |
| 6 | 20:1 | 15% | 80 | 7 | 77% | 14% | 18.6 | 2.6 | 7200 | 10,700 | 1.32 | |
| 7 | 20:1 | 25% | 80 | 7 | 84% | 24% | 18.7 | 4.5 | 7400 | 10,500 | 1.25 | |
| 8 | 100:1 | 5% | 80 | 7 | 65% | 4% | 90.1 | 3.6 | 30,700 | 44,400 | 1.36 |
| Sample | Entry | Sample Info. | 5 wt%-loss T/°C | Onset T/°C | Tg, DSC/°C a |
|---|---|---|---|---|---|
| ILA | - | monomer | 348 | 348 | - |
| UPyA | - | monomer | 201 | 198 | - |
| POIL- | 2 | 11 k | 348 | 353 | −13 |
| 3 | 40 k | 360 | 357 | 7 | |
| CPILU- | 5 | 4%, 12 k | 338 | 198, 360 | 9 |
| 6 | 14%, 11 k | 297 | 212, 365 | 13 | |
| 7 | 24%, 10 k | 263 | 215, 363 | 30 |
| Sample | Entry | Sample Info. | Tg, DSC/°C a | σTg/S·cm−1b | Rσ, Tg c | η0, 80 °C Pa·s d | σ80 °C/S·cm−1e |
|---|---|---|---|---|---|---|---|
| POIL- | 2 | 11 k | −13 | 3.07 × 10−9 (−10 °C) | 6.5 | 43 | 2.46 × 10−4 |
| 3 | 40 k | 7 | 2.04 × 10−8 (10 °C) | 7.3 | 368 | 9.09 × 10−5 | |
| CPILU- | 5 | 4%, 12 k | 9 | 2.55 × 10−7 (10 °C) | 8.4 | 37 | 2.19 × 10−4 |
| 6 | 14%, 11 k | 13 | 2.19 × 10−8 (20 °C) | 7.3 | 2.76 × 103 | 4.85 × 10−5 | |
| 7 | 24%, 10 k | 30 | 3.27 × 10−8 (30 °C) | 7.5 | 2.84 × 105 | 1.84 × 10−5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Bhandary, R.; Marinow, A.; Ivanov, D.; Du, M.; Androsch, R.; Binder, W.H. Synthesis and Characterization of Quadrupolar-Hydrogen-Bonded Polymeric Ionic Liquids for Potential Self-Healing Electrolytes. Polymers 2022, 14, 4090. https://doi.org/10.3390/polym14194090
Li C, Bhandary R, Marinow A, Ivanov D, Du M, Androsch R, Binder WH. Synthesis and Characterization of Quadrupolar-Hydrogen-Bonded Polymeric Ionic Liquids for Potential Self-Healing Electrolytes. Polymers. 2022; 14(19):4090. https://doi.org/10.3390/polym14194090
Chicago/Turabian StyleLi, Chenming, Rajesh Bhandary, Anja Marinow, Dmitrii Ivanov, Mengxue Du, René Androsch, and Wolfgang H. Binder. 2022. "Synthesis and Characterization of Quadrupolar-Hydrogen-Bonded Polymeric Ionic Liquids for Potential Self-Healing Electrolytes" Polymers 14, no. 19: 4090. https://doi.org/10.3390/polym14194090