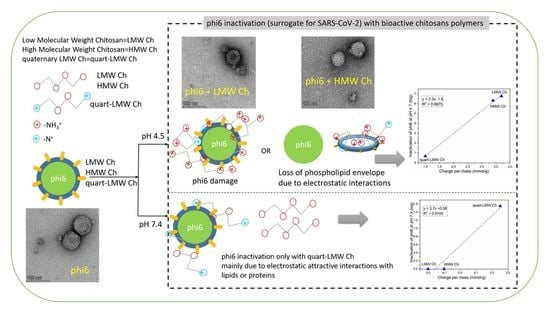
First Insights into the Antiviral Activity of Chitosan-Based Bioactive Polymers towards the Bacteriophage Phi6: Physicochemical Characterization, Inactivation Potential, and Inhibitory Mechanisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of LMW and HMW Chs Colloidal Macromolecular Formulation
2.3. Quart-LMW Ch Preparation
2.4. Chitosan-Based Polymers Characterization
2.4.1. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.4.2. X-ray Photoelectron Microscopy (XPS)
2.4.3. Potentiometric and Polyelectrolyte Titration
2.5. Determination of Antiviral Activity of Chitosans
2.5.1. Infectivity Assays
Double-Layer Plaque Assay (DAL)
2.6. Interaction of Chitosans with Phi6 and Mechanisms of Inactivation
2.6.1. Observation of Virus Morphology with Transmission Electron Microscopy (TEM)
2.6.2. ATR-FTIR of Antiviral Agent-Model Virus
2.6.3. Dynamic Light Scattering and Electrophoresis Experiments in Antiviral Agent-Model Virus Suspension
3. Results and Discussion
3.1. Physicochemical Characterization of Chitosan-Based Polymers as Potential Antiviral Agents
3.1.1. ATR-FTIR of Chitosans
3.1.2. X-ray Photoelectron Spectroscopy (XPS)
3.1.3. Potentiometric and Polyelectrolyte Titration
3.2. Antiviral Activity of Bioactive Chitosans against Phi6
3.3. Interactions between Phi6 and Chitosans, and Mechanism of Inactivation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, Y.; Bu, F.; Zhou, H.; Wang, Y.; Cui, J.; Wang, X.; Nie, G.; Xiao, H. Biosafety materials: An emerging new research direction of materials science from the COVID-19 outbreak. Mater. Chem. Front. 2020, 4, 1930–1953. [Google Scholar] [CrossRef]
- Chua, M.H.; Cheng, W.; Goh, S.S.; Kong, J.; Li, B.; Lim, J.Y.C.; Mao, L.; Wang, S.; Xue, K.; Yang, L.; et al. Face Masks in the New COVID-19 Normal: Materials, Testing, and Perspectives. Research 2020, 2020, 7286735. [Google Scholar] [CrossRef]
- Tharayil, A.; Rajakumari, R.; Mozetic, M.; Primc, G.; Thomas, S. Contact transmission of SARS-CoV-2 on fomite surfaces: Surface survival and risk reduction. Interface Focus 2021, 12, 20210042. [Google Scholar] [CrossRef]
- Pitol, A.K.; Julian, T.R. Community Transmission of SARS-CoV-2 by Surfaces: Risks and Risk Reduction Strategies. Environ. Sci. Technol. Lett. 2021, 8, 263–269. [Google Scholar] [CrossRef]
- Sun, Z.; Ostrikov, K.K. Future antiviral surfaces: Lessons from COVID-19 pandemic. Sustain. Mater. Technol. 2020, 25, e00203. [Google Scholar] [CrossRef]
- Lishchynskyi, O.; Shymborska, Y.; Stetsyshyn, Y.; Raczkowska, J.; Skirtach, A.G.; Peretiatko, T.; Budkowski, A. Passive antifouling and active self-disinfecting antiviral surfaces. Chem. Eng. J. 2022, 446, 137048. [Google Scholar] [CrossRef] [PubMed]
- Rakowska, P.D.; Tiddia, M.; Faruqui, N.; Bankier, C.; Pei, Y.; Pollard, A.J.; Zhang, J.; Gilmore, I.S. Antiviral surfaces and coatings and their mechanisms of action. Commun. Mater. 2021, 2, 53. [Google Scholar] [CrossRef]
