A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds
Abstract
:1. Introduction
2. Scaffolding and Its Importance in Biomedical Applications (Regenerative Engineering)
3. Polymers as Biomaterials for Scaffolding
- Support for new tissue growth.
- Prevention of cellular activity.
- Guided tissue response.
- Improvement of cell connection and consequent cellular activation.
- Inhibition of cellular attachment and/or activation.
- Prevention of a biological response.
3.1. Natural Biopolymer-Based Scaffolds
- Polypeptide- and protein-based: collagen, fibrin, fibrinogen, gelatin, silk, elastin, myosin, keratin, and actin.
- Polysaccharide-based: chitin, chitosan, alginate, hyaluronic acid, cellulose, agarose, dextran, and glycosaminoglycans.
- Polynucleotide-based: DNA, linear plasmid DNA, and RNA.
3.1.1. Polypeptide- and Protein-Based Scaffolds
3.1.2. Polysaccharide-Based Scaffolds
3.2. Synthetic Biopolymer-Based Scaffolds
3.3. Natural–Natural Biopolymer Composites
3.4. Natural–Synthetic Biopolymer Composites
- High batch-to-batch inconsistency owing to complicated isolation techniques from inconsistent sources.
- Poor processability and solubility blocking the utilization of industrial fabrication processes.
- Possibility of contamination by pyrogens and pathogens.
- Poor or limited material properties like elasticity, ductility, strength, and shelf life.
- High cost.
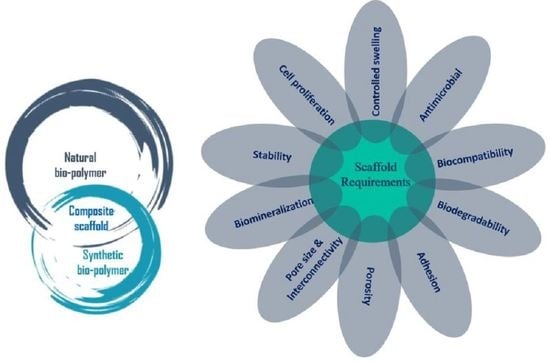
4. Properties of Polymer Scaffolds
4.1. Bioactivity
4.2. Biocompatibility
4.3. Biodegradability
4.4. Porosity and Pore Size
4.5. Morphology
4.6. Mechanical Properties
5. Commercial Status of Biopolymers
6. Scaffold Fabrication Techniques
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Berthiaume, F.; Yarmush, M.L. Tissue Engineering. In Encyclopedia of Physical Science and Technology; Elsevier: Amsterdam, The Netherlands, 2003; pp. 817–842. [Google Scholar]
- Furth, M.E.; Atala, A. Tissue Engineering. In Principles of Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2014; pp. 83–123. [Google Scholar]
- Caddeo, S.; Boffito, M.; Sartori, S. Tissue Engineering Approaches in the Design of Healthy and Pathological In Vitro Tissue Models. Front. Bioeng. Biotechnol. 2017, 5, 40. [Google Scholar] [CrossRef] [Green Version]
- Bonassar, L.J.; Vacanti, C.A. Tissue Engineering: The First Decade and Beyond. J. Cell. Biochem. 1998, 30–31, 297–303. [Google Scholar] [CrossRef]
- Bell, E. Tissue Engineering, An Overview. In Tissue Engineering; Bell, E., Ed.; Birkhäuser: Boston, MA, USA, 1993; pp. 3–15. [Google Scholar]
- Ude, C.C.; Miskon, A.; Idrus, R.B.H.; Abu Bakar, M.B. Application of Stem Cells in Tissue Engineering for Defense Medicine. Mil. Med. Res. 2018, 5, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solchaga, L.A.; Goldberg, V.M.; Caplan, A.I. Cartilage Regeneration Using Principles of Tissue Engineering. Clin. Orthop. Relat. Res. 2001, 391, S161–S170. [Google Scholar] [CrossRef]
- Ikada, Y. Challenges in Tissue Engineering. J. R. Soc. Interface 2006, 3, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Bianco, P.; Robey, P.G. Stem Cells in Tissue Engineering. Nature 2001, 414, 118–121. [Google Scholar] [CrossRef]
- Howard, D.; Buttery, L.D.; Shakesheff, K.M.; Roberts, S.J. Tissue Engineering: Strategies, Stem Cells and Scaffolds. J. Anat. 2008, 213, 66–72. [Google Scholar] [CrossRef]
- Ripamonti, U. Soluble, Insoluble and Geometric Signals Sculpt the Architecture of Mineralized Tissues. J. Cell Mol. Med. 2004, 8, 169–180. [Google Scholar] [CrossRef]
- Giardino, R.; Nicoli Aldini, N.; Torricelli, P.; Fini, M.; Giavaresi, G.; Rocca, M.; Martini, L. A Resorbable Biomaterial Shaped as a Tubular Chamber and Containing Stem Cells: A Pilot Study on Artificial Bone Regeneration. Int. J. Artif. Organs 2000, 23, 331–337. [Google Scholar] [CrossRef]
- Ma, P.X. Scaffolds for Tissue Fabrication. Mater. Today 2004, 7, 30–40. [Google Scholar] [CrossRef]
- Langer, R.; Vacanti, J.P. Tissue Engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [Green Version]
- Stock, U.A.; Vacanti, J.P. Tissue Engineering: Current State and Prospects. Annu. Rev. Med. 2001, 52, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Di Nardo, P.; Minieri, M.; Ahluwalia, A. Engineering the Stem Cell Niche and the Differentiative Micro- and Macroenvironment: Technologies and Tools for Applying Biochemical, Physical and Structural Stimuli and Their Effects on Stem Cells. In Stem Cell Engineering; Artmann, G.M., Minger, S., Hescheler, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 41–59. [Google Scholar]
- Liao, S.; Chan, C.K.; Ramakrishna, S. Stem Cells and Biomimetic Materials Strategies for Tissue Engineering. Mater. Sci. Eng. C 2008, 28, 1189–1202. [Google Scholar] [CrossRef]
- Place, E.S.; George, J.H.; Williams, C.K.; Stevens, M.M. Synthetic Polymer Scaffolds for Tissue Engineering. Chem. Soc. Rev. 2009, 38, 1139. [Google Scholar] [CrossRef]
- Langer, R.; Tirrell, D.A. Designing Materials for Biology and Medicine. Nature 2004, 428, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Boccaccini, A.R.; Blaker, J.J. Bioactive Composite Materials for Tissue Engineering Scaffolds. Expert Rev. Med. Devices 2005, 2, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Sultana, N. Biodegradable Polymer-Based Scaffolds for Bone Tissue Engineering; SpringerBriefs in Applied Sciences and Technology; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 978-3-642-34801-3. [Google Scholar]
- Mariani, E.; Lisignoli, G.; Borzì, R.M.; Pulsatelli, L. Biomaterials: Foreign Bodies or Tuners for the Immune Response? Int. J. Mol. Sci. 2019, 20, 636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutmacher, D.W. Scaffolds in Tissue Engineering Bone and Cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Freed, L.E.; Vunjak-Novakovic, G.; Biron, R.J.; Eagles, D.B.; Lesnoy, D.C.; Barlow, S.K.; Langer, R. Biodegradable Polymer Scaffolds for Tissue Engineering. Biotechnology 1994, 12, 689–693. [Google Scholar] [CrossRef]
- Olivares, A.L.; Lacroix, D. Computational Methods in the Modeling of Scaffolds for Tissue Engineering. In Computational Modeling in Tissue Engineering; Geris, L., Ed.; Studies in Mechanobiology, Tissue Engineering and Biomaterials; Springer: Berlin/Heidelberg, Germany, 2012; Volume 10, pp. 107–126. [Google Scholar]
- Giannitelli, S.M.; Accoto, D.; Trombetta, M.; Rainer, A. Current Trends in the Design of Scaffolds for Computer-Aided Tissue Engineering. Acta Biomater. 2014, 10, 580–594. [Google Scholar] [CrossRef]
- Prasadh, S.; Wong, R.C.W. Unraveling the Mechanical Strength of Biomaterials Used as a Bone Scaffold in Oral and Maxillofacial Defects. Oral Sci. Int. 2018, 15, 48–55. [Google Scholar] [CrossRef]
- Morouço, P.; Biscaia, S.; Viana, T.; Franco, M.; Malça, C.; Mateus, A.; Moura, C.; Ferreira, F.C.; Mitchell, G.; Alves, N.M. Fabrication of Poly(ε -Caprolactone) Scaffolds Reinforced with Cellulose Nanofibers, with and without the Addition of Hydroxyapatite Nanoparticles. BioMed Res. Int. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, S.; Ding, F.; Gong, L.; Gu, X. Extracellular Matrix Scaffolds for Tissue Engineering and Regenerative Medicine. Curr. Stem Cell Res. Ther. 2017, 12, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Hosseinkhani, M.; Mehrabani, D.; Karimfar, M.H.; Bakhtiyari, S.; Manafi, A.; Shirazi, R. Tissue Engineered Scaffolds in Regenerative Medicine. World J. Plast. Surg. 2014, 3, 3–7. [Google Scholar]
- Abbasian, M.; Massoumi, B.; Mohammad-Rezaei, R.; Samadian, H.; Jaymand, M. Scaffolding Polymeric Biomaterials: Are Naturally Occurring Biological Macromolecules More Appropriate for Tissue Engineering? Int. J. Biol. Macromol. 2019, 134, 673–694. [Google Scholar] [CrossRef]
- Mooney, D.J.; Sano, K.; Kaufmann, P.M.; Majahod, K.; Schloo, B.; Vacanti, J.P.; Langer, R. Long-Term Engraftment of Hepatocytes Transplanted on Biodegradable Polymer Sponges. J. Biomed. Mater. Res. 1997, 37, 413–420. [Google Scholar] [CrossRef]
- Prakasam, M.; Popescu, M.; Piticescu, R.; Largeteau, A. Fabrication Methodologies of Biomimetic and Bioactive Scaffolds for Tissue Engineering Applications. In Scaffolds in Tissue Engineering—Materials, Technologies and Clinical Applications; Baino, F., Ed.; InTech: London, UK, 2017; ISBN 978-953-51-3641-5. [Google Scholar]
- Sanz-Herrera, J.A.; García-Aznar, J.M.; Doblaré, M. On Scaffold Designing for Bone Regeneration: A Computational Multiscale Approach. Acta Biomater. 2009, 5, 219–229. [Google Scholar] [CrossRef]
- Yang, S.; Leong, K.F.; Du, Z.; Chua, C.K. The Design of Scaffolds for Use in Tissue Engineering. Part I. Traditional Factors. Tissue Eng. 2001, 7, 679–689. [Google Scholar] [CrossRef] [Green Version]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Ogueri, K.S.; Laurencin, C.T. Nanofiber Technology for Regenerative Engineering. ACS Nano 2020, 14, 9347–9363. [Google Scholar] [CrossRef]
- Ogueri, K.S.; Jafari, T.; Escobar Ivirico, J.L.; Laurencin, C.T. Polymeric biomaterials for scaffold-based bone regenerative engineering. Regen. Eng. Transl. Med. 2019, 5, 128–154. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.K. (Ed.) Engineering Biomaterials for Regenerative Medicine; Springer: New York, NY, USA, 2012; ISBN 978-1-4614-1079-9. [Google Scholar]
- Lanza, R.P.; Langer, R.S.; Vacanti, J.; Atala, A. Principles of Tissue Engineering; Academic Press: Cambridge, MA, USA, 2020; ISBN 978-0-12-821401-5. [Google Scholar]
- Zia, K.M.; Tabasum, S.; Nasif, M.; Sultan, N.; Aslam, N.; Noreen, A.; Zuber, M. A Review on Synthesis, Properties and Applications of Natural Polymer Based Carrageenan Blends and Composites. Int. J. Biol. Macromol. 2017, 96, 282–301. [Google Scholar] [CrossRef] [PubMed]
- Pina, S.; Ribeiro, V.P.; Marques, C.F.; Maia, F.R.; Silva, T.H.; Reis, R.L.; Oliveira, J.M. Scaffolding Strategies for Tissue Engineering and Regenerative Medicine Applications. Materials 2019, 12, 1824. [Google Scholar] [CrossRef] [Green Version]
- Ghassemi, T.; Shahroodi, A.; Ebrahimzadeh, M.H.; Mousavian, A.; Movaffagh, J.; Moradi, A. Current Concepts in Scaffolding for Bone Tissue Engineering. Arch. Bone Jt Surg. 2018, 6, 90–99. [Google Scholar]
