Performance Evaluation of CNT Reinforcement on Electroless Plating on Solid Free-Form-Fabricated PETG Specimens for Prosthetic Limb Application
Abstract
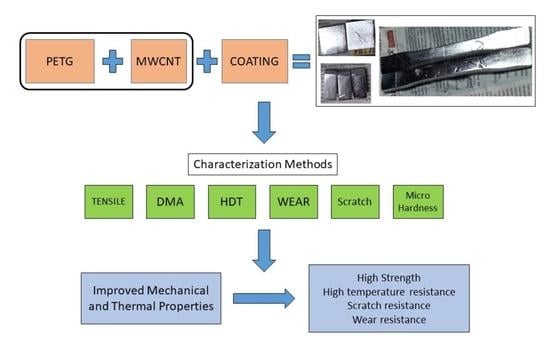
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Fabrication of 3D-Printed Specimens
2.2.2. Electroless Copper Metallization of 3D-Printed PETG-MWCNT Samples
2.2.3. Tensile Test
2.2.4. Dynamic Mechanical Analysis
2.2.5. Heat Distortion Temperature
2.2.6. Wear Test
2.2.7. Water Contact Angle Measurements (Wettability Test)
2.2.8. Scratch Test
2.2.9. Microhardness
3. Results and Discussion
3.1. Tensile Strength
3.2. Dynamic Mechanical Analysis
3.3. Heat Distortion Test
3.4. Wear
3.5. Contact Angle
3.6. Scratch Test
- P = applied load;
- w = width of the scratch measured from the microscope;
- = constant value.

3.7. Microhardness
4. Conclusions
- With the addition of MWCNTs to the PETG, there was an improvement in the base material properties. Furthermore, the electroless metal layer coating enhanced the strength of the PETG material.
- The heat distortion temperature value increased due to the coating on the PETG-MWCNT compared to the uncoated PETG sample, showing that the material can resist high temperatures; hence, the blended PETG+MWCNT polymer with the coating can be extensively used for various applications.
- The wear characterization specified that the initial wear rate was considerably decreased due to the thickness of the electroless metal layer coating on the PETG-MWCNT specimen, and through the contact angle measurement, it was evident that, with a greater coating thickness on the PETG-MWCNT specimen, the wettability was maximum.
- From the scratch test, it was noted that the PETG-MWCNT substrates coated with Ni had the lowest penetration depth and lowest friction coefficient. The microhardness test indicated the high indentation hardness and, hence, proved that it is superior in terms of abrasion resistance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dvorak, K.; Zarybnicka, L.; Dvorakova, J. Quality Parameters of 3D Print Products by the DMLS Method. Manuf. Technol. 2019, 19, 209–215. [Google Scholar] [CrossRef]
- Siddikali, P.; Sreekanth, P.R. Modeling of pneumatic controlled biomimetic articulated passive prosthetic spring-loaded knee mechanism for transfemoral amputees. Mater. Today Proc. 2020, 27, 829–834. [Google Scholar] [CrossRef]
- Sudin, M.N.; Ramli, F.R.; Alkahari, M.R.; Abdullah, M.A. Comparison of wear behavior of ABS and ABS composite parts fabricated via fused deposition modeling. Int. J. Adv. Appl. Sci. 2018, 5, 164–169. [Google Scholar] [CrossRef]
- Ryspayeva, A.; Jones, T.D.; Khan, S.R.; Esfahani, M.N.; Shuttleworth, M.P.; Harris, R.A.; Marques-Hueso, J. Selective Metallization of 3D Printable Thermoplastic Polyurethanes. IEEE Access 2019, 7, 104947–104955. [Google Scholar] [CrossRef]
- Lin, S.; Guo, W.; Chen, C.; Ma, J.; Wang, B. Mechanical properties and morphology of biodegradable poly(lactic acid)/poly(butylene adipate-co-terephthalate) blends compatibilized by transesterification. Mater. Des. 2012, 36, 604–608. [Google Scholar] [CrossRef]
- Bembenek, M.; Kowalski, Ł.; Kosoń-Schab, A. Research on the Influence of Processing Parameters on the Specific Tensile Strength of FDM Additive Manufactured PET-G and PLA Materials. Polymers 2022, 14, 2446. [Google Scholar] [CrossRef] [PubMed]
- Erden, M.A.; Akgul, Y.; Kayabas, O.; Ahlatci, H.; Cetinkaya, K.; Ozturk, F.H. Mechanical properties of graphene-nanoparticle and carbon-nanotube-reinforced pe-matrix nanocomposites. Mater. Tehnol. 2019, 53, 785–789. [Google Scholar] [CrossRef]
- Dalai, N.; Sreekanth, P.R. UHMWPE/nanodiamond nanocomposites for orthopaedic applications: A novel sandwich configuration based approach. J. Mech. Behav. Biomed. Mater. 2021, 116, 104327. [Google Scholar] [CrossRef] [PubMed]