- Shirvanimoghaddam, K.; Akbari, M.K.; Yadav, R.; Al-tamimi, A.K.; Naebe, M. Fight against COVID-19: The case of antiviral surfaces. APL Mater. 2021, 9, 031112. [Google Scholar] [CrossRef]
- Otto, D.P.; De Villiers, M.M. Layer-By-Layer Nanocoating of Antiviral Polysaccharides on Surfaces to Prevent Coronavirus Infections. Molecules 2020, 25, 3415. [Google Scholar] [CrossRef]
- Chen, X.; Han, W.; Wang, G.; Zhao, X. Application prospect of polysaccharides in the development of anti-novel coronavirus drugs and vaccines. Int. J. Biol. Macromol. 2020, 164, 331–343. [Google Scholar] [CrossRef]
- Mallakpour, S.; Azadi, E.; Hussain, M.C. Chitosan, alginate, hyaluronic acid, gums, and β-glucan as potent adjuvants and vaccine delivery systems for viral threats including SARS-CoV-2: A review. Int. J. Biol. Macromol. 2021, 182, 1931–1940. [Google Scholar] [CrossRef] [PubMed]
- Mallakpour, S.; Azadi, E.; Hussain, C.M. Protection, disinfection, and immunization for healthcare during the COVID-19 pandemic: Role of natural and synthetic macromolecules. Sci. Total Environ. 2021, 776, 145989. [Google Scholar] [CrossRef]
- Wan, M.; Qin, W.; Lei, C.; Li, Q.; Meng, M.; Fang, M.; Song, W.; Chen, J.; Tay, F.; Niu, L. Bioactive Materials Biomaterials from the sea: Future building blocks for biomedical applications. Bioact. Mater. 2021, 6, 4255–4285. [Google Scholar] [CrossRef] [PubMed]
- Nilsen-Nygaard, J.; Fernandez, E.N.; Radusek, T.; Rotabakk, B.T.; Sarfraz, J.; Sharmin, N.; Sivertsvik, M.; Sone, I.; Pettersen, M.K. Current status of biobased and biodegradable food packaging materials: Impact on food quality and effect of innovative processing technologies. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1333–1380. [Google Scholar] [CrossRef] [PubMed]
- Zemljič, L.F.; Plohl, O.; Vesel, A.; Luxbacher, T.; Potrč, S. Physicochemical characterization of packaging foils coated by chitosan and polyphenols colloidal formulations. Int. J. Mol. Sci. 2020, 21, 495. [Google Scholar] [CrossRef] [PubMed]
- Glaser, T.K.; Plohl, O.; Vesel, A.; Ajdnik, U.; Ulrih, N.P.; Hrnčič, M.K.; Bren, U.; Zemljič, L.F. Functionalization of Polyethylene (PE) and Polypropylene (PP) Material Using Chitosan Nanoparticles with Incorporated Resveratrol as Potential Active Packaging. Materials 2019, 12, 2118. [Google Scholar] [CrossRef] [PubMed]
- Ajdnik, U.; Zemljič, L.F.; Plohl, O.; Pérez, L.; Trček, J.; Bračič, M.; Mohan, T. Bioactive Functional Nanolayers of Chitosan–Lysine Surfactant with Single- and Mixed-Protein-Repellent and Antibiofilm Properties for Medical Implants. ACS Appl. Mater. Interfaces 2021, 13, 23352–23368. [Google Scholar] [CrossRef] [PubMed]
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, chemical modification and characterization of chitin and chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. [Google Scholar] [CrossRef]