- Singh, M.R.; Patel, S.; Singh, D. Natural polymer-based hydrogels as scaffolds for tissue engineering. In Nanobiomaterials in Soft Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2016; pp. 231–260. [Google Scholar]
- Heidary Rouchi, A.; Mahdavi-Mazdeh, M. Regenerative Medicine in Organ and Tissue Transplantation: Shortly and Practically Achievable? Int. J. Organ. Transpl. Med. 2015, 6, 93–98. [Google Scholar]
- Klimek, K.; Ginalska, G. Proteins and Peptides as Important Modifiers of the Polymer Scaffolds for Tissue Engineering Applications-A Review. Polymers 2020, 12, 844. [Google Scholar] [CrossRef] [Green Version]
- Mondal, M.I.H. Cellulose-Based Superabsorbent Hydrogels; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2019; ISBN 978-3-319-77829-7. [Google Scholar]
- Barbosa, M.A.; Martins, M.C.L. (Eds.) Peptides and Proteins as Biomaterials for Tissue Regeneration and Repair; Woodhead Publishing Series in Biomaterials; Woodhead Publishing is an Imprint of Elsevier: Duxford, UK, 2018; ISBN 978-0-08-100803-4. [Google Scholar]
- Gagner, J.E.; Kim, W.; Chaikof, E.L. Designing Protein-Based Biomaterials for Medical Applications. Acta Biomater. 2014, 10, 1542–1557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abascal, N.C.; Regan, L. The Past, Present and Future of Protein-Based Materials. Open Biol. 2018, 8, 180113. [Google Scholar] [CrossRef] [Green Version]
- James, P.; Numat, K. Polymerization of Peptide Polymers for Biomaterial Applications. In Polymer Science; Ylmaz, F., Ed.; InTech: London, UK, 2013; ISBN 978-953-51-0941-9. [Google Scholar]
- Reis, R.L.; Neves, N.M. (Eds.) Natural-Based Polymers for Biomedical Applications; Woodhead Publishing in Materials; CRC Press: Boca Raton, FL, USA, 2008; ISBN 978-1-84569-481-4. [Google Scholar]
- Higuchi, A.; Ling, Q.-D.; Hsu, S.-T.; Umezawa, A. Biomimetic Cell Culture Proteins as Extracellular Matrices for Stem Cell Differentiation. Chem. Rev. 2012, 112, 4507–4540. [Google Scholar] [CrossRef]
- Pieper, J.S.; van der Kraan, P.M.; Hafmans, T.; Kamp, J.; Buma, P.; van Susante, J.L.C.; van den Berg, W.B.; Veerkamp, J.H.; van Kuppevelt, T.H. Crosslinked Type II Collagen Matrices: Preparation, Characterization, and Potential for Cartilage Engineering. Biomaterials 2002, 23, 3183–3192. [Google Scholar] [CrossRef]
- Irawan, V.; Sung, T.-C.; Higuchi, A.; Ikoma, T. Collagen Scaffolds in Cartilage Tissue Engineering and Relevant Approaches for Future Development. Tissue Eng. Regen. Med. 2018, 15, 673–697. [Google Scholar] [CrossRef]
- Lu, Z.; Doulabi, B.Z.; Huang, C.; Bank, R.A.; Helder, M.N. Collagen Type II Enhances Chondrogenesis in Adipose Tissue-Derived Stem Cells by Affecting Cell Shape. Tissue Eng. Part A 2010, 16, 81–90. [Google Scholar] [CrossRef]
- Matsiko, A.; Levingstone, T.J.; O’Brien, F.J.; Gleeson, J.P. Addition of Hyaluronic Acid Improves Cellular Infiltration and Promotes Early-Stage Chondrogenesis in a Collagen-Based Scaffold for Cartilage Tissue Engineering. J. Mech. Behav. Biomed. Mater. 2012, 11, 41–52. [Google Scholar] [CrossRef]
- Djagny, V.B.; Wang, Z.; Xu, S. Gelatin: A Valuable Protein for Food and Pharmaceutical Industries: Review. Crit. Rev. Food Sci. Nutr. 2001, 41, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.E.; Nuge, T.; Yeow, T.K.; Nordin, N.; Prasad, R.G. Gelatin Based Scaffolds for Tissue Engineering—A review. Polym. Res. J. 2015, 9, 15–32. [Google Scholar]
- Aldana, A.A.; Abraham, G.A. Current Advances in Electrospun Gelatin-Based Scaffolds for Tissue Engineering Applications. Int. J. Pharm. 2017, 523, 441–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raucci, M.G.; D’Amora, U.; Ronca, A.; Demitri, C.; Ambrosio, L. Bioactivation Routes of Gelatin-Based Scaffolds to Enhance at Nanoscale Level Bone Tissue Regeneration. Front. Bioeng. Biotechnol. 2019, 7, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.H.; Kim, D.K.; Song, J.E.; Oliveira, J.M.; Reis, R.L.; Khang, G. Silk Fibroin-Based Scaffold for Bone Tissue Engineering. Adv. Exp. Med. Biol. 2018, 1077, 371–387. [Google Scholar]
- Cheng, G.; Davoudi, Z.; Xing, X.; Yu, X.; Cheng, X.; Li, Z.; Deng, H.; Wang, Q. Advanced Silk Fibroin Biomaterials for Cartilage Regeneration. ACS Biomater. Sci. Eng. 2018, 4, 2704–2715. [Google Scholar] [CrossRef]
- Tandon, S.; Kandasubramanian, B.; Ibrahim, S.M. Silk-Based Composite Scaffolds for Tissue Engineering Applications. Ind. Eng. Chem. Res. 2020, 59, 17593–17611. [Google Scholar] [CrossRef]
- Sproul, E.; Nandi, S.; Brown, A. Fibrin biomaterials for tissue regeneration and repair. In Peptides and Proteins as Biomaterials for Tissue Regeneration and Repair; Elsevier: Amsterdam, The Netherlands, 2018; pp. 151–173. [Google Scholar]
- Park, C.H.; Woo, K.M. Fibrin-Based Biomaterial Applications in Tissue Engineering and Regenerative Medicine. Adv. Exp. Med. Biol. 2018, 1064, 253–261. [Google Scholar]
- Rajangam, T.; An, S.S.A. Fibrinogen and Fibrin Based Micro and Nano Scaffolds Incorporated with Drugs, Proteins, Cells and Genes for Therapeutic Biomedical Applications. Int. J. Nanomed. 2013, 8, 3641–3662. [Google Scholar]
- Bencherif, S.A.; Gsib, O.; Egles, C. Fibrin: An underrated biopolymer for skin tissue engineering. J. Mol. Biol. Biotech. 2017, 2, 1. [Google Scholar]
- Tchobanian, A.; Van Oosterwyck, H.; Fardim, P. Polysaccharides for Tissue Engineering: Current Landscape and Future Prospects. Carbohydr. Polym. 2019, 205, 601–625. [Google Scholar] [CrossRef] [PubMed]
- Wahab, I.F.; Razak, S.I.A. Polysaccharides as Composite Biomaterials. In Composites from Renewable and Sustainable Materials; Poletto, M., Ed.; InTech: London, UK, 2016; ISBN 978-953-51-2793-2. [Google Scholar]
- Tiwari, S.; Patil, R.; Bahadur, P. Polysaccharide Based Scaffolds for Soft Tissue Engineering Applications. Polymers 2018, 11, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, R.; Shitiz, K.; Singh, A. Chitin and Chitosan: Biopolymers for Wound Management. Int. Wound J. 2017, 14, 1276–1289. [Google Scholar] [CrossRef]
- Islam, S.; Bhuiyan, M.A.R.; Islam, M.N. Chitin and Chitosan: Structure, Properties and Applications in Biomedical Engineering. J. Polym. Environ. 2017, 25, 854–866. [Google Scholar] [CrossRef]
- Azuma, K.; Ifuku, S.; Osaki, T.; Okamoto, Y.; Minami, S. Preparation and Biomedical Applications of Chitin and Chitosan Nanofibers. J. Biomed. Nanotechnol. 2014, 10, 2891–2920. [Google Scholar] [CrossRef] [PubMed]
- Gnavi, S.; Barwig, C.; Freier, T.; Haastert-Talini, K.; Grothe, C.; Geuna, S. The Use of Chitosan-Based Scaffolds to Enhance Regeneration in the Nervous System. Int. Rev. Neurobiol. 2013, 109, 1–62. [Google Scholar]
- Shalumon, K.T.; Anulekha, K.H.; Chennazhi, K.P.; Tamura, H.; Nair, S.V.; Jayakumar, R. Fabrication of Chitosan/Poly(Caprolactone) Nanofibrous Scaffold for Bone and Skin Tissue Engineering. Int. J. Biol. Macromol. 2011, 48, 571–576. [Google Scholar] [CrossRef]
- Tao, F.; Cheng, Y.; Shi, X.; Zheng, H.; Du, Y.; Xiang, W.; Deng, H. Applications of Chitin and Chitosan Nanofibers in Bone Regenerative Engineering. Carbohydr. Polym. 2020, 230, 115658. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Ali, A.; Sheikh, J. A Review on Chitosan Centred Scaffolds and Their Applications in Tissue Engineering. Int. J. Biol. Macromol. 2018, 116, 849–862. [Google Scholar] [CrossRef]
- Sieni, E.; Bazzolo, B.; Pieretti, F.; Zamuner, A.; Tasso, A.; Dettin, M.; Conconi, M.T. Breast Cancer Cells Grown on Hyaluronic Acid-Based Scaffolds as 3D in Vitro Model for Electroporation. Bioelectrochemistry 2020, 136, 107626. [Google Scholar] [CrossRef]
- Ehsanipour, A.; Nguyen, T.; Aboufadel, T.; Sathialingam, M.; Cox, P.; Xiao, W.; Walthers, C.M.; Seidlits, S.K. Injectable, Hyaluronic Acid-Based Scaffolds with Macroporous Architecture for Gene Delivery. Cell Mol. BioEng. 2019, 12, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.N.; Birkinshaw, C. Hyaluronic Acid Based Scaffolds for Tissue Engineering—A Review. Carbohydr. Polym. 2013, 92, 1262–1279. [Google Scholar] [CrossRef]
- Manna, F.; Dentini, M.; Desideri, P.; De Pità, O.; Mortilla, E.; Maras, B. Comparative Chemical Evaluation of Two Commercially Available Derivatives of Hyaluronic Acid (Hylaform from Rooster Combs and Restylane from Streptococcus) Used for Soft Tissue Augmentation. J. Eur. Acad. Derm. Venereol. 1999, 13, 183–192. [Google Scholar] [CrossRef]
- Zhu, J. Bioactive Modification of Poly(Ethylene Glycol) Hydrogels for Tissue Engineering. Biomaterials 2010, 31, 4639–4656. [Google Scholar] [CrossRef] [Green Version]
- Geckil, H.; Xu, F.; Zhang, X.; Moon, S.; Demirci, U. Engineering Hydrogels as Extracellular Matrix Mimics. Nanomedicine 2010, 5, 469–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kluge, J.A.; Mauck, R.L. Synthetic/Biopolymer Nanofibrous Composites as Dynamic Tissue Engineering Scaffolds. In Biomedical Applications of Polymeric Nanofibers; Jayakumar, R., Nair, S., Eds.; Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2011; Volume 246, pp. 101–130. [Google Scholar]
- Cascone, M.G.; Barbani, N.; Cristallini, C.; Giusti, P.; Ciardelli, G.; Lazzeri, L. Bioartificial Polymeric Materials Based on Polysaccharides. J. Biomater. Sci. Polym. Ed. 2001, 12, 267–281. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Nair, S. (Eds.) Biomedical Applications of Polymeric Nanofibers; Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2012; Volume 246, ISBN 978-3-642-27147-2. [Google Scholar]
- Ciardelli, G.; Chiono, V.; Vozzi, G.; Pracella, M.; Ahluwalia, A.; Barbani, N.; Cristallini, C.; Giusti, P. Blends of Poly-(Epsilon-Caprolactone) and Polysaccharides in Tissue Engineering Applications. Biomacromolecules 2005, 6, 1961–1976. [Google Scholar] [CrossRef]
- Migliaresi, C.; De Lollis, A.; Fambri, L.; Cohn, D. The Effect of Thermal History on the Crystallinity of Different Molecular Weight PLLA Biodegradable Polymers. Clin. Mater. 1991, 8, 111–118. [Google Scholar] [CrossRef]
- Li, R.; Yao, D. Preparation of Single Poly(Lactic Acid) Composites. J. Appl. Polym. Sci. 2008, 107, 2909–2916. [Google Scholar] [CrossRef]
