Extruded PLA Nanocomposites Modified by Graphene Oxide and Ionic Liquid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Samples Preparation
2.3. Characterization Methods
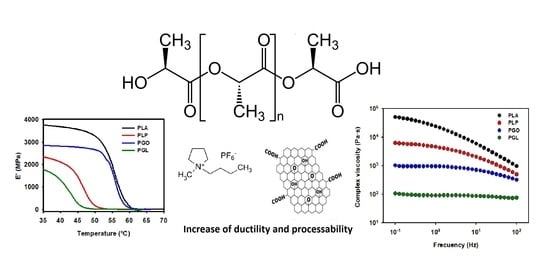
3. Results
3.1. Processing and Characterization of Pellets
3.1.1. Optimization of the Extrusion of PLA and Its Derivatives
3.1.2. RAMAN and FT-IR
3.1.3. Thermal Analysis: TGA and DSC
3.2. Viscoelastic Behavior
3.2.1. Dynamic Mechanical Analysis (DMA)
3.2.2. Rheology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Albertsson, A.C.K. Chemistry and biochemistry of polymer biodegradation. In Chemistry and Technology of Biodegradable Polymers; Griffin, G.J.L., Ed.; Blackie: Glasgow, UK, 1994; p. 7. [Google Scholar]
- Ali, S.A.M.; Doherty, P.J.; Williams, D.F. The Mechanisms of Oxidative-Degradation of Biomedical Polymers by Free-Radicals. J. Appl. Polym. Sci. 1994, 51, 1389–1398. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Park, H.-M.; Ha, C.-S. Bio-nanocomposites for food packaging applications. Prog. Polym. Sci. 2013, 38, 1629–1652. [Google Scholar] [CrossRef]
- Alfei, S.; Schito, A.M.; Zuccari, G. Biodegradable and Compostable Shopping Bags under Investigation by FTIR Spectroscopy. Appl. Sci. 2021, 11, 621. [Google Scholar] [CrossRef]
- Nampoothiri, K.M.; Nair, N.R.; John, R.P. An overview of the recent developments in polylactide (PLA) research. Bioresour. Technol. 2010, 101, 8493–8501. [Google Scholar] [CrossRef]
- Castro-Aguirre, E.; Íñiguez-Franco, F.; Samsudin, H.; Fang, X.; Auras, R. Poly(lactic acid)-Mass production, processing, industrial applications, and end of life. Adv. Drug Deliv. Rev. 2016, 107, 333–366. [Google Scholar] [CrossRef] [Green Version]
- Mazur, K.; Singh, R.; Hatice, R.P.; Genç, H.; Unterweger, H.; Sałasińska, K.; Bogucki, R.; Kuciel, S.; Cicha, I. The Effect of Antibacterial Particle Incorporation on the Mechanical Properties, Biodegradability, and Biocompatibility of PLA and PHBV Composites. Macromol. Mater. Eng. 2020, 305, 2000244. [Google Scholar] [CrossRef]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly(lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Ramot, Y.; Haim-Zada, M.; Domb, A.J.; Nyska, A. Biocompatibility and safety of PLA and its copolymers. Adv. Drug Deliv. Rev. 2016, 107, 153–162. [Google Scholar] [CrossRef]
- Auras, R.A.; Harte, B.; Selke, S.; Hernandez, R. Mechanical, physical, and barrier properties of poly(lactide) films. J. Plast. Film Sheeting 2003, 19, 123–135. [Google Scholar] [CrossRef]
- Weber, C.J.; Haugaard, V.; Festersen, R.; Bertelsen, G. Production and applications of biobased packaging materials for the food industry. Food Addit. Contam. 2002, 19, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Nazrin, A.; Sapuan, S.M.; Zuhri, M.Y.M.; Ilyas, R.A.; Syafiq, R.; Sherwani, S.F.K. Nanocellulose Reinforced Thermoplastic Starch (TPS), Polylactic Acid (PLA), and Polybutylene Succinate (PBS) for Food Packaging Applications. Front. Chem. 2020, 8, 213. [Google Scholar] [CrossRef]
- Raquez, J.-M.; Habibi, Y.; Murariu, M.; Dubois, P. Polylactide (PLA)-based nanocomposites. Prog. Polym. Sci. 2013, 38, 1504–1542. [Google Scholar] [CrossRef]