- Islam, M.; Biswas, S.; Sakib, N.; Ur, T. Chitosan based bioactive materials in tissue engineering applications-A review. Bioact. Mater. 2020, 5, 164–183. [Google Scholar] [CrossRef]
- Chi, J.; Zhang, X.; Chen, C.; Shao, C.; Zhao, Y.; Wang, Y. Antibacterial and angiogenic chitosan microneedle array patch for promoting wound healing. Bioact. Mater. 2020, 5, 253–259. [Google Scholar] [CrossRef]
- Plohl, O.; Ajdnik, U.; Gyergyek, S.; Ban, I.; Vesel, A.; Glaser, T.K.; Zemljič, L.F. Superior stability and high biosorbent efficiency of carboxymethylchitosan covalently linked to silica-coated core-shell magnetic nanoparticles for application in copper removal. J. Environ. Chem. Eng. 2019, 7, 102913. [Google Scholar] [CrossRef]
- Upadhyay, U.; Sreedhar, I.; Singh, S.A.; Patel, C.M.; Anitha, K.L. Recent advances in heavy metal removal by chitosan based adsorbents. Carbohydr. Polym. 2021, 251, 117000. [Google Scholar] [CrossRef] [PubMed]
- Chirkov, S.N. The antiviral activity of chitosan (review). Appl. Biochem. Microbiol. 2002, 38, 5–13. [Google Scholar] [CrossRef]
- Jaber, N.; Al-Remawi, M.; Al-Akayleh, F.; Al-Muhtaseb, N.; Al-Adham, I.S.I.; Collier, P.J. A review of the antiviral activity of Chitosan, including patented applications and its potential use against COVID-19. J. Appl. Microbiol. 2021, 132, 41–58. [Google Scholar] [CrossRef]
- Kochkina, Z.M.; Chirkov, S.N. Effect of Chitosan Derivatives on the Reproduction of Coliphages T2 and T7. Microbiology 2000, 69, 257–260. [Google Scholar] [CrossRef]
- Kochkina, Z.M.; Chirkov, S.N. Influence of chitosan derivatives on the development of phage infection in the Bacillus thuringiensis culture. Microbiology 2000, 69, 217–219. [Google Scholar] [CrossRef]
- Su, X.; Zivanovic, S.; Souza, D.H.D. Effect of Chitosan on the Infectivity of Murine Norovirus, Feline Calicivirus, and Bacteriophage MS2. J. Food Prod. 2009, 72, 2623–2628. [Google Scholar] [CrossRef]
- Davis, R.; Zivanovic, S.; Souza, D.H.D.; Davidson, P.M. Effectiveness of chitosan on the inactivation of enteric viral surrogates. Food Microbiol. 2012, 32, 57–62. [Google Scholar] [CrossRef]
- Amankwaah, C.; Li, J.; Lee, J.; Pascall, M.A. Antimicrobial Activity of Chitosan-Based Films Enriched with Green Tea Extracts on Murine Norovirus, Escherichia coli, and Listeria innocua. Int. J. Food Sci. 2020, 2020, 3941924. [Google Scholar] [CrossRef]
- He, X.; Xing, R.; Liu, S.; Qin, Y.; Li, K.; Yu, H. The improved antiviral activities of amino- modified chitosan derivatives on Newcastle virus. Drug Chem. Toxicol. 2021, 44, 335–340. [Google Scholar] [CrossRef]
- Davydova, V.N.; Nagorskaya, V.P.; Gorbach, V.I.; Kalitnik, A.A.; Reunov, A.V.; Solov’eva, T.F.; Ermak, I.M. Chitosan antiviral activity: Dependence on structure and depolymerization method. Appl. Biochem. Microbiol. 2011, 47, 103–108. [Google Scholar] [CrossRef]