- Narayanan, G.; Vernekar, V.N.; Kuyinu, E.L.; Laurencin, C.T. Poly (Lactic Acid)-Based Biomaterials for Orthopaedic Regenerative Engineering. Adv. Drug Deliv. Rev. 2016, 107, 247–276. [Google Scholar] [CrossRef] [PubMed]
- Ghalia, M.A.; Dahman, Y. Biodegradable Poly(Lactic Acid)-Based Scaffolds: Synthesis and Biomedical Applications. J. Polym. Res. 2017, 24, 74. [Google Scholar] [CrossRef]
- Lasprilla, A.J.R.; Martinez, G.A.R.; Lunelli, B.H.; Jardini, A.L.; Filho, R.M. Poly-Lactic Acid Synthesis for Application in Biomedical Devices—A Review. Biotechnol. Adv. 2012, 30, 321–328. [Google Scholar] [CrossRef]
- Gunatillake, P.A.; Adhikari, R. Biodegradable Synthetic Polymers for Tissue Engineering. Eur. Cell Mater. 2003, 5, 1–16; discussion 16. [Google Scholar] [CrossRef]
- Haghighat, F.; Ravandi, S.A.H. Mechanical Properties and in Vitro Degradation of PLGA Suture Manufactured via Electrospinning. Fibers Polym. 2014, 15, 71–77. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, X.; Ren, L.; Yao, Y.; Zhang, F.; Wang, D.-A. Poly(Lactide-Co-Glycolide)/Titania Composite Microsphere-Sintered Scaffolds for Bone Tissue Engineering Applications. J. Biomed. Mater. Res. 2010, 93, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaz, A.; Jayasuriya, A.C. Recent Trends in the Application of Widely Used Natural and Synthetic Polymer Nanocomposites in Bone Tissue Regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110698. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh-Osgouei, M.; Li, Y.; Wen, C. A Comprehensive Review of Biodegradable Synthetic Polymer-Ceramic Composites and Their Manufacture for Biomedical Applications. Bioact. Mater. 2019, 4, 22–36. [Google Scholar] [CrossRef]
- Okamoto, M.; John, B. Synthetic Biopolymer Nanocomposites for Tissue Engineering Scaffolds. Prog. Polym. Sci. 2013, 38, 1487–1503. [Google Scholar] [CrossRef]
- Liu, C.; He, H. (Eds.) Developments and Applications of Calcium Phosphate Bone Cements; Springer Series in Biomaterials Science and Engineering; Springer: Singapore, 2018; Volume 9, ISBN 978-981-10-5974-2. [Google Scholar]
- Martin, C.; Winet, H.; Bao, J.Y. Acidity near Eroding Polylactide-Polyglycolide in Vitro and in Vivo in Rabbit Tibial Bone Chambers. Biomaterials 1996, 17, 2373–2380. [Google Scholar] [CrossRef]
- Lopes, M.S.; Jardini, A.L.; Filho, R.M. Poly (Lactic Acid) Production for Tissue Engineering Applications. Procedia Eng. 2012, 42, 1402–1413. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.R.; Bei, J.Z.; Wang, S.G. Polycaprolactone Microparticles and Their Biodegradation. Polym. Degrad. Stab. 2000, 67, 455–459. [Google Scholar] [CrossRef]
- Pitt, C.G.; Gratzl, M.M.; Kimmel, G.L.; Surles, J.; Schindler, A. Aliphatic Polyesters II. The Degradation of Poly (DL-Lactide), Poly (Epsilon-Caprolactone), and Their Copolymers in Vivo. Biomaterials 1981, 2, 215–220. [Google Scholar] [CrossRef]
- Shimao, M. Biodegradation of Plastics. Curr. Opin. Biotechnol. 2001, 12, 242–247. [Google Scholar] [CrossRef]
- Zhu, J.; Marchant, R.E. Design Properties of Hydrogel Tissue-Engineering Scaffolds. Expert Rev. Med. Devices 2011, 8, 607–626. [Google Scholar] [CrossRef]
- Merrill, E.W.; Salzman, W. Polyethylene oxide as a biomaterial. ASAIO J. 1983, 6, 60–64. [Google Scholar]
- Ostuni, E.; Chapman, R.G.; Holmlin, R.E.; Takayama, S.; Whitesides, G.M. A Survey of Structure−Property Relationships of Surfaces That Resist the Adsorption of Protein. Langmuir 2001, 17, 5605–5620. [Google Scholar] [CrossRef]
- Kasper, F.K.; Tanahashi, K.; Fisher, J.P.; Mikos, A.G. Synthesis of Poly(Propylene Fumarate). Nat. Protoc. 2009, 4, 518–525. [Google Scholar] [CrossRef]
- Tan, H.; Marra, K.G. Injectable, Biodegradable Hydrogels for Tissue Engineering Applications. Materials 2010, 3, 1746–1767. [Google Scholar] [CrossRef]
- Chavarría-Gaytán, M.C.; Olivas-Armendáriz, I.; García-Casillas, P.E.; Martínez-Villafañe, A.; Martínez-Pérez, C.A. Synthesis and Characterization of Polyurethane Scaffolds for Biomedical Applications. MRS Proc. 2009, 1243, 19. [Google Scholar] [CrossRef]
- Burke, A.; Hasirci, N. Polyurethanes in Biomedical Applications. In Biomaterials; Hasirci, N., Hasirci, V., Eds.; Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 2004; Volume 553, pp. 83–101. [Google Scholar]
- Ahmad, S. Polyurethane: A Versatile Scaffold for Biomedical Applications. SBB 2018, 2. [Google Scholar] [CrossRef]
- Wendels, S.; Avérous, L. Biobased Polyurethanes for Biomedical Applications. Bioact. Mater. 2021, 6, 1083–1106. [Google Scholar] [CrossRef] [PubMed]
- Mondal, D.; Griffith, M.; Venkatraman, S.S. Polycaprolactone-Based Biomaterials for Tissue Engineering and Drug Delivery: Current Scenario and Challenges. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 255–265. [Google Scholar] [CrossRef]
- Rai, P.; Mehrotra, S.; Priya, S.; Gnansounou, E.; Sharma, S.K. Recent Advances in the Sustainable Design and Applications of Biodegradable Polymers. Bioresour. Technol. 2021, 325, 124739. [Google Scholar] [CrossRef]
- Pektok, E.; Nottelet, B.; Tille, J.-C.; Gurny, R.; Kalangos, A.; Moeller, M.; Walpoth, B.H. Degradation and Healing Characteristics of Small-Diameter Poly(ε-Caprolactone) Vascular Grafts in the Rat Systemic Arterial Circulation. Circulation 2008, 118, 2563–2570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, X.; Mikos, A.G. Poly(propylene fumarate). In An Introduction to Biomaterials; Guelcher, S.A., Hollinger, J.O., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 205–218. [Google Scholar]
- Mistry, A.S.; Mikos, A.G. Tissue Engineering Strategies for Bone Regeneration. Adv. Biochem. Eng. Biotechnol. 2005, 94, 1–22. [Google Scholar] [PubMed]
- Garlotta, D. A Literature Review of Poly(Lactic Acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Saito, Y.; Doi, Y. Microbial Synthesis and Properties of Poly(3-Hydroxybutyrate-Co-4-Hydroxybutyrate) in Comamonas Acidovorans. Int. J. Biol. Macromol. 1994, 16, 99–104. [Google Scholar] [CrossRef]
- Pillai, C.K.S. Challenges for Natural Monomers and Polymers: Novel Design Strategies and Engineering to Develop Advanced Polymers. Des. Monomers Polym. 2010, 13, 87–121. [Google Scholar] [CrossRef]
- Sheikholeslam, M.; Wright, M.E.E.; Jeschke, M.G.; Amini-Nik, S. Biomaterials for Skin Substitutes. Adv. Healthc Mater. 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Pamfil, D.; Schick, C.; Vasile, C. New Hydrogels Based on Substituted Anhydride Modified Collagen and 2-Hydroxyethyl Methacrylate. Synthesis and Characterization. Ind. Eng. Chem. Res. 2014, 53, 11239–11248. [Google Scholar] [CrossRef]
- Yu, L.; Wei, M. Biomineralization of Collagen-Based Materials for Hard Tissue Repair. IJMS 2021, 22, 944. [Google Scholar] [CrossRef]
- Liu, B.; Song, Y.; Jin, L.; Wang, Z.; Pu, D.; Lin, S.; Zhou, C.; You, H.; Ma, Y.; Li, J.; et al. Silk Structure and Degradation. Colloids Surf. B Biointerfaces 2015, 131, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Santi, S.; Mancini, I.; Dirè, S.; Callone, E.; Speranza, G.; Pugno, N.; Migliaresi, C.; Motta, A. A Bio-Inspired Multifunctionalized Silk Fibroin. ACS Biomater. Sci. Eng. 2021, 7, 507–516. [Google Scholar] [CrossRef]
- Conboy, I.; Freimer, J.; Weisenstein, L. Tissue Engineering of Muscle Tissue. Compr. Biomater. 2011, 345–359. [Google Scholar] [CrossRef]
- Ahmed, T.A.E.; Dare, E.V.; Hincke, M. Fibrin: A Versatile Scaffold for Tissue Engineering Applications. Tissue Eng. Part B Rev. 2008, 14, 199–215. [Google Scholar] [CrossRef]
- Ducret, M.; Costantini, A.; Gobert, S.; Farges, J.-C.; Bekhouche, M. Fibrin-Based Scaffolds for Dental Pulp Regeneration: From Biology to Nanotherapeutics. Eur. Cell Mater. 2021, 41, 1–14. [Google Scholar] [CrossRef]
- Nagarajan, S.; Radhakrishnan, S.; Kalkura, S.N.; Balme, S.; Miele, P.; Bechelany, M. Overview of Protein-Based Biopolymers for Biomedical Application. Macromol. Chem. Phys. 2019, 220, 1900126. [Google Scholar] [CrossRef]
- Kang, J.I.; Park, K.M. Advances in Gelatin-Based Hydrogels for Wound Management. J. Mater. Chem. B 2021, 9, 1503–1520. [Google Scholar] [CrossRef]
- Rouse, J.G.; Van Dyke, M.E. A Review of Keratin-Based Biomaterials for Biomedical Applications. Materials 2010, 3, 999–1014. [Google Scholar] [CrossRef] [Green Version]
- Hill, P.; Brantley, H.; Van Dyke, M. Some Properties of Keratin Biomaterials: Kerateines. Biomaterials 2010, 31, 585–593. [Google Scholar] [CrossRef]
- Roslan, M.R.; Nasir, N.F.M.; Cheng, E.M.; Amin, N.A.M. Tissue Engineering Scaffold Based on Starch: A Review. In Proceedings of the 2016 International Conference on Electrical, Electronics, and Optimization Techniques (ICEEOT), Chennai, India, 3–5 March 2016; pp. 1857–1860. [Google Scholar]
- Gomes, M.E.; Oliveira, J.T.; Rodrigues, M.T.; Santos, M.I.; Tuzlakoglu, K.; Viegas, C.A.; Dias, I.R.; Reis, R.L. Starch-polycaprolactone based scaffolds in bone and cartilage tissue engineering approaches. In Natural-Based Polymers for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2008; pp. 337–356. [Google Scholar]
- Ahmed, S.; Ikram, S. Chitosan Based Scaffolds and Their Applications in Wound Healing. Achiev. Life Sci. 2016, 10, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Pita-López, M.L.; Fletes-Vargas, G.; Espinosa-Andrews, H.; Rodríguez-Rodríguez, R. Physically Cross-Linked Chitosan-Based Hydrogels for Tissue Engineering Applications: A State-of-the-Art Review. Eur. Polym. J. 2021, 145, 110176. [Google Scholar] [CrossRef]
- Sivashankari, P.R.; Prabaharan, M. Prospects of Chitosan-Based Scaffolds for Growth Factor Release in Tissue Engineering. Int. J. Biol. Macromol. 2016, 93, 1382–1389. [Google Scholar] [CrossRef] [PubMed]
- Zarrintaj, P.; Manouchehri, S.; Ahmadi, Z.; Saeb, M.R.; Urbanska, A.M.; Kaplan, D.L.; Mozafari, M. Agarose-Based Biomaterials for Tissue Engineering. Carbohydr. Polym. 2018, 187, 66–84. [Google Scholar] [CrossRef]
- Salati, M.A.; Khazai, J.; Tahmuri, A.M.; Samadi, A.; Taghizadeh, A.; Taghizadeh, M.; Zarrintaj, P.; Ramsey, J.D.; Habibzadeh, S.; Seidi, F.; et al. Agarose-Based Biomaterials: Opportunities and Challenges in Cartilage Tissue Engineering. Polymers 2020, 12, 1150. [Google Scholar] [CrossRef]