- Luo, F.H.; Fortenberry, A.; Ren, J.; Qiang, Z. Recent Progress in Enhancing Poly(Lactic Acid) Stereocomplex Formation for Material Property Improvement. Front. Chem. 2020, 8, 688. [Google Scholar] [CrossRef] [PubMed]
- Sessini, V.; Palenzuela, M.; Damian, J.; Mosquera, M.E.G. Bio-based polyether from limonene oxide catalytic ROP as green polymeric plasticizer for PLA. Polymer 2020, 210, 123003. [Google Scholar] [CrossRef]
- Alberts, E.; Ballentine, M.; Barnes, E.; Kennedy, A. Impact of metal additives on particle emission profiles from a fused filament fabrication 3D printer. Atmos. Environ. 2021, 244, 117956. [Google Scholar] [CrossRef]
- Torimoto, T.; Tsuda, T.; Okazaki, K.-I.; Kuwabata, S. New Frontiers in Materials Science Opened by Ionic Liquids. Adv. Mater. 2010, 22, 1196–1221. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez, M.D.; Jiménez, A.E.; Sanes, J.; Carrión, F. Ionic liquids as advanced lubricant fluids. Molecules 2009, 14, 2888–2908. [Google Scholar] [CrossRef]
- Cai, M.R.; Yu, Q.L.; Liu, W.M. Ionic liquid lubricants: When chemistry meets tribology. Chem. Soc. Rev. 2020, 49, 7753. [Google Scholar] [CrossRef]
- Greer, A.J.; Jacquemin, J.M.; Hardacre, C. Industrial Applications of Ionic Liquids. Molecules 2020, 25, 5207. [Google Scholar] [CrossRef]
- Chen, B.-K.; Wu, T.-Y.; Chang, Y.-M.; Chen, A.F. Ductile polylactic acid prepared with ionic liquids. Chem. Eng. J. 2013, 215–216, 886–893. [Google Scholar] [CrossRef]
- Park, K.; Ha, J.U.; Xanthos, M. Ionic Liquids as Plasticizers/Lubricants for Polylactic Acid. Pol. Eng. Sci. 2010, 50, 1105–1110. [Google Scholar] [CrossRef]
- Espejo, C.; Carrión-Vilches, F.J.; Bermúdez, M.D. Viscoelastic properties and long-term stability of polystyrene-carbon nanotube nanocomposites. Effect of the nature of the carbon nanotubes and modification by ionic liquid. Pol. Degrad. Stab. 2014, 103, 42–48. [Google Scholar] [CrossRef]
- Pereira, E.C.L.; Fernandes da Silva, M.E.C.; Pontes, K.; Soares, B.G. Influence of Protonic Ionic Liquid on the Dispersion of Carbon Nanotube in PLA/EVA Blends and Blend Compatibilization. Front. Mater. 2019, 6, 234. [Google Scholar] [CrossRef]
- Sanusi, O.M.; Benelfellah, A.; Bikiaris, D.N.; Hocine, N.A. Effect of rigid nanoparticles and preparation techniques on the performances of poly(lactic acid) nanocomposites: A review. Polym. Adv. Technol. 2020. [Google Scholar] [CrossRef]
- Singha, S.; Hedenqvist, M.S. A Review on Barrier Properties of Poly(Lactic Acid)/Clay Nanocomposites. Polymers 2020, 12, 1095. [Google Scholar] [CrossRef]
- Alfei, S.; Marengo, B.; Zuccari, G. Nanotechnology application in food packaging: A plethora of opportunities versus pending risks assessment and public concerns. Food Res. Int. 2020, 137, 109664. [Google Scholar] [CrossRef]
- Liu, W.; Ullah, B.; Kuo, C.-C.; Cai, X. Two-Dimensional Nanomaterials-Based Polymer Composites: Fabrication and Energy Storage Applications. Adv. Polym. Technol. 2019, 4294306. [Google Scholar] [CrossRef]