- Sharma, N.; Modak, C.; Singh, P.K.; Kumar, R.; Khatri, D.; Singh, S.B. Underscoring the immense potential of chitosan in fighting a wide spectrum of viruses: A plausible molecule against SARS-CoV-2? Int. J. Biol. Macromol. 2021, 179, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Pyrć, K.; Milewska, A.; Duran, E.B.; Botwina, P.; Dabrowska, A.; Jedrysik, M.; Benedyk, M.; Lopes, R.; Arenas-Pinto, A.; Badr, M.; et al. SARS-CoV-2 inhibition using a mucoadhesive, amphiphilic chitosan that may serve as an anti-viral nasal spray. Sci. Rep. 2021, 11, 20012. [Google Scholar] [CrossRef]
- Milewska, A.; Ciejka, J.; Kaminski, K.; Karewicz, A.; Bielska, D.; Zeglen, S.; Karolak, W.; Nowakowska, M.; Potempa, J.; Bosch, B.J.; et al. Novel polymeric inhibitors of HCoV-NL63. Antivir. Res. 2013, 97, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Milewska, A.; Kaminski, K.; Ciejka, J.; Kosowicz, K.; Zeglen, S.; Wojarski, J.; Nowakowska, M.; Szczubiałka, K.; Pyrc, K. HTCC: Broad range inhibitor of coronavirus entry. PLoS ONE 2016, 11, e0156552. [Google Scholar] [CrossRef]
- Milewska, A.; Chi, Y.; Szczepanski, A.; Barreto-Duran, E.; Liu, K.; Liu, D.; Guo, X.; Ge, Y.; Li, J.; Cui, L.; et al. HTCC as a highly effective polymeric inhibitor of SARS-CoV-2 and MERS-CoV. bioRxiv 2020. [Google Scholar] [CrossRef]
- Fedorenko, A.; Grinberg, M.; Orevi, T.; Kashtan, N. Survival of the enveloped bacteriophage Phi6 (a surrogate for SARS-CoV-2) in evaporated saliva microdroplets deposited on glass surfaces. Sci. Rep. 2020, 10, 22419. [Google Scholar] [CrossRef]
- Szczubia, K.; Zazakowny, K.; Lach, R.; Nowakowska, M. Chitosan Derivatives as Novel Potential Heparin Reversal Agents. J. Med. Chem. 2010, 53, 4141–4147. [Google Scholar] [CrossRef]
- Pyrć, K.; Milewska, A.; Nowakowska, M.; Szczubialka, K.; Kaminski, K. The Use of Chitosan Polymer in the Treatment and Prevention of Infections Caused by Coronaviruses. U.S. Patent US 2015/0164938A1, 18 June 2015. [Google Scholar]
- Čakara, D.; Fras, L.; Bračič, M.; Kleinschek, K.S. Protonation behavior of cotton fabric with irreversibly adsorbed chitosan: A potentiometric titration study. Carbohydr. Polym. 2009, 78, 36–40. [Google Scholar] [CrossRef]
- Esteban, P.P.; Jenkins, A.T.A.; Arnot, T.C. Elucidation of the mechanisms of action of Bacteriophage K/nano-emulsion formulations against S. aureus via measurement of particle size and zeta potential. Colloids Surf. B 2016, 139, 87–94. [Google Scholar] [CrossRef]
- Rwei, S.; Chen, Y.; Lin, W.; Chiang, W. Synthesis and Rheological Characterization of Water-Soluble Glycidyltrimethylammonium-Chitosan. Mar. Drugs 2014, 12, 5547–5562. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Wan, Y.; Wang, X.; Zha, Q.; Liu, H.; Qiu, Z.; Zhang, S. Synthesis and characterization of N-(2-hydroxy)propyl-3-trimethyl ammonium chitosan chloride for potential application in gene delivery. Colloids Surf. B 2012, 91, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Block, K.A.; Trusiak, A.; Katz, A.; Gottlieb, P.; Alimova, A.; Wei, H.; Morales, J.; Rice, W.J.; Steiner, J.C. Disassembly of the cystovirus φ6 envelope by montmorillonite clay. Microbiologyopen 2014, 3, 42–51. [Google Scholar] [CrossRef]