- Gallastegui, A.; Dominguez-Alfaro, A.; Lezama, L.; Alegret, N.; Prato, M.; Gómez, M.L.; Mecerreyes, D. Fast Visible-Light Photopolymerization in the Presence of Multiwalled Carbon Nanotubes: Toward 3D Printing Conducting Nanocomposites. ACS Macro Lett. 2022, 11, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.N.; Yap, S.L.; Samsudin, F.N.D.B.; Khan, M.Z.; Srinivasa, R.S.P. Effect of Argon Plasma Treatment on Tribological Properties of UHMWPE/MWCNT Nanocomposites. Polymers 2016, 8, 295. [Google Scholar] [CrossRef]
- Rallini, M.; Kenny, J.M. 3-nanofillers in polymers. In Modification of Polymer Properties; William Andrew Pub.: Norwich, NY, USA, 2017. [Google Scholar]
- Zhu, Y.; Ramadani, E.; Egap, E. Thiol ligand capped quantum dot as an efficient and oxygen tolerance photoinitiator for aqueous phase radical polymerization and 3D printing under visible light. Polym. Chem. 2021, 12, 5106–5116. [Google Scholar] [CrossRef]
- Padhi, S.K.; Mahapatra, S.S.; Padhi, R.; Das, H.C. Performance analysis of a thick copper-electroplated FDM ABS plastic rapid tool EDM electrode. Adv. Manuf. 2018, 6, 442–456. [Google Scholar] [CrossRef]
- Szykiedans, K.; Credo, W.; Osiński, D. Selected Mechanical Properties of PETG 3D Prints. Procedia Eng. 2017, 177, 455–461. [Google Scholar] [CrossRef]
- Hamidi, A.; Tadesse, Y. Single step 3D printing of bioinspired structures via metal reinforced thermoplastic and highly stretchable elastomer. Compos. Struct. 2019, 210, 250–261. [Google Scholar] [CrossRef]
- Guessasma, S.; Belhabib, S.; Nouri, H. Printability and Tensile Performance of 3D Printed Polyethylene Terephthalate Glycol Using Fused Deposition Modelling. Polymers 2019, 11, 1220. [Google Scholar] [CrossRef]
- Dalai, N.; Sreekanth, P.R. Mechanical properties of graphene and nano-diamond reinforced ultra high molecular weight polyethylene. Mater. Today: Proc. 2019, 27, 1013–1016. [Google Scholar] [CrossRef]
- Yesaswi, C.S.; Sreekanth, P.S.R. Characterisation of Silver-coated Teflon fabric-reinforced Nafion ionic polymer metal composite with carbon nanotubes and graphene nanoparticles. Iran. Polym. J. 2022, 31, 485–502. [Google Scholar] [CrossRef]
- Carneiro, Í.; Simões, S. Effect of Morphology and Structure of MWCNTs on Metal Matrix Nanocomposites. Materials 2020, 13, 5557. [Google Scholar] [CrossRef]
- Casanova, E.G.O.; Mandujano, H.A.T.; Aguirre, M.R. Microscopy and Spectroscopy Characterization of Carbon Nanotubes Grown at Different Temperatures Using Cyclohexanol as Carbon Source. J. Spectrosc. 2019, 2019, 6043523. [Google Scholar] [CrossRef]
- Huang, X.; Farra, R.; Schlögl, R.; Willinger, M.-G. Growth and Termination Dynamics of Multiwalled Carbon Nanotubes at Near Ambient Pressure: An in Situ Transmission Electron Microscopy Study. Nano Lett. 2019, 19, 5380–5387. [Google Scholar] [CrossRef]
- Krishna Satya, S.; Rama Sreekanth, P.S. An experimental study on recycled polypropylene and high-density polyethylene and evaluation of their mechanical properties. Mater. Today Proc. 2020, 27, 920–924. [Google Scholar] [CrossRef]
- Kannan, S.; Senthilkumaran, D. Investigating the influence of electroplating layer thickness on the tensile strength for fused deposition processed abs thermoplastics. Int. J. Eng. Technol. 2014, 6, 1047–1052. [Google Scholar]
- Charbonnier, M.; Romand, M.; Goepfert, Y.; Léonard, D.; Bouadi, M. Copper metallization of polymers by a palladium-free elec-troless process. Surf. Coat. Technol. 2006, 200, 5478–5486. [Google Scholar] [CrossRef]