- Sanes, J.; Sánchez, C.; Pamies, R.; Avilés, M.D.; Bermúdez, M.D. Extrusion of Polymer Nanocomposites with Graphene and Graphene Derivative Nanofillers: An Overview of Recent Developments. Materials 2020, 13, 549. [Google Scholar] [CrossRef] [Green Version]
- de Armentia, S.L.; del Real, J.C.; Paz, E.; Dunne, N. Advances in Biodegradable 3D Printed Scaffolds with Carbon-Based Nanomaterials for Bone Regeneration. Materials 2020, 13, 5083. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Chen, D.; Feng, H.; Li, G.J. Graphene Oxide: Preparation, Functionalization, and Electrochemical Applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef] [PubMed]
- Chua, C.K.; Pumera, M. Covalent chemistry on graphene. Chem. Soc. Rev. 2013, 42, 3222–3233. [Google Scholar] [CrossRef]
- Haubner, K.; Murawski, J.; Olk, P.; Eng, L.M.; Ziegler, C.; Adolphi, B.; Jaehne, E. The Route to Functional Graphene Oxide. Chem. Phys. Chem. 2010, 11, 2131–2139. [Google Scholar] [CrossRef]
- Gardella, L.; Furfaro, D.; Galimbert, M.; Monticelli, O. On the development of a facile approach based on the use of ionic liquids: Preparation of PLLA (sc-PLA)/high surface area nano-graphite systems. Green Chem. 2015, 17, 4082–4088. [Google Scholar] [CrossRef]
- Gui, H.; Wang, J.; Yang, X.; Bahadera, A.; Ding, Y. Synergistic effect of graphene and an ionic liquid containing phosphonium on the thermal stability and flame retardancy of polylactide. RSC Adv. 2015, 5, 27814–27822. [Google Scholar] [CrossRef]
- Delogu, F.; Gorrasi, F.; Sorrentino, A. Fabrication of polymer nanocomposites via ball milling: Present status and future perspectives. Prog. Mater. Sci. 2017, 8, 75–126. [Google Scholar] [CrossRef]
- Barrau, S.; Vanmansart, C.; Moreau, M.; Addad, A.; Stoclet, G.; Lefebvre†, J.-M.; Seguela, R. Crystallization Behavior of Carbon Nanotube-Polylactide Nanocomposites. Macromolecules 2011, 44, 6496–6502. [Google Scholar] [CrossRef]
- Ali, A.M.; Ahmad, S.H. Effect of Processing Parameter and Filler Content on Tensile Properties of Multi-walled Carbon Nanotubes Reinforced Polylactic Acid Nanocomposite. AIP Conf. Proc. 2013, 1528, 254. [Google Scholar] [CrossRef]
- Chieng, B.W.; Ibrahim, N.A.; Yunus, W.Z.M.W.; Hussein, M.Z. Poly(lactic acid)/Poly(ethylene glycol) Polymer Nanocomposites: Effects of Graphene Nanoplatelets. Polymers 2014, 6, 93–104. [Google Scholar] [CrossRef] [Green Version]
- Bao, C.; Song, L.; Xing, W.; Yuan, B.; Wilkie, C.A.; Huang, J.; Guo, J.; Hu, Y. Preparation of graphene by pressurized oxidation and multiplex reduction and its polymer nanocomposites by masterbatch-based melt blending. J. Mater. Chem. 2012, 22, 6088–6096. [Google Scholar] [CrossRef]
- Maciejewska, M.; Zaborski, M. Effect of ionic liquids on the dispersion of zinc oxide andsilica nanoparticles, vulcanisation behaviour and properties of NBR composites. Express Polym. Lett. 2014, 8, 932–940. [Google Scholar] [CrossRef] [Green Version]
- Fedosse Zornio, C.; Livi, S.; Duchet-Rumeau, J.; Gerard, J.-F. Ionic Liquid-Nanostructured Poly(Methyl Methacrylate). Nanomaterials 2019, 9, 1376. [Google Scholar] [CrossRef] [Green Version]