- Kochkina, Z.M.; Surgucheva, N.A.; Chirkov, S.N. Inactivation of Coliphages by Chitosan Derivatives. Microbiology 2000, 69, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Laurinavičius, S.; Käkelä, R.; Bamford, D.H.; Somerharju, P. The origin of phospholipids of the enveloped bacteriophage phi6. Virology 2004, 326, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar] [CrossRef]
- Kim, K.; Jothikumar, N.; Sen, A.; Murphy, J.L.; Chellam, S. Removal and Inactivation of an Enveloped Virus Surrogate by Iron Conventional Coagulation and Electrocoagulation. Environ. Sci. Technol. 2021, 55, 2674–2683. [Google Scholar] [CrossRef] [PubMed]
- Movasaghi, Z.; Rehman, S.; Rehman, I.U. Fourier transform infrared (FTIR) spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
- Laurinavičius, S.; Bamford, D.H.; Somerharju, P. Transbilayer distribution of phospholipids in bacteriophage membranes. Biochim. Biophys. Acta Biomembr. 2007, 1768, 2568–2577. [Google Scholar] [CrossRef]
- Kozlowski, L.P. Proteome-pI: Proteome isoelectric point database. Nucleic Acids Res. 2017, 45, D1112–D1116. [Google Scholar] [CrossRef]
- Dika, C.; Duval, J.F.L.; Merlin, C.; Gantzer, C. Impact of Internal RNA on Aggregation and Electrokinetics of Viruses: Comparison between MS2 Phage and Corresponding Virus-Like Particles. Appl. Environ. Microbiol. 2011, 77, 4939–4948. [Google Scholar] [CrossRef]
- Potrč, S.; Sterniša, M.; Smole Možina, S.; Knez Hrnčič, M.; Zemljič, L.F. Bioactive Characterization of Packaging Foils Coated by Chitosan and Polyphenol Colloidal Formulations. Int. J. Mol. Sci. 2020, 21, 2610. [Google Scholar] [CrossRef] [PubMed]
- Whitworth, C.; Mu, Y.; Houston, H.; Martinez-Smith, M.; Noble-Wang, J.; Coulliette-Salmond, A.; Rose, L. Persistence of bacteriophage phi6 on porous and nonporous surfaces and the potential for its use as an ebola virus or coronavirus surrogate. Appl. Environ. Microbiol. 2020, 86, e01482-20. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, J.F.; Tzagoloff, A.; Levine, D.; Mindich, L. Proteins of bacteriophage phi6. J. Virol. 1975, 16, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Gerba, C.P. Quaternary ammonium biocides: Efficacy in Application application. Appl. Environ. Microbiol. 2015, 81, 464–469. [Google Scholar] [CrossRef]
- Hutchison, J.B.; Plummer, C.; Gamer, G.; Sehgal, A.; Purevdorj-Gage, L.; Ramdani, K. Bacteriophage Φ 6 (Phi6) as a Surrogate for Enveloped Viral pathogens in Standard and Long- lasting Virucidal Efficacy Tests. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Alhakamy, N.A.; Ahmed, O.A.A.; Ibrahim, T.S.; Aldawsari, H.M.; Eljaaly, K.; Fahmy, U.A.; Alaofi, A.L.; Caraci, F.; Caruso, G. Evaluation of the Antiviral Activity of Sitagliptin-Glatiramer Acetate Nano-Conjugates against SARS-CoV-2 Virus. Pharmaceuticals 2021, 14, 178. [Google Scholar] [CrossRef] [PubMed]