- Felfel, R.M.; Gideon-Adeniyi, M.J.; Zakir Hossain, K.M.; Roberts, G.A.F.; Grant, D.M. Structural, Mechanical and Swelling Characteristics of 3D Scaffolds from Chitosan-Agarose Blends. Carbohydr. Polym. 2019, 204, 59–67. [Google Scholar] [CrossRef]
- Farokhi, M.; Jonidi Shariatzadeh, F.; Solouk, A.; Mirzadeh, H. Alginate Based Scaffolds for Cartilage Tissue Engineering: A Review. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 230–247. [Google Scholar] [CrossRef]
- Valente, J.F.A.; Valente, T.A.M.; Alves, P.; Ferreira, P.; Silva, A.; Correia, I.J. Alginate Based Scaffolds for Bone Tissue Engineering. Mater. Sci. Eng. C 2012, 32, 2596–2603. [Google Scholar] [CrossRef]
- Steiner, D.; Lingens, L.; Fischer, L.; Köhn, K.; Detsch, R.; Boccaccini, A.R.; Fey, T.; Greil, P.; Weis, C.; Beier, J.P.; et al. Encapsulation of Mesenchymal Stem Cells Improves Vascularization of Alginate-Based Scaffolds. Tissue Eng. Part A 2018, 24, 1320–1331. [Google Scholar] [CrossRef]
- Müller, F.A.; Müller, L.; Hofmann, I.; Greil, P.; Wenzel, M.M.; Staudenmaier, R. Cellulose-Based Scaffold Materials for Cartilage Tissue Engineering. Biomaterials 2006, 27, 3955–3963. [Google Scholar] [CrossRef]
- Novotna, K.; Havelka, P.; Sopuch, T.; Kolarova, K.; Vosmanska, V.; Lisa, V.; Svorcik, V.; Bacakova, L. Cellulose-Based Materials as Scaffolds for Tissue Engineering. Cellulose 2013, 20, 2263–2278. [Google Scholar] [CrossRef] [Green Version]
- Salmoria, G.V.; Klauss, P.; Paggi, R.A.; Kanis, L.A.; Lago, A. Structure and Mechanical Properties of Cellulose Based Scaffolds Fabricated by Selective Laser Sintering. Polym. Test. 2009, 28, 648–652. [Google Scholar] [CrossRef]
- Castro, K.C.; Campos, M.G.N.; Mei, L.H.I. Hyaluronic Acid Electrospinning: Challenges, Applications in Wound Dressings and New Perspectives. Int. J. Biol. Macromol. 2021, 173, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-W.; Spector, M. Development of Hyaluronic Acid-Based Scaffolds for Brain Tissue Engineering. Acta Biomater. 2009, 5, 2371–2384. [Google Scholar] [CrossRef]
- Wang, X.; He, J.; Wang, Y.; Cui, F.-Z. Hyaluronic Acid-Based Scaffold for Central Neural Tissue Engineering. Interface Focus 2012, 2, 278–291. [Google Scholar] [CrossRef] [Green Version]
- Köwitsch, A.; Zhou, G.; Groth, T. Medical Application of Glycosaminoglycans: A Review. J. Tissue Eng. Regen. Med. 2018, 12, e23–e41. [Google Scholar] [CrossRef]
- Sandri, G.; Rossi, S.; Bonferoni, M.C.; Miele, D.; Faccendini, A.; Del Favero, E.; Di Cola, E.; Icaro Cornaglia, A.; Boselli, C.; Luxbacher, T.; et al. Chitosan/Glycosaminoglycan Scaffolds for Skin Reparation. Carbohydr. Polym. 2019, 220, 219–227. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Venugopal, J.; Huang, Z.-M.; Lim, C.T.; Ramakrishna, S. Characterization of the Surface Biocompatibility of the Electrospun PCL-Collagen Nanofibers Using Fibroblasts. Biomacromolecules 2005, 6, 2583–2589. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.; Augustine, R.; Kalarikkal, N.; Thomas, S.; Seantier, B.; Grohens, Y. Recent Advances in Electrospun Polycaprolactone Based Scaffolds for Wound Healing and Skin Bioengineering Applications. Mater. Today Commun. 2019, 19, 319–335. [Google Scholar] [CrossRef]
- Osathanon, T.; Chuenjitkuntaworn, B.; Nowwarote, N.; Supaphol, P.; Sastravaha, P.; Subbalekha, K.; Pavasant, P. The Responses of Human Adipose-Derived Mesenchymal Stem Cells on Polycaprolactone-Based Scaffolds: An in Vitro Study. Tissue Eng. Regen. Med. 2014, 11, 239–246. [Google Scholar] [CrossRef]
- Hyon, S.-H.; Jamshidi, K.; Ikada, Y. Synthesis of Polylactides with Different Molecular Weights. Biomaterials 1997, 18, 1503–1508. [Google Scholar] [CrossRef]
- Kiran, I.; Shad, N.A.; Sajid, M.M.; Jamil, Y.; Javed, Y.; Hussain, M.I.; Akhtar, K. Graphene Functionalized PLA Nanocomposites and Their Biomedical Applications. In Graphene Based Biopolymer Nanocomposites; Sharma, B., Jain, P., Eds.; Composites Science and Technology; Springer: Singapore, 2021; pp. 83–105. [Google Scholar]
- Niaounakis, M. Biopolymers: Processing and Products; William Andrew: Norwich, NY, USA, 2014; ISBN 978-0-323-27938-3. [Google Scholar]
- Zhang, J.; Yang, S.; Yang, X.; Xi, Z.; Zhao, L.; Cen, L.; Lu, E.; Yang, Y. Novel Fabricating Process for Porous Polyglycolic Acid Scaffolds by Melt-Foaming Using Supercritical Carbon Dioxide. ACS Biomater. Sci. Eng. 2018, 4, 694–706. [Google Scholar] [CrossRef]
- Wu, Y.; Xia, H.; Zhang, B.; Zhao, Y.; Wang, Y. Assessment of Polyglycolic Acid Scaffolds for Periodontal Ligament Regeneration. Biotechnol. Biotechnol. Equip. 2018, 32, 701–706. [Google Scholar] [CrossRef] [Green Version]
- Khorasani, M.T.; Mirmohammadi, S.A.; Irani, S. Polyhydroxybutyrate (PHB) Scaffolds as a Model for Nerve Tissue Engineering Application: Fabrication and In Vitro Assay. Int. J. Polym. Mater. 2011, 60, 562–575. [Google Scholar] [CrossRef]
- Israni, N.; Shivakumar, S. Polyhydroxybutyrate. In Materials for Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 405–444. [Google Scholar]
- Diez-Pascual, A.M. Tissue Engineering Bionanocomposites Based on Poly(Propylene Fumarate). Polymers 2017, 9, 260. [Google Scholar] [CrossRef] [Green Version]
- Hassan, N.; Chaudhery, I.; Rehman, A.; Ahmed, N. Polymeric nanoparticles used in tissue engineering. In Advances in Polymeric Nanomaterials for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 191–224. ISBN 978-0-12-814657-6. [Google Scholar]
- Vafaei, A.; Rahbarghazi, R.; Kharaziha, M.; Avval, N.A.; Rezabakhsh, A.; Karimipour, M. Polycaprolactone Fumarate Acts as an Artificial Neural Network to Promote the Biological Behavior of Neural Stem Cells. J. Biomed. Mater. Res. 2021, 109, 246–256. [Google Scholar] [CrossRef]
- Han, D.K.; Park, K.D.; Hubbell, J.A.; Kim, Y.H. Surface Characteristics and Biocompatibility of Lactide-Based Poly(Ethylene Glycol) Scaffolds for Tissue Engineering. J. Biomater. Sci. Polym. Ed. 1998, 9, 667–680. [Google Scholar] [PubMed]
- Wang, H.; Liu, X.; Christiansen, D.E.; Fattahpour, S.; Wang, K.; Song, H.; Mehraeen, S.; Cheng, G. Thermoplastic Polyurethane with Controllable Degradation and Critical Anti-Fouling Properties. Biomater. Sci. 2021, 9, 1381–1396. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Qi, R.; Shen, M.; Cao, X.; Guo, R.; Zhang, Y.; Shi, X. Improved Cellular Response on Multiwalled Carbon Nanotube-Incorporated Electrospun Polyvinyl Alcohol/Chitosan Nanofibrous Scaffolds. Colloids Surf. B Biointerfaces 2011, 84, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Negi, Y.S.; Choudhary, V.; Bhardwaj, N.K. Microstructural and Mechanical Properties of Porous Biocomposite Scaffolds Based on Polyvinyl Alcohol, Nano-Hydroxyapatite and Cellulose Nanocrystals. Cellulose 2014, 21, 3409–3426. [Google Scholar] [CrossRef]
- Kumar, A.; Han, S.S. PVA-Based Hydrogels for Tissue Engineering: A Review. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 159–182. [Google Scholar] [CrossRef]
- Li, X.H.; Meng, Y.Z.; Wang, S.J.; Rajulu, A.V.; Tjong, S.C. Completely Biodegradable Composites of Poly(Propylene Carbonate) and Short, Lignocellulose Fiber Hildegardia Populifolia: Completely Biodegradable Composites. J. Polym. Sci. B Polym. Phys. 2004, 42, 666–675. [Google Scholar] [CrossRef]
- Li, X.H.; Meng, Y.Z.; Zhu, Q.; Xu, Y.; Tjong, S.C. Melt Processable and Biodegradable Aliphatic Polycarbonate Derived from Carbon Dioxide and Propylene Oxide. J. Appl. Polym. Sci. 2003, 89, 3301–3308. [Google Scholar] [CrossRef]
- Sasanuma, Y.; Takahashi, Y. Structure–Property Relationships of Poly(Ethylene Carbonate) and Poly(Propylene Carbonate). ACS Omega 2017, 2, 4808–4819. [Google Scholar] [CrossRef]
- Parikh, S.N. Bone Graft Substitutes: Past, Present, Future. J. Postgrad. Med. 2002, 48, 142–148. [Google Scholar]
- Zhang, Q.; Lu, H.; Kawazoe, N.; Chen, G. Pore Size Effect of Collagen Scaffolds on Cartilage Regeneration. Acta Biomater. 2014, 10, 2005–2013. [Google Scholar] [CrossRef]
- Radhika Rajasree, S.R.; Gobalakrishnan, M.; Aranganathan, L.; Karthih, M.G. Fabrication and Characterization of Chitosan Based Collagen/ Gelatin Composite Scaffolds from Big Eye Snapper Priacanthus Hamrur Skin for Antimicrobial and Anti Oxidant Applications. Mater. Sci. Eng. C 2020, 107, 110270. [Google Scholar] [CrossRef]
- Satish Kumar, T.; Vijaya Ramu, D.; Sampath Kumar, N.S. Preparation and Characterization of Biodegradable Collagen—Chitosan Scaffolds. Mater. Today Proc. 2019, 19, 2587–2590. [Google Scholar] [CrossRef]
- Noh, Y.K.; Dos Santos Da Costa, A.; Park, Y.S.; Du, P.; Kim, I.-H.; Park, K. Fabrication of Bacterial Cellulose-Collagen Composite Scaffolds and Their Osteogenic Effect on Human Mesenchymal Stem Cells. Carbohydr. Polym. 2019, 219, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, H.; Li, X.; Yan, S.; Xu, A.; You, R.; Zhang, Q. Natural Silk Nanofibrils as Reinforcements for the Preparation of Chitosan-Based Bionanocomposites. Carbohydr. Polym. 2021, 253, 117214. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Gong, K.; Zheng, Z.; Wang, A.; Ao, Q.; Gong, Y.; Zhang, X. Chitosan/Silk Fibroin-Based Tissue-Engineered Graft Seeded with Adipose-Derived Stem Cells Enhances Nerve Regeneration in a Rat Model. J. Mater. Sci. Mater. Med. 2011, 22, 1947–1964. [Google Scholar] [CrossRef]
- Asadpour, S.; Kargozar, S.; Moradi, L.; Ai, A.; Nosrati, H.; Ai, J. Natural Biomacromolecule Based Composite Scaffolds from Silk Fibroin, Gelatin and Chitosan toward Tissue Engineering Applications. Int. J. Biol. Macromol. 2020, 154, 1285–1294. [Google Scholar] [CrossRef]
- Hajiabbas, M.; Alemzadeh, I.; Vossoughi, M. A Porous Hydrogel-Electrospun Composite Scaffold Made of Oxidized Alginate/Gelatin/Silk Fibroin for Tissue Engineering Application. Carbohydr. Polym. 2020, 245, 116465. [Google Scholar] [CrossRef] [PubMed]