- Alatabe, M.J.A.; Hameed, M.A.R.; Al-Zobai, K.M.M. Exfoliate apricot kernels, natural low-cost bio-sorbent for rapid and efficient adsorption of CN- ions from aqueous solutions. Isotherm, kinetic and thermodynamic models. Int. J. Appl. Sci. Eng. 2021, 18, 1–11. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, H.; Kim, H.-J.; Chung, C.J.; Choi, Y.J.; Kim, S.-J.; Cha, J.-Y. Thermo-mechanical properties of 3D printed photocurable shape memory resin for clear aligners. Sci. Rep. 2022, 12, 1–10. [Google Scholar] [CrossRef]
- Badgayan, N.D.; Sahu, S.K.; Samanta, S.; Sreekanth, P.S.R. Evaluation of Dynamic Mechanical and Thermal Behavior of HDPE Reinforced with MWCNT/h-BNNP: An Attempt to Find Possible Substitute for a Metallic Knee in Transfemoral Prosthesis. Int. J. Thermophys. 2019, 40, 1–20. [Google Scholar] [CrossRef]
- Bhandari, S.; Lopez-Anido, R.A.; Gardner, D.J. Enhancing the interlayer tensile strength of 3D printed short carbon fiber reinforced PETG and PLA composites via annealing. Addit. Manuf. 2019, 30, 100922. [Google Scholar] [CrossRef]
- Madarvoni, S.; PS Rama, S. Dynamic mechanical behaviour of graphene, hexagonal boron nitride rein-forced carbon-kevlar, hybrid fabric-based epoxy nanocomposites. Polym. Polym. Compos. 2022, 30, 1–14. [Google Scholar]
- Zhao, X.; Wang, T.; Li, Y.; Huang, L.; Handschuh-Wang, S. Polydimethylsiloxane/Nanodiamond Composite Sponge for Enhanced Mechanical or Wettability Performance. Polymers 2019, 11, 948. [Google Scholar] [CrossRef]
- Faraji, S.; Faraji, A.H.; Noori, S.R. An investigation on electroless Cu–P composite coatings with micro and nano-SiC particles. Mater. Des. 2014, 54, 570–575. [Google Scholar] [CrossRef]
- Syafiq, A.; Vengadaesvaran, B.; Rahim, N.; Pandey, A.; Bushroa, A.; Ramesh, K.; Ramesh, S. An extensive study of the adhesion and antifogging of the transparent polydimethylsiloxane/Sylgard coating system. In Energy Materials; Elsevier: Amsterdam, The Netherlands, 2021; pp. 83–103. [Google Scholar] [CrossRef]
- Petrova, M.; Georgieva, M.; Lazarova, D.; Dobrev, D.; Pavlov, T. Electroless metallisation of ABS polymer samples produced by different technologies. Trans. IMF 2021, 99, 188–193. [Google Scholar] [CrossRef]
- Nemane, V.; Chatterjee, S. Evaluation of microstructural, mechanical, and tribological characteristics of Ni-BW-SiC electroless composite coatings involving multi-pass scratch test. Mater. Charact. 2021, 180, 111414. [Google Scholar] [CrossRef]
- Gyawali, G.; Joshi, B.; Tripathi, K.; Lee, S.W. Preparation of Ni–W–Si3N4 composite coatings and evaluation of their scratch resistance properties. Ceram. Int. 2016, 42, 3497–3503. [Google Scholar] [CrossRef]





















| Scratch No. | Load (N) | Stroke Length (mm) | Scratch Velocity (mm/s) | Scratch Offset (mm) |
|---|---|---|---|---|
| 1 | 10.0 | 10 | 1.0 | 0.50 |
| 2 | 20.0 | 10 | 1.0 | 0.50 |
| 3 | 30.0 | 10 | 1.0 | 0.50 |
| Material | UTS | Young’s Modulus (MPa) |
|---|---|---|
| PETG | 29.41 | 490.03 |
| Coated PETG | 32.37 | 491.04 |
| PETG+MWCNT | 34.86 | 516.87 |
| Coated PETG+MWCNT | 37.72 | 571.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddikali, P.; Sreekanth, P.S.R. Performance Evaluation of CNT Reinforcement on Electroless Plating on Solid Free-Form-Fabricated PETG Specimens for Prosthetic Limb Application. Polymers 2022, 14, 3366. https://doi.org/10.3390/polym14163366
Siddikali P, Sreekanth PSR. Performance Evaluation of CNT Reinforcement on Electroless Plating on Solid Free-Form-Fabricated PETG Specimens for Prosthetic Limb Application. Polymers. 2022; 14(16):3366. https://doi.org/10.3390/polym14163366
Chicago/Turabian StyleSiddikali, Palaiam, and P. S. Rama Sreekanth. 2022. "Performance Evaluation of CNT Reinforcement on Electroless Plating on Solid Free-Form-Fabricated PETG Specimens for Prosthetic Limb Application" Polymers 14, no. 16: 3366. https://doi.org/10.3390/polym14163366