| Antiviral Agent | Elemental Composition (at.%) | |||||
|---|---|---|---|---|---|---|
| C | N | O | Si | Cl | Ca | |
| HMW Ch | 70.6 | 4.6 | 23.8 | 0.7 | / | 0.4 |
| LMW Ch | 63.0 | 6.7 | 30.3 | / | / | / |
| quart-LMW Ch | 72.4 | 4.8 | 20.1 | 1.2 | 1.5 | / |
| Antiviral Agent | pH | Inactivation | |||
|---|---|---|---|---|---|
| Log | Average (log) | % | Average (%) | ||
| LMW Ch | 4.5 ± 0.3 | 6.80 | 6.77 | 100.0 | 100.0 |
| 6.74 | 100.0 | ||||
| 7.4 ± 0.4 | 0 | 0 | 0 | 0 | |
| Quart-LMW Ch | 4.5 ± 0.3 | 0.67 | 0.67 | 78.76 | 78.76 |
| 7.4 ± 0.4 | 1.60 | 1.55 | 97.48 | 97.15 | |
| 1.59 | 97.42 | ||||
| 1.46 | 96.56 | ||||
| HMW Ch | 4.5 ± 0.3 | 6.19 | 6.32 | 99.99994 | 99.99995 |
| 6.45 | 99.99996 | ||||
| 7.4 ± 0.4 | 0 | 0 | 0 | 0 | |
| Model Virus (106 PFU/mL) | Antiviral Agent (1.25 mg/mL) | pH in One Point | Transmittance (%) | HD-Average (nm) | PDI (%) | Increased HD with Respect to Ch (%) |
|---|---|---|---|---|---|---|
| phi6 | / | 7.4 + 0.4 | 89 | 3874 ± 1343 | 36 | / |
| phi6 | / | 4.5 ± 0.3 | 89 | 600 ± 23 | 25 | / |
| / | quart-LMW Ch | 4.5 ± 0.3 | 89 | 1250 ± 163 | 21 | / |
| / | HMW Ch | 90 | 964 ± 371 | 27 | / | |
| / | LMW Ch | 89 | 1598 ± 548 | 31 | / | |
| / | quart-LMW Ch | 7.4 + 0.4 | 85 | 1577 ± 412 | 28 | / |
| / | HMW Ch | 87 | 1573 ± 415 | 34 | / | |
| / | LMW Ch | 84 | 8380 ± 1439 | 39 | / | |
| phi6 | quart-LMW Ch | 4.5 ± 0.3 | 80 | 2271 ± 1164 | 36 | 82 |
| phi6 | HMW Ch | 88 | 5405 ± 730 | 48 | 461 | |
| phi6 | LMW Ch | 86 | 6417 ± 282 | 30 | 302 | |
| phi6 | quart-LMW Ch | 7.4 + 0.4 | 78 | 2207 ± 296 | 37 | 77 |
| phi6 | HMW Ch | 83 | 1787 ± 660 | 39 | 85 | |
| phi6 | LMW Ch | 86 | 2751 ± 88 | 29 | 72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plohl, O.; Fric, K.; Filipić, A.; Kogovšek, P.; Tušek Žnidarič, M.; Zemljič, L.F. First Insights into the Antiviral Activity of Chitosan-Based Bioactive Polymers towards the Bacteriophage Phi6: Physicochemical Characterization, Inactivation Potential, and Inhibitory Mechanisms. Polymers 2022, 14, 3357. https://doi.org/10.3390/polym14163357
Plohl O, Fric K, Filipić A, Kogovšek P, Tušek Žnidarič M, Zemljič LF. First Insights into the Antiviral Activity of Chitosan-Based Bioactive Polymers towards the Bacteriophage Phi6: Physicochemical Characterization, Inactivation Potential, and Inhibitory Mechanisms. Polymers. 2022; 14(16):3357. https://doi.org/10.3390/polym14163357
Chicago/Turabian StylePlohl, Olivija, Katja Fric, Arijana Filipić, Polona Kogovšek, Magda Tušek Žnidarič, and Lidija Fras Zemljič. 2022. "First Insights into the Antiviral Activity of Chitosan-Based Bioactive Polymers towards the Bacteriophage Phi6: Physicochemical Characterization, Inactivation Potential, and Inhibitory Mechanisms" Polymers 14, no. 16: 3357. https://doi.org/10.3390/polym14163357