- Davidenko, N.; Campbell, J.J.; Thian, E.S.; Watson, C.J.; Cameron, R.E. Collagen–Hyaluronic Acid Scaffolds for Adipose Tissue Engineering. Acta Biomater. 2010, 6, 3957–3968. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Chen, X.; Feng, M.; Shi, Z.; Zhang, D.; Lin, Q. Layer-by-Layer Assembly of 3D Alginate-Chitosan-Gelatin Composite Scaffold Incorporating Bacterial Cellulose Nanocrystals for Bone Tissue Engineering. Mater. Lett. 2017, 209, 492–496. [Google Scholar] [CrossRef]
- Tran, T.T.; Hamid, Z.A.; Cheong, K.Y. A Review of Mechanical Properties of Scaffold in Tissue Engineering: Aloe Vera Composites. J. Phys. Conf. Ser. 2018, 1082, 012080. [Google Scholar] [CrossRef]
- Tillman, B.W.; Yazdani, S.K.; Lee, S.J.; Geary, R.L.; Atala, A.; Yoo, J.J. The in Vivo Stability of Electrospun Polycaprolactone-Collagen Scaffolds in Vascular Reconstruction. Biomaterials 2009, 30, 583–588. [Google Scholar] [CrossRef]
- Mallick, S.P.; Singh, B.N.; Rastogi, A.; Srivastava, P. Design and Evaluation of Chitosan/Poly(l-Lactide)/Pectin Based Composite Scaffolds for Cartilage Tissue Regeneration. Int. J. Biol. Macromol. 2018, 112, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, G.; Prakash, C.; Ramakrishna, S.; Lamberti, L.; Pruncu, C.I. 3D Printed Biodegradable Composites: An Insight into Mechanical Properties of PLA/Chitosan Scaffold. Polym. Test. 2020, 89, 106722. [Google Scholar] [CrossRef]
- Bahcecioglu, G.; Hasirci, N.; Hasirci, V. Cell Behavior on the Alginate-Coated PLLA/PLGA Scaffolds. Int. J. Biol. Macromol. 2019, 124, 444–450. [Google Scholar] [CrossRef]
- Rajzer, I.; Kurowska, A.; Jabłoński, A.; Jatteau, S.; Śliwka, M.; Ziąbka, M.; Menaszek, E. Layered Gelatin/PLLA Scaffolds Fabricated by Electrospinning and 3D Printing- for Nasal Cartilages and Subchondral Bone Reconstruction. Mater. Des. 2018, 155, 297–306. [Google Scholar] [CrossRef]
- de Siqueira, L.; Ribeiro, N.; Paredes, M.B.A.; Grenho, L.; Cunha-Reis, C.; Trichês, E.S.; Fernandes, M.H.; Sousa, S.R.; Monteiro, F.J. Influence of PLLA/PCL/HA Scaffold Fiber Orientation on Mechanical Properties and Osteoblast Behavior. Materials 2019, 12, 3879. [Google Scholar] [CrossRef] [Green Version]
- Kanimozhi, K.; Khaleel Basha, S.; Sugantha Kumari, V. Processing and Characterization of Chitosan/PVA and Methylcellulose Porous Scaffolds for Tissue Engineering. Mater. Sci. Eng. C 2016, 61, 484–491. [Google Scholar] [CrossRef]
- Maharjan, B.; Kaliannagounder, V.K.; Jang, S.R.; Awasthi, G.P.; Bhattarai, D.P.; Choukrani, G.; Park, C.H.; Kim, C.S. In-Situ Polymerized Polypyrrole Nanoparticles Immobilized Poly(ε-Caprolactone) Electrospun Conductive Scaffolds for Bone Tissue Engineering. Mater. Sci. Eng. C 2020, 114, 111056. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, Y.; Zhang, N.; Shi, J.; Zhang, X.; Qi, C.; Midgley, A.C.; Wang, S. Potentials of Sandwich-like Chitosan/Polycaprolactone/Gelatin Scaffolds for Guided Tissue Regeneration Membrane. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 109, 110618. [Google Scholar] [CrossRef]
- Li, K.; Wang, D.; Zhao, K.; Song, K.; Liang, J. Electrohydrodynamic Jet 3D Printing of PCL/PVP Composite Scaffold for Cell Culture. Talanta 2020, 211, 120750. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, T.; Hua, W.; Li, P.; Wang, X. 3D Porous Poly(Lactic Acid)/Regenerated Cellulose Composite Scaffolds Based on Electrospun Nanofibers for Biomineralization. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124048. [Google Scholar] [CrossRef]
- Patel, D.K.; Dutta, S.D.; Hexiu, J.; Ganguly, K.; Lim, K.-T. Bioactive Electrospun Nanocomposite Scaffolds of Poly(Lactic Acid)/Cellulose Nanocrystals for Bone Tissue Engineering. Int. J. Biol. Macromol. 2020, 162, 1429–1441. [Google Scholar] [CrossRef]
- Wibowo, A.; Vyas, C.; Cooper, G.; Qulub, F.; Suratman, R.; Mahyuddin, A.I.; Dirgantara, T.; Bartolo, P. 3D Printing of Polycaprolactone-Polyaniline Electroactive Scaffolds for Bone Tissue Engineering. Materials 2020, 13, 512. [Google Scholar] [CrossRef] [Green Version]
- Ju, J.; Gu, Z.; Liu, X.; Zhang, S.; Peng, X.; Kuang, T. Fabrication of Bimodal Open-Porous Poly (Butylene Succinate)/Cellulose Nanocrystals Composite Scaffolds for Tissue Engineering Application. Int. J. Biol. Macromol. 2020, 147, 1164–1173. [Google Scholar] [CrossRef]
- Gomes, M.; Azevedo, H.; Malafaya, P.; Silva, S.; Oliveira, J.; Silva, G.; Sousa, R.; Mano, J.; Reis, R. Natural Polymers in tissue engineering applications. In Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2008; pp. 145–192. [Google Scholar]
- Van Krevelen, D.W. Properties of Polymers: Their Correlation with Chemical Structure; Their Numerial Estimation and Prediction from Additive Group Contributions; Elsevier Science: Oxford, UK, 1997; ISBN 978-0-444-59612-3. [Google Scholar]
- Niaounakis, M. Biopolymers: Applications and Trends; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 978-0-323-35399-1. [Google Scholar]
- George, A.M.; Reddy Peddireddy, S.P.; Thakur, G.; Rodrigues, F.C. Biopolymer-based scaffolds. In Biopolymer-Based Formulations; Elsevier: Amsterdam, The Netherlands, 2020; pp. 717–749. [Google Scholar]
- Kim, S.; Uroz, M.; Bays, J.L.; Chen, C.S. Harnessing Mechanobiology for Tissue Engineering. Dev. Cell 2021, 56, 180–191. [Google Scholar] [CrossRef]
- Stratton, S.; Shelke, N.B.; Hoshino, K.; Rudraiah, S.; Kumbar, S.G. Bioactive Polymeric Scaffolds for Tissue Engineering. Bioact. Mater. 2016, 1, 93–108. [Google Scholar] [CrossRef]
- Tripathy, N.; Perumal, E.; Ahmad, R.; Song, J.E.; Khang, G. Hybrid Composite Biomaterials. In Principles of Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 695–714. [Google Scholar]
- Raut, H.K.; Das, R.; Liu, Z.; Liu, X.; Ramakrishna, S. Biocompatibility of Biomaterials for Tissue Regeneration or Replacement. Biotechnol. J. 2020, 15, 2000160. [Google Scholar] [CrossRef]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current Development of Biodegradable Polymeric Materials for Biomedical Applications. Drug Des. Devel. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef] [Green Version]
- Puls, J.; Wilson, S.A.; Hölter, D. Degradation of Cellulose Acetate-Based Materials: A Review. J. Polym. Environ. 2011, 19, 152–165. [Google Scholar] [CrossRef] [Green Version]
- John, M.; Thomas, S. Biofibres and Biocomposites. Carbohydr. Polym. 2008, 71, 343–364. [Google Scholar] [CrossRef]
- Williams, S.; Peoples, O. Biodegradable plastics from plants. ChemTech 1996, 26, 38–44. [Google Scholar]
- De Wilde, B. International norms on biodegradability and certification procedures. In Handbook of Biodegradable Polymers; Bastioli, C., Ed.; De Gruyter: Berlin, Germany; Boston, MA, USA, 2005; Chapter 5. [Google Scholar]
- Samantaray, P.K.; Little, A.; Haddleton, D.M.; McNally, T.; Tan, B.; Sun, Z.; Huang, W.; Ji, Y.; Wan, C. Poly(Glycolic Acid) (PGA): A Versatile Building Block Expanding High Performance and Sustainable Bioplastic Applications. Green Chem. 2020, 22, 4055–4081. [Google Scholar] [CrossRef]
- Hsu, S.-H.; Hung, K.-C.; Chen, C.-W. Biodegradable Polymer Scaffolds. J. Mater. Chem. B 2016, 4, 7493–7505. [Google Scholar] [CrossRef] [PubMed]
- Itävaara, M.; Siika-aho, M.; Viikari, L. Enzymatic Degradation of Cellulose-Based Materials. J. Polym. Environ. 1999, 7, 67–73. [Google Scholar] [CrossRef]
- Bahrami, N.; Farzin, A.; Bayat, F.; Goodarzi, A.; Salehi, M.; Karimi, R.; Mohamadnia, A.; Parhiz, A.; Ai, J. Optimization of 3D Alginate Scaffold Properties with Interconnected Porosity Using Freeze-Drying Method for Cartilage Tissue Engineering Application. Arch. Neurosci. 2019, 6. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Liu, Y.; Li, X.; Wen, P.; Zhang, Y.; Long, Y.; Wang, X.; Guo, Y.; Xing, F.; Gao, J. Preparation of Aligned Porous Gelatin Scaffolds by Unidirectional Freeze-Drying Method. Acta Biomater. 2010, 6, 1167–1177. [Google Scholar] [CrossRef]
- Hollister, S.J.; Maddox, R.D.; Taboas, J.M. Optimal Design and Fabrication of Scaffolds to Mimic Tissue Properties and Satisfy Biological Constraints. Biomaterials 2002, 23, 4095–4103. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Silk Fibroin Protein and Chitosan Polyelectrolyte Complex Porous Scaffolds for Tissue Engineering Applications. Carbohydr. Polym. 2011, 85, 325–333. [Google Scholar] [CrossRef]
- Guan, Y.; You, H.; Cai, J.; Zhang, Q.; Yan, S.; You, R. Physically Crosslinked Silk Fibroin/Hyaluronic Acid Scaffolds. Carbohydr. Polym. 2020, 239, 116232. [Google Scholar] [CrossRef]
- Nieto-Suárez, M.; López-Quintela, M.A.; Lazzari, M. Preparation and Characterization of Crosslinked Chitosan/Gelatin Scaffolds by Ice Segregation Induced Self-Assembly. Carbohydr. Polym. 2016, 141, 175–183. [Google Scholar] [CrossRef]
- Baniasadi, H.; Ramazani S A, A.; Mashayekhan, S. Fabrication and Characterization of Conductive Chitosan/Gelatin-Based Scaffolds for Nerve Tissue Engineering. Int. J. Biol. Macromol. 2015, 74, 360–366. [Google Scholar] [CrossRef]
- Xu, C.; Lu, W.; Bian, S.; Liang, J.; Fan, Y.; Zhang, X. Porous Collagen Scaffold Reinforced with Surfaced Activated PLLA Nanoparticles. Sci. World J. 2012, 2012, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Mirab, F.; Eslamian, M.; Bagheri, R. Fabrication and Characterization of a Starch-Based Nanocomposite Scaffold with Highly Porous and Gradient Structure for Bone Tissue Engineering. Biomed. Phys. Eng. Express 2018, 4, 055021. [Google Scholar] [CrossRef] [Green Version]
- Saeedi Garakani, S.; Davachi, S.M.; Bagher, Z.; Heraji Esfahani, A.; Jenabi, N.; Atoufi, Z.; Khanmohammadi, M.; Abbaspourrad, A.; Rashedi, H.; Jalessi, M. Fabrication of Chitosan/Polyvinylpyrrolidone Hydrogel Scaffolds Containing PLGA Microparticles Loaded with Dexamethasone for Biomedical Applications. Int. J. Biol. Macromol. 2020, 164, 356–370. [Google Scholar] [CrossRef]
- Guo, Z.; Yang, C.; Zhou, Z.; Chen, S.; Li, F. Characterization of Biodegradable Poly(Lactic Acid) Porous Scaffolds Prepared Using Selective Enzymatic Degradation for Tissue Engineering. RSC Adv. 2017, 7, 34063–34070. [Google Scholar] [CrossRef] [Green Version]
- Shih, Y. Effects Of Degradation On Mechanical Properties Of Tissue-Engineering Poly(glycolic Acid) Scaffolds. Yale Medicine Thesis Digital Library. 2015. Available online: http://elischolar.library.yale.edu/ymtdl/2011 (accessed on 28 December 2020).
- Lam, C.X.F.; Hutmacher, D.W.; Schantz, J.-T.; Woodruff, M.A.; Teoh, S.H. Evaluation of Polycaprolactone Scaffold Degradation for 6 Months in Vitro and in Vivo. J. Biomed. Mater. Res. A 2009, 90, 906–919. [Google Scholar] [CrossRef]
- You, Y.; Min, B.-M.; Lee, S.J.; Lee, T.S.; Park, W.H. In Vitro Degradation Behavior of Electrospun Polyglycolide, Polylactide, and Poly(Lactide-Co-Glycolide). J. Appl. Polym. Sci. 2005, 95, 193–200. [Google Scholar] [CrossRef]
- Guarino, V.; Causa, F.; Taddei, P.; di Foggia, M.; Ciapetti, G.; Martini, D.; Fagnano, C.; Baldini, N.; Ambrosio, L. Polylactic Acid Fibre-Reinforced Polycaprolactone Scaffolds for Bone Tissue Engineering. Biomaterials 2008, 29, 3662–3670. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Martin, D. Hydrolytic Degradation of Segmented Polyurethane Copolymers for Biomedical Applications. Polym. Degrad. Stab. 2012, 97, 1553–1561. [Google Scholar] [CrossRef]
- Jing, X.; Mi, H.-Y.; Salick, M.R.; Cordie, T.; Crone, W.C.; Peng, X.-F.; Turng, L.-S. Morphology, Mechanical Properties, and Shape Memory Effects of Poly(Lactic Acid)/ Thermoplastic Polyurethane Blend Scaffolds Prepared by Thermally Induced Phase Separation. J. Cell. Plast. 2014, 50, 361–379. [Google Scholar] [CrossRef]
- Yadav, P.; Beniwal, G.; Saxena, K.K. A Review on Pore and Porosity in Tissue Engineering. Mater. Today Proc. 2021, S2214785320403785. [Google Scholar]
- Polo-Corrales, L.; Latorre-Esteves, M.; Ramirez-Vick, J.E. Scaffold Design for Bone Regeneration. J. NanoSci. Nanotechnol. 2014, 14, 15–56. [Google Scholar] [CrossRef] [Green Version]
- Chan, B.P.; Leong, K.W. Scaffolding in Tissue Engineering: General Approaches and Tissue-Specific Considerations. Eur. Spine J. 2008, 17 (Suppl. 4), 467–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabir, M.I.; Xu, X.; Li, L. A Review on Biodegradable Polymeric Materials for Bone Tissue Engineering Applications. J. Mater. Sci. 2009, 44, 5713–5724. [Google Scholar] [CrossRef]
- Hollister, S.J. Porous Scaffold Design for Tissue Engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef]
- Babaie, E.; Bhaduri, S.B. Fabrication Aspects of Porous Biomaterials in Orthopedic Applications: A Review. ACS Biomater. Sci. Eng. 2018, 4, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D Biomaterial Scaffolds and Osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Ibara, A.; Miyaji, H.; Fugetsu, B.; Nishida, E.; Takita, H.; Tanaka, S.; Sugaya, T.; Kawanami, M. Osteoconductivity and Biodegradability of Collagen Scaffold Coated with Nano- β -TCP and Fibroblast Growth Factor 2. J. Nanomater. 2013, 2013, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Soliman, S.; Sant, S.; Nichol, J.W.; Khabiry, M.; Traversa, E.; Khademhosseini, A. Controlling the Porosity of Fibrous Scaffolds by Modulating the Fiber Diameter and Packing Density. J. Biomed. Mater. Res. A 2011, 96, 566–574. [Google Scholar] [CrossRef]
- Sarker, B.; Li, W.; Zheng, K.; Detsch, R.; Boccaccini, A.R. Designing Porous Bone Tissue Engineering Scaffolds with Enhanced Mechanical Properties from Composite Hydrogels Composed of Modified Alginate, Gelatin, and Bioactive Glass. ACS Biomater. Sci. Eng. 2016, 2, 2240–2254. [Google Scholar] [CrossRef]
- Zhong, X.; Dehghani, F. Fabrication of Biomimetic Poly(Propylene Carbonate) Scaffolds by Using Carbon Dioxide as a Solvent, Monomer and Foaming Agent. Green Chem. 2012, 14, 2523. [Google Scholar] [CrossRef]
- Liu, Y.; Nelson, T.; Chakroff, J.; Cromeens, B.; Johnson, J.; Lannutti, J.; Besner, G.E. Comparison of Polyglycolic Acid, Polycaprolactone, and Collagen as Scaffolds for the Production of Tissue Engineered Intestine. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 750–760. [Google Scholar] [CrossRef]
- Le, X.; Poinern, G.E.J.; Ali, N.; Berry, C.M.; Fawcett, D. Engineering a Biocompatible Scaffold with Either Micrometre or Nanometre Scale Surface Topography for Promoting Protein Adsorption and Cellular Response. Int. J. Biomater. 2013, 2013, 1–16. [Google Scholar] [CrossRef]
- Carletti, E.; Motta, A.; Migliaresi, C. Scaffolds for Tissue Engineering and 3D Cell Culture. In 3D Cell Culture; Haycock, J.W., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2011; Volume 695, pp. 17–39. [Google Scholar]
- Campuzano, S.; Pelling, A.E. Scaffolds for 3D Cell Culture and Cellular Agriculture Applications Derived From Non-Animal Sources. Front. Sustain. Food Syst. 2019, 3, 38. [Google Scholar] [CrossRef] [Green Version]
- Vincent, L.; Engler, A.J. 5.5 Effect of Substrate Modulus on Cell Function and Differentiation. In Comprehensive Biomaterials II; Elsevier: Amsterdam, The Netherlands, 2017; pp. 88–101. [Google Scholar]
- Chen, F.-M.; Liu, X. Advancing Biomaterials of Human Origin for Tissue Engineering. Prog. Polym. Sci. 2016, 53, 86–168. [Google Scholar] [CrossRef] [Green Version]
- Pilia, M.; Guda, T.; Appleford, M. Development of Composite Scaffolds for Load-Bearing Segmental Bone Defects. BioMed. Res. Int. 2013, 2013, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Goonoo, N.; Bhaw-Luximon, A.; Bowlin, G.L.; Jhurry, D. An Assessment of Biopolymer- and Synthetic Polymer-Based Scaffolds for Bone and Vascular Tissue Engineering: Polymer-Based Scaffolds for Bone and Vascular Tissue Engineering. Polym. Int. 2013, 62, 523–533. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and Mechanical Properties of PLA, and Their Functions in Widespread Applications—A Comprehensive Review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thavornyutikarn, B.; Chantarapanich, N.; Sitthiseripratip, K.; Thouas, G.A.; Chen, Q. Bone Tissue Engineering Scaffolding: Computer-Aided Scaffolding Techniques. Prog. Biomater. 2014, 3, 61–102. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Leong, K.-F.; Du, Z.; Chua, C.-K. The Design of Scaffolds for Use in Tissue Engineering. Part II. Rapid Prototyping Techniques. Tissue Eng. 2002, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Donnaloja, F.; Jacchetti, E.; Soncini, M.; Raimondi, M.T. Natural and Synthetic Polymers for Bone Scaffolds Optimization. Polymers 2020, 12, 905. [Google Scholar] [CrossRef] [Green Version]
- Taddei, P.; Chiono, V.; Anghileri, A.; Vozzi, G.; Freddi, G.; Ciardelli, G. Silk Fibroin/Gelatin Blend Films Crosslinked with Enzymes for Biomedical Applications. Macromol. BioSci. 2013, 13, 1492–1510. [Google Scholar] [CrossRef]
- Lohrasbi, S.; Mirzaei, E.; Karimizade, A.; Takallu, S.; Rezaei, A. Collagen/Cellulose Nanofiber Hydrogel Scaffold: Physical, Mechanical and Cell Biocompatibility Properties. Cellulose 2020, 27, 927–940. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, G. Collagen/Alginate Scaffolds Comprising Core (PCL)–Shell (Collagen/Alginate) Struts for Hard Tissue Regeneration: Fabrication, Characterisation, and Cellular Activities. J. Mater. Chem. B 2013, 1, 3185. [Google Scholar] [CrossRef] [PubMed]
- Unal, S.; Arslan, S.; Yilmaz, B.K.; Oktar, F.N.; Ficai, D.; Ficai, A.; Gunduz, O. Polycaprolactone/Gelatin/Hyaluronic Acid Electrospun Scaffolds to Mimic Glioblastoma Extracellular Matrix. Materials 2020, 13, 2661. [Google Scholar] [CrossRef]
- Zhang, Y.; Ouyang, H.; Lim, C.T.; Ramakrishna, S.; Huang, Z.-M. Electrospinning of Gelatin Fibers and Gelatin/PCL Composite Fibrous Scaffolds. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 72, 156–165. [Google Scholar] [CrossRef]
- Liao, G.; Jiang, K.; Jiang, S.; Xia, H. Synthesis and Characterization of Biodegradable Poly(ϵ-Caprolactone)-b-Poly(L-Lactide) and Study on Their Electrospun Scaffolds. J. Macromol. Sci. Part A 2010, 47, 1116–1122. [Google Scholar] [CrossRef]
- Jem, K.J.; Tan, B. The Development and Challenges of Poly (Lactic Acid) and Poly (Glycolic Acid). Adv. Ind. Eng. Polym. Res. 2020, 3, 60–70. [Google Scholar] [CrossRef]
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, Structure and Properties of Polyhydroxyalkanoates: Biological Polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555. [Google Scholar] [CrossRef]
- Plackett, D.; Siró, I. Polyhydroxyalkanoates (PHAs) for food packaging. In Multifunctional and Nanoreinforced Polymers for Food Packaging; Elsevier: Amsterdam, The Netherlands, 2011; pp. 498–526. [Google Scholar]
- Khanna, S.; Srivastava, A.K. Recent Advances in Microbial Polyhydroxyalkanoates. Process. Biochem. 2005, 40, 607–619. [Google Scholar] [CrossRef]
- Jem, K.J.; van der Pol, J.F.; de Vos, S. Microbial Lactic Acid, Its Polymer Poly(lactic acid), and Their Industrial Applications. In Plastics from Bacteria; Chen, G.G.-Q., Ed.; Microbiology Monographs; Springer: Berlin/Heidelberg, Germany, 2010; Volume 14, pp. 323–346. [Google Scholar]
- Lee, S.J.; Liu, J.; Oh, S.H.; Soker, S.; Atala, A.; Yoo, J.J. Development of a Composite Vascular Scaffolding System That Withstands Physiological Vascular Conditions. Biomaterials 2008, 29, 2891–2898. [Google Scholar] [CrossRef] [PubMed]
- Gilding, D.K.; Reed, A.M. Biodegradable Polymers for Use in Surgery—Polyglycolic/Poly(Actic Acid) Homo- and Copolymers: 1. Polymer 1979, 20, 1459–1464. [Google Scholar]
- Dobrzynski, P.; Kasperczyk, J.; Janeczek, H.; Bero, M. Synthesis of Biodegradable Copolymers with the Use of Low Toxic Zirconium Compounds. 1. Copolymerization of Glycolide with l -Lactide Initiated by Zr(Acac)4. Macromolecules 2001, 34, 5090–5098. [Google Scholar] [CrossRef]
- Wahit, M.U.; Hassan, A.; Ishak, Z.A.M.; Czigany, T. Toughening of polyamide 6 nanocomposites: Effect of organoclay and maleic anhydride grafted polyethylene octene loading on morphology and mechanical properties. Int. J. Mech. Mater. Eng. 2009, 4, 76–87. [Google Scholar]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric Scaffolds in Tissue Engineering Application: A Review. Int. J. Polym. Sci. 2011, 2011, 1–19. [Google Scholar] [CrossRef]
- Garg, T.; Goyal, A.K. Biomaterial-Based Scaffolds--Current Status and Future Directions. Expert Opin. Drug Deliv. 2014, 11, 767–789. [Google Scholar] [CrossRef]
- Lin, S.T.; Kimble, L.; Bhattacharyya, D. Polymer Blends and Composites for Biomedical Applications. In Biomaterials for Implants and Scaffolds; Li, Q., Mai, Y.-W., Eds.; Springer Series in Biomaterials Science and Engineering; Springer: Berlin/Heidelberg, Germany, 2017; Volume 8, pp. 195–235. [Google Scholar]
- Bettinger, C.J.; Borenstein, J.T.; Langer, R. Micro- and Nanofabricated Scaffolds. In Principles of Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2007; pp. 341–358. [Google Scholar]
- Altuntaş, E.; Özkan, B.; Yener, G. Porous scaffolds. In Nanobiomaterials Science, Development and Evaluation; Elsevier: Amsterdam, The Netherlands, 2017; pp. 27–59. [Google Scholar]
- Simionescu, B.C.; Ivanov, D. Natural and Synthetic Polymers for Designing Composite Materials. In Handbook of Bioceramics and Biocomposites; Antoniac, I.V., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–54. [Google Scholar]
- Subia, B.; Kundu, J.; Kundu, S.C. Biomaterial Scaffold Fabrication Techniques for Potential Tissue Engineering Applications. In Tissue Engineering; Eberli, D., Ed.; InTech: London, UK, 2010; ISBN 978-953-307-079-7. [Google Scholar]
- Prasad, A.; Sankar, M.R.; Katiyar, V. State of Art on Solvent Casting Particulate Leaching Method for Orthopedic ScaffoldsFabrication. Mater. Today Proc. 2017, 4, 898–907. [Google Scholar] [CrossRef]
- Sola, A.; Bertacchini, J.; D’Avella, D.; Anselmi, L.; Maraldi, T.; Marmiroli, S.; Messori, M. Development of Solvent-Casting Particulate Leaching (SCPL) Polymer Scaffolds as Improved Three-Dimensional Supports to Mimic the Bone Marrow Niche. Mater. Sci. Eng. C 2019, 96, 153–165. [Google Scholar] [CrossRef] [Green Version]
- Allaf, R.M. Melt-molding technologies for 3D scaffold engineering. In Functional 3D Tissue Engineering Scaffolds; Elsevier: Amsterdam, The Netherlands, 2018; pp. 75–100. [Google Scholar]
- Salerno, A.; Oliviero, M.; Di Maio, E.; Iannace, S.; Netti, P.A. Design of Porous Polymeric Scaffolds by Gas Foaming of Heterogeneous Blends. J. Mater. Sci. Mater. Med. 2009, 20, 2043–2051. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.S.; Yoon, J.J.; Park, T.G. A Novel Fabrication Method of Macroporous Biodegradable Polymer Scaffolds Using Gas Foaming Salt as a Porogen Additive. J. Biomed. Mater. Res. 2000, 53, 1–7. [Google Scholar] [CrossRef]
- Brougham, C.M.; Levingstone, T.J.; Shen, N.; Cooney, G.M.; Jockenhoevel, S.; Flanagan, T.C.; O’Brien, F.J. Freeze-Drying as a Novel Biofabrication Method for Achieving a Controlled Microarchitecture within Large, Complex Natural Biomaterial Scaffolds. Adv. Healthc. Mater. 2017, 6, 1700598. [Google Scholar] [CrossRef] [PubMed]
- Fereshteh, Z. Freeze-drying technologies for 3D scaffold engineering. In Functional 3D Tissue Engineering Scaffolds; Elsevier: Amsterdam, The Netherlands, 2018; pp. 151–174. [Google Scholar]
- Lannutti, J.; Reneker, D.; Ma, T.; Tomasko, D.; Farson, D. Electrospinning for Tissue Engineering Scaffolds. Mater. Sci. Eng. C 2007, 27, 504–509. [Google Scholar] [CrossRef]
- Nair, L.S.; Bhattacharyya, S.; Laurencin, C.T. Development of Novel Tissue Engineering Scaffolds via Electrospinning. Expert Opin. Biol. Ther. 2004, 4, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Skoog, S.A.; Goering, P.L.; Narayan, R.J. Stereolithography in Tissue Engineering. J. Mater. Sci. Mater. Med. 2014, 25, 845–856. [Google Scholar] [CrossRef]
- Melchels, F.P.W.; Feijen, J.; Grijpma, D.W. A Review on Stereolithography and Its Applications in Biomedical Engineering. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Do, A.-V.; Smith, R.; Acri, T.M.; Geary, S.M.; Salem, A.K. 3D printing technologies for 3D scaffold engineering. In Functional 3D Tissue Engineering Scaffolds; Elsevier: Amsterdam, The Netherlands, 2018; pp. 203–234. [Google Scholar]
- Duan, B.; Wang, M.; Zhou, W.Y.; Cheung, W.L.; Li, Z.Y.; Lu, W.W. Three-Dimensional Nanocomposite Scaffolds Fabricated via Selective Laser Sintering for Bone Tissue Engineering. Acta Biomater. 2010, 6, 4495–4505. [Google Scholar] [CrossRef]
- Mazzoli, A. Selective Laser Sintering in Biomedical Engineering. Med. Biol. Eng. Comput 2013, 51, 245–256. [Google Scholar] [CrossRef]
- Zein, I.; Hutmacher, D.W.; Tan, K.C.; Teoh, S.H. Fused Deposition Modeling of Novel Scaffold Architectures for Tissue Engineering Applications. Biomaterials 2002, 23, 1169–1185. [Google Scholar] [CrossRef]
- Korpela, J.; Kokkari, A.; Korhonen, H.; Malin, M.; Närhi, T.; Seppälä, J. Biodegradable and Bioactive Porous Scaffold Structures Prepared Using Fused Deposition Modeling. J. Biomed. Mater. Res. 2013, 101, 610–619. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, M.; Zhou, Z.; Gou, J.; Hui, D. 3D Printing of Polymer Matrix Composites: A Review and Prospective. Compos. Part B Eng. 2017, 110, 442–458. [Google Scholar] [CrossRef]
- An, J.; Teoh, J.E.M.; Suntornnond, R.; Chua, C.K. Design and 3D Printing of Scaffolds and Tissues. Engineering 2015, 1, 261–268. [Google Scholar] [CrossRef] [Green Version]
- Kačarević, Ž.; Rider, P.; Alkildani, S.; Retnasingh, S.; Smeets, R.; Jung, O.; Ivanišević, Z.; Barbeck, M. An Introduction to 3D Bioprinting: Possibilities, Challenges and Future Aspects. Materials 2018, 11, 2199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arslan-Yildiz, A.; Assal, R.E.; Chen, P.; Guven, S.; Inci, F.; Demirci, U. Towards Artificial Tissue Models: Past, Present, and Future of 3D Bioprinting. Biofabrication 2016, 8, 014103. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Chen, Q.; Liu, C.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Wang, X. Natural Polymers for Organ 3D Bioprinting. Polymers 2018, 10, 1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, Z.; Jin, S.; Ye, K. Tissue and Organ 3D Bioprinting. SLAS Technol. 2018, 23, 301–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Polymer | Structure | Desirable Properties and Advantages | Disadvantages | Ref | ||
|---|---|---|---|---|---|---|
| Natural polymer-, | Polypeptide-, and Protein-based scaffolds | Collagen |
Glycine Proline Hydroxyproline |
|
| [52,124,125] |
Silk fibroin |
|
|
| [64,126,127] | ||
Fibrinogen and fibrin |
|
|
| [67,128,129,130] | ||
Gelatin |
|
|
| [60,131,132] | ||
| Keratin |
|
|
| [133,134] | ||
| Polysaccharide-based scaffolds | Starch |
|
|
| [135,136] | |
Chitin/chitosan |
|
|
| [75,137,138,139,140,141,142,143,144,145,146,147,148,149] | ||
Agarose |
|
|
| [140,141,142] | ||
Alginate |
|
|
| [143,144,145] | ||
Cellulose |
|
|
| [146,147,148] | ||
Hyaluronic acid |
|
|
| [81,149,150,151] | ||
Glycosaminoglycans |
|
|
| [152,153] | ||
| Synthetic polymers | Poly(ƹ-caprolactone) (PCL) |
|
|
| [154,155,156] | |
Polylactic acid (PLA) |
|
|
| [92,157,158] | ||
Polylactic-co-glycolic acid (PLGA) |
|
|
| [159] | ||
Polyglycolic acid (PGA) |
|
|
| [160,161] | ||
Polyhydroxybutyrate (PHB) |
|
|
| [159,162,163] | ||
Polypropylene fumarate (PPF) |
|
|
| [159,164,165] | ||
Poly(ethylene glycol) (PEG) |
|
|
| [123,166,167] | ||
Polyurethane (PU) |
|
|
| [113,168] | ||
Polyvinyl alcohol (PVA) |
|
|
| [169,170,171] | ||
Polypropylene carbonate (PPC) |
|
|
| [172,173,174] | ||
| Natural–Natural Biopolymer Composite Scaffold Material | Fabrication Method | Properties Considered | Biological Assessment | Characteristics | Scaffold Application | Ref. |
|---|---|---|---|---|---|---|
| Collagen | Freeze-drying |
|
|
| Cartilage regeneration | [176] |
| Collagen/gelatin/chitosan (40–20–40%) | Freeze-drying |
|
|
| Tissue engineering | [177] |
| Collagen–chitosan (7:3) | Lyophilization |
|
|
| Tissue regeneration | [178] |
| Cellulose–collagen (5:1) | Freeze-drying |
|
|
| Bone tissue engineering | [179] |
| Silk fibrils/chitosan (3:4) | Freeze-drying |
|
|
| Would dressing, tissue engineering scaffolds, flexible biodevices | [180] |
| Chitosan/SF (7:3) | Lyophilization |
|
|
| Nerve regeneration, cartilage regeneration | [181] |
| SF (7 w/v%)/chitosan–gelatin (1:2) cross-linked with methanol and glutaraldehyde | Freeze-drying |
|
|
| Tissue engineering | [182] |
| Oxidized alginate/gelatin/SF (13:17:10 w/v%) | Electrospinning |
|
|
| Regenerative medicine, skin tissue engineering | [183] |
| Collagen–HA (15 wt.%) | Freeze-drying |
|
|
| Brain tissue engineering | [184] |
| Alginate/cellulose nanocrystals–chitosan–gelatin | Layer-by-layer assembly and then freeze-drying |
|
|
| Bone tissue engineering | [185] |
| Natural–Synthetic Biopolymer Composite Scaffold Material | Fabrication Method | Properties Considered | Biological Assessment | Characteristics | Scaffold Application | Ref. |
|---|---|---|---|---|---|---|
| PCL/collagen | Electrospinning |
|
|
| Vascular tissue engineering | [187] |
| Chitosan/PLLA/pectin (50:25:25) | Freeze drying |
|
|
| Neo-cartilage tissue regeneration, surgical manipulation | [188] |
| PLA/chitosan | Fused filament fabrication (3D printing) |
|
|
| Clinical purposes | [189] |
| Alginate-coated PLLA/PLGA (95:5, w/w) | Lyophilization |
|
|
| Designing engineered tissues | [190] |
| PLLA/gelatin (6%)/osteo (1.5%) | Electrospinning and 3D printing (FDM: Fused deposition modeling) |
|
|
| Nasal cartilages and subchondral bone reconstruction | [191] |
| PLLA/PCL/HA | Electrospinning associated with electrospray |
|
|
| Tissue engineering | [192] |
| CS/PVA/ methylcellulose | Combination of film casting and lyophilization methods |
|
|
| Drug delivery vehicles and skin tissue engineering | [193] |
| PCL/PPy | Electrospinning (ES) |
|
|
| Bone tissue engineering | [194] |
| Chitosan(CS)/PCL(P)/gelatin(G) | Electrospinning followed by freeze-drying |
|
|
| Periodontal regeneration | [195] |
| PCL/PVP (polyvinylpyrrolidone) | E-jet 3D printing |
|
|
| Cartilage regeneration | [196] |
| PLA/regenerated cellulose (RC) | Electrospinning and freeze-drying techniques |
|
|
| Bone tissue engineering | [197] |
| PLA/cellulose nanocrystals | Electrospinning |
|
|
| Bone tissue engineering | [198] |
| PCL/polyaniline (0.1 wt.%) | Screw-assisted extrusion-based 3D printing |
|
|
| Bone tissue engineering | [199] |
| PBS/cellulose nanocrystals (5 wt.%) | Two-step depressurization in a supercritical carbon dioxide (Sc-CO2) foaming process |
|
|
| Tissue engineering | [200] |
| Scaffold Material | Degradation Mechanism | Degradation Duration (Weeks) | Degradation Rate (%) | Solvent | Application | Ref. |
|---|---|---|---|---|---|---|
| Alginate | Enzymatic | 4 | >70 | DMEM + FBS | Bone and cartilage tissue substitutes | [217] |
| Gelatin | Hydrolysis, dissolving, transformation, and enzyme-catalyzed decomposition | 2.5 | 94.9 | Lysozyme | Cartilage cells | [218] |
| Chitosan/gelatin | Enzymatic | 4 | 28 ± 3.5 | PBS | Tissue engineering | [219] |
| Chitosan | Enzymatic | 4 | ∼60 | Lysozyme | Cartilage regeneration | [220] |
| Silk fibroin/chitosan | 50 | |||||
| Silk fibroin/hyaluronic acid | Enzymatic | 3 | ∼47 | Collagenase IA solution | Soft tissue engineering | [221] |
| Silk fibroin | ∼72 | |||||
| Chitosan/gelatin | Enzymatic | 3 | 50–60 | PBS with lysozyme | Biomedical applications | [222,223] |
| Collagen | Enzymatic | 2 | 71 | PBS | Tissue engineering | [224] |
| Collagen/PLLA | Hydrolysis and enzymatic | 5 | ||||
| Starch/PVA | Hydrolytic | 4 | 27.1 | Simulated body fluid (SBF) | Bone tissue engineering | [225] |
| Chitosan/PVP–PLGA | Hydrolytic | 4–6 | 100 | PBS | Allergic rhinitis and chronic sinusitis | [226] |
| PLA | Enzymatic | 32 | 80 | Simulated body fluid (SBF) | Tissue engineering | [227] |
| PGA | Hydrolytic | 1–6 | 50 | PBS | Tissue-engineered vascular grafts | [228] |
| PCL | Hydrolytic (surface erosion) | 24 | 7 | PBS | Drug delivery and tissue engineering | [229] |
| PLGA | Hydrolytic | 6 | ~50 | PBS | Tissue engineering, drug carriers, and sensors | [230] |
| PGA | 3 | 60 | ||||
| PCL/PLLA | Hydrolytic | 5 | 14 | NaOH solution | Bone tissue engineering | [231] |
| Polyurethane copolymers | Hydrolytic | 8 | ∼10 | PBS | Soft tissue engineering | [232] |
| PLA/thermoplastic polyurethane | Hydrolytic | 4 | ∼10 | PBS | Medical and tissue engineering | [233] |
| Scaffold Material | Fabrication Method | Pore Size (μm) | Porosity (%) | Application | Ref. |
|---|---|---|---|---|---|
| Trabecular bone | NA | / | 50–90 | NA | [240] |
| Cortical bone | / | 3–12 | |||
| Collagen | Freeze-drying | 150–250 | 98.8 ± 0.1 | Cartilage regeneration | [177] |
| Collagen | Freeze-drying | / | 96.05 ± 0.11 | Bone tissue engineering | [241] |
| Gelatin | Freeze-drying | ∼50–100 | ~98 | Cartilage cells | [218] |
| Collagen/chitosan | Freeze-drying | 2–5 | 41.5% ± 2.69 | Tissue engineering | [178] |
| Gelatin/chitosan | 5–10 | 81.02% ± 1.04 | |||
| Collagen/gelatin/chitosan | 10–20 | 61.34% ± 2.53 | |||
| Silk fibroin | Freeze-drying | 70 ± 23 | 92 | Tissue engineering | [183] |
| Chitosan/gelatin | 280 ± 31 | 67 | |||
| Silk fibroin/chitosan/gelatin | 153 ± 18 | 80 | |||
| PCL | Electrospinning | ~44–64 | ~90 | ECM for tissue engineering | [242] |
| PCL | Fused deposition modelling | / | 70 | Bone regeneration | [229] |
| PCL | Extrusion | / | 49.0 ± 1.4 | Biomedical applications | [28] |
| PCL/cellulose nanofibers | / | 49.5 ± 2.1 | |||
| Alginate | Freeze-drying | 250–320 | 85 ± 3.1 | Bone and cartilage tissue engineering | [217] |
| Alginate dialdehyde–gelatin (ADA–GEL) | Freeze-drying | ~200 | ~90 | Bone tissue engineering | [243] |
| PLA | Melt blending and hot pressing | 80.01 | 79.88 | Tissue engineering | [227] |
| PPC | Gas foaming–salt leaching method | 418 ± 84 | 92.4 | Tissue engineering | [244] |
| PGA | Electrospinning | 157.9 ± 30.5 | 91.5 ± 4.1 | Tissue-engineered intestines (TEI) | [245] |
| PCL | 45.0 ± 12.6 | 67.9 ± 2.9 | |||
| PGA/PLLA | 84.7 ± 23.2 | 81.9 ± 3.3 | |||
| CollaTape | 54.4 ± 10.6 | 86.7 ± 3.4 | |||
| CollaTape/PLLA | 45.2 ± 22.5 | 76.6 ± 3.9 | |||
| Collagen/PLLA | Lyophilizing | 150–250 | >95 | Tissue engineering | [224] |
| Scaffold Material | Scaffold Fabrication Method | Young’s Modulus (MPa) | Strength (MPa) | Elongation (%) at Break | Scaffold Application | Ref. |
|---|---|---|---|---|---|---|
| Cortical bone | NA | 15–20 × 103 | 100–230 | / | NA | [35,256] |
| Trabecular bone | 0.1–2 × 103 | 2–12 | / | |||
| Cancellous bone | 20–500 | 4–12 | / | |||
| Cartilage | 0.7–15.3 | 3.7–10.5 | / | |||
| Tendon | 0.143–2.31 × 103 | 24–112 | / | |||
| Silk fibroin (SF) | Solvent casting | 310 ± 90 | 22.8 ± 13.7 | 1.3 ± 0.3 | Soft tissue engineering | [257] |
| Gelatin (G) | 370 ± 80 | 95.3 ± 25.6 | 5.3 ± 1.4 | |||
| SF/G (50/50) | 460 ± 70 | 89.4 ± 12.9 | 3.2 ± 0.6 | |||
| Collagen | Solution casting | / | 57 ± 6 | 16.3 ± 1.3 | Biomedical applications | [258] |
| Collagen/cellulose nanofibers (8%) | 156 ± 5 | 23.06 ± 1.3 | ||||
| Alginate | Freeze-drying | 65 ± 13 ×10−3 | 326 ± 49 × 10−3 | / | Bone tissue engineering | [243] |
| Alginate–gelatin–bioglass (5 w/v%) | 417 ± 33 × 10−3 | 908 ± 117 ×10−3 | ||||
| Silk fibroin | Freeze-drying | 70 ± 1.01 × 10−3 | 14 ± 2 × 10−3 | 27.5 ± 6.2 | Tissue engineering | [183] |
| Chitosan/gelatin | 20 ± 1.3 × 10−3 | 5.6 ± 0.2 × 10−3 | 37.9 ± 3.8 | |||
| Silk fibroin/chitosan/gelatin | 27 ± 1.4 × 10−3 | 7.4 ± 0.3 × 10−3 | 36.6 ± 3.5 | |||
| Chitosan | Lyophilization | 6.8 ± 0.5 | 4.7 ± 0.4 | 62 ± 8.7 | Nerve regeneration, cartilage regeneration | [259] |
| Chitosan/silk fibroin (7:3) | 5.3 ± 0.2 | 3.1 ± 0.7 | 56 ± 7.4 | |||
| Chitosan/silk fibroin (5:5) | 3.4 ± 0.3 | 2.1 ± 0.5 | 33 ± 4.8 | |||
| PCL | Cryogenic plotting/melt plotting | 17.00 ± 0.75 | 1.71 ± 0.37 | / | Hard tissue regeneration | [260] |
| Collagen | 0.55 ± 0.03 | 0.024 ± 0.003 | ||||
| Core (PCL)–shell (collagen/alginate) (shell–core = 0.18) | 8.68 ± 1.14 | 1.28 ± 0.17 | ||||
| PCL/gelatin/hyaluronic acid fibers | Electrospinning | / | 7.9 ± 0.8 | 69 | Glioblastoma extracellular matrix | [261] |
| Gelatin | Electrospinning | 105 | 2.50 | 64 | Tissue engineering | [262] |
| PCL | 4.98 | 2.70 | 126 | |||
| Gelatin/PCL | 30.8 | 1.29 | 138 | |||
| PLLA | Electrospinning | 55.93 ± 2.11 | 3.05 ± 0.21 | 37.3 ± 3.9 | Tissue engineering | [263] |
| PLLA/PCL (90/10) | 18.11 ± 0.94 | 2.75 ± 0.09 | 66.5 ± 8.6 | |||
| PLLA/PCL (50/50) | 6.21 ± 0.64 | 1.58 ± 0.16 | 94.6 ± 7.5 | |||
| PGA | Melt compounding or lamination | 7000 | 115 | 16.4 | Biomedical applications | [264] |
| PHB | Solution-cast | 3500 | 40 | 5 | Therapeutic applications | [265] |
| Polypropylene | / | 1700 | 38 | 400 | Therapeutic applications | [266,267] |
| Low-density polyethylene | / | 200 | 10 | 620 | Therapeutic applications | [265] |
| Polystyrene | / | 3100 | 50 | / | Biomedical applications | [267] |
| PVC | / | 300–2400 | 10–60 | 12–32 | Biomedical applications | [266] |
| PLA | / | 2400 | 53 | 5 | Biomedical applications | [268] |
| PCL | Electrospinning | 7.5 ± 0.7 | 1.5 ± 0.7 | 417 ± 58 | Vascular cells | [269] |
| PCL/collagen (dry) | 3.8 ± 5.6 | 8.3 ± 1.2 | 62 ± 5 | |||
| PCL/collagen (wet) | 2.7 ± 1.2 | 4.0 ± 0.4 | 140 ± 13 | |||
| PLGA | / | 2000–4000 | 40–90 | < 10 | Biomedical applications | [270,271] |
| PET | / | 3500 | 47 | 2–83 | Biomedical applications | [264] |
| PA 6 | Melt compounding followed by injection moulding | 1947 ± 164 | 56 ± 1.0 | 70 | Biomedical applications | [182] |
| Polymer | Biomedical Application | Trade Name |
|---|---|---|
| Collagen | Provides an increased surface area for cell attachment, growth, and migration for tissue engineering applications | SpongeCol® |
| SphereCol® provides a 3D bio-scaffold which is optimal in many cell culture procedures | SphereCol® | |
| VitroCol® is especially ideal for human cell culture systems as a coating for surfaces, for providing preparations of thin layers of cultured cells, or for use as a solid gel. | VitroCol® | |
| Skin replacement product | TransCyte® | |
| Gelatin | A medical device intended for application to bleeding surfaces as a hemostatic | Gelfoam® |
| Silk | Therapeutic clothing | DermaSilk® |
| Chitosan | Natural wound care for animals—big and small | ChitoClear® |
| Natural healing and scar recovery | ChitoCare® | |
| Hyaluronic acid | Cell culture scaffolds | HyStem™ |
| PGA | Mainly applied for absorbable sutures and also for stents, adhesion barriers, absorbable reinforcement for artificial dura, and scaffolds | BioDegmer® PGA |
| The first biodegradable synthetic suture (1969) | DEXON | |
| Bone internal fixation devices | Biofix® | |
| Medical device applications | PURASORB® PG | |
| Absorbable mesh for temporary wound and organ support | Safil® Mesh | |
| PLA | Meniscus repair fixation devices | The Meniscus Arrow (Bionx Implants, Inc., Blue Bell, PA), Clearfix Screw (Mitek, Norwood, MA) |
| Fixed installations such as bone plates, bone screws, surgical sutures, spinning | Revode 100 series Revode 200 series | |
| PGLA (Poly(glycolide-co-L-lactide)) | Mainly applied for absorbable sutures and also for stents, scaffolds, adhesion barriers, artificial dura, and guided tissue regeneration (GTR) membranes | BioDegmer® PGLA |
| A temporary wound or organ support | VICRYL™ (polyglactin 910) Woven Mesh | |
| PGDLLA (Poly(glycolide-co-DL-lactide)) | Mainly applied for GTR membranes (porous membranes) for regeneration and adhesion of lost periodontal supporting tissues caused by periodontal disease | BioDegmer® PGDLLA |
| PLLA | Mainly applied for absorbable bone fixture and utilized for stents, scaffolds, and adhesion barriers | BioDegmer® PLLA |
| Orthopedic fixation devices | Bio-Anchor® | |
| Medical device applications | PURASORB® PL grades | |
| Fabrication of medical research devices and tissue engineering research solutions, such as orthopedic or soft tissue fixation devices. | Resomer® series L 206 S L 207 S L 209 S L 210 S | |
| PDLA | Bone fixture material | BioDegmer® PDLA |
| Medical device applications | PURASORB® PD grades | |
| PDLLA | Mainly applied for the coating of suture | BioDegmer® PDLLA |
| Medical device applications | PURASORB® PDL grades | |
| Form scaffolds make it a useful biomaterial in biomedical and tissue engineering | Resomer® R 207 S | |
| PCL | Medical device applications | PURASORB® PC |
| 67% PGA: 33% trimethylene carbonate (TMC) | Soft tissue reinforcement | BIO-A® |
| Fabrication Method | Advantages | Disadvantages | Materials | Ref. |
|---|---|---|---|---|
| Solvent casting and particulate leaching: Polymer solution poured into the mold along with an appropriate porogen. A porous scaffold is obtained at high pressure and after evaporation of organic solvents |
|
| Different classes of synthetic polymers (e.g., PLLA, PLGA, or PEG) and natural polymers | [279,280] |
| Melt molding: Both polymers and a suitable porogen are melted together, then by cooling the polymer mixture the scaffold is obtained. In this process, the porosity is attained by dissolving the porogen in water |
|
| PLA, PGA, PLGA–gelatin, PA | [281] |
| Gas foaming: Polymer gel paste along with sieved effervescent salt particles poured into a mold and immersed into hot water. Formation of the porous matrix after the evolution of ammonia and carbon dioxide gas from salt particles of the solidifying polymer matrix |
|
| PLA, PLLA, or PLGA | [282,283] |
| Freeze-drying: A polymer solution is poured into a suitable mold and solvents are removed using a lyophiliser. This technique is mainly based on the sublimation process |
|
| Natural polymers like alginate, agarose, gelatin, chitosan, etc., and PGA, PLLA, PLGA, PLGA/PPF blends | [284,285] |
| Electrospinning: The electrospinning process draws a continuous narrow stream of material from a reservoir of polymer melt or solution to a collecting plate, where the material accumulates, producing the fibrous mat. This is accomplished by inducing charge buildup on the surface of the solution through the application of strong voltages |
|
| Synthetic polymers (PEO, PLGA, PLLA, PCL, PVA) and natural polymers (collagen, silk fibroin, elastin, fibrinogen, chitosan) and their composites | [286,287] |
| Stereolithography (SLA): In SLA, an object is created by selectively curing a polymer resin layer-by-layer using an ultraviolet (UV) laser beam |
|
| PPF, PEO, PEG | [288,289,290] |
| Selective laser sintering: This method selectively sinters thin layers of polymer-based mixtures in the powder form, creating solid 3D composite objects with macro-and microscale features |
|
| Nondegradable or degradable biopolymers (e.g., PE, PCL, PLLA, PLGA, etc.), and composites can be processed into scaffolds for TE | [290,291,292] |
| Fused deposition modeling (FDM): FDM uses a layer-by-layer deposition technique, in which molten polymers or ceramics are extruded through a nozzle with a small orifice and merge with the material on the previous layer |
|
| Biodegradable materials used for this method include PCL, PLGA, polycarbonate, polypropylene, and various polyesters | [290,293,294] |
| 3D printing: It is a process of reconstruction of a 3D physical model by the successive addition of material layers resulting in a 3D solid object based on CAD model design |
|
| PEO, PCL, and PLGA | [290,295,296] |
| 3D bioprinting: It is the 3D printing process of generating layer-by-layer 3D tissue-like structures using viable cells, an encapsulation biomaterial, and growth and differentiation factors to create a bio-printed pre-tissue that is further transferred to an incubator where it matures into a tissue |
|
| Common biomaterials include natural and/or synthetic polymers and decellularized ECM | [297,298,299,300] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. https://doi.org/10.3390/polym13071105
Reddy MSB, Ponnamma D, Choudhary R, Sadasivuni KK. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers. 2021; 13(7):1105. https://doi.org/10.3390/polym13071105
Chicago/Turabian StyleReddy, M. Sai Bhargava, Deepalekshmi Ponnamma, Rajan Choudhary, and Kishor Kumar Sadasivuni. 2021. "A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds" Polymers 13, no. 7: 1105. https://doi.org/10.3390/polym13071105