- Sanes, J.; Ojados, G.; Pamies, R.; Bermúdez, M.D. PMMA nanocomposites with graphene oxide hybrid nanofillers. Express Polym. Lett. 2019, 10, 910–922. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Hussain, S.; Alamri, M.S.; Ibraheem, M.A.; Qasem, A.A.A. Specific Mechanical Energy and Thermal Degradation of Poly(lactic acid) and Poly(caprolactone)/Date Pits Composites. Int. J. Polym. Sci. 2018, 7493545. [Google Scholar] [CrossRef] [Green Version]
- Yuniarto, K.; Purwanto, Y.A.; Purwanto, S.; Welt, B.A.; Purwadaria, H.K.; Sunarti, T.C. Infrared and Raman Studies on Polylactide Acid and Polyethylene Glycol-400 Blend. Infrared and Raman studies on polylactide acid and polyethylene glycol-400 blend. AIP Conf. Proc. 2016, 1725, 020101. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.; Lv, J.; Feng, J. Spectral Characterization of Four Kinds of Biodegradable Plastics: Poly (Lactic Acid), Poly (Butylenes Adipate-Co-Terephthalate), Poly (Hydroxybutyrate-Co-Hydroxyvalerate) and Poly (Butylenes Succinate) with FTIR and Raman Spectroscopy. J. Pol. Environ. 2013, 21, 108–114. [Google Scholar] [CrossRef]
- Mofokeng, J.P.; Luyt, A.S.; Tábi, T.; Kovács, J. Comparison of injection moulded, natural fibre-reinforced composites with PP and PLA as matrices. J. Therm. Comp. Mat. 2012, 25, 927–948. [Google Scholar] [CrossRef]
- Quynh, T.M.; Mai, H.H.; Lan, P.N. Stereocomplexation of low molecular weight poly(L-lactic acid) and high molecular weight poly(D-lactic acid), radiation crosslinking PLLA/PDLA stereocomplexes and their characterization. Rad. Phys. Chem. 2013, 83, 105–110. [Google Scholar] [CrossRef]
- Babushkina, O.B.; Ekres, S.; Nauer, G.E. Spectroscopy and Electrochemistry of Tantalum(V) in 1-Butyl-1-methylpyrrolidinium Trifluoromethanesulfonate. Z. Nat. A 2008, 63, 73–80. [Google Scholar] [CrossRef]
- Haddad, B.; Villemin, D.; Belarbi, E.H.; Bar, N.; Rahmouni, M. New dicationic piperidinium hexafluorophosphate ILs, synthesis, characterization and dielectric measurements. Arab. J. Chem. 2014, 7, 781–787. [Google Scholar] [CrossRef] [Green Version]
- Dulgerbaki, C.; Maslakci, N.N.; Komur, A.I.; Oksuz, A.U. Chemical synthesis and characterization of poly(3,4-ethylenedioxythiophene)/tungsten composite materials in the presence of ionic liquids. Compos. Interfaces 2016, 23, 297–307. [Google Scholar] [CrossRef]
- Moumene, T.; Belarbi, E.H.; Haddad, B.; Villemin, D.; Abbas, O.; Khelifa, B.; Bresson, S. Study of imidazolium dicationic ionic liquids by Raman and FTIR spectroscopies: The effect of the nature of the anion. J. Mol. Struct. 2015, 1083, 179–186. [Google Scholar] [CrossRef]
- Matsuo, Y.; Okada, H.; Ueno, H. Endohedral Lithium-Containing Fullerenes: Preparation, Derivatization, and Application; Springer: Singapore, 2017. [Google Scholar]
- Hosseini, M.A.; Malekie, S.; Ebrahimi, N. The analysis of linear dose-responses in gamma-irradiated graphene oxide: Can FTIR analysis be considered a novel approach to examining the linear dose-responses in carbon nanostructures? Radiat. Phys. Chem. 2020, 176, 109067. [Google Scholar] [CrossRef]
- Khalili, D. Graphene oxide: A promising carbocatalyst for the regioselective thiocyanation of aromatic amines, phenols, anisols and enolizable ketones by hydrogen peroxide/KSCN in water. New J. Chem. 2016, 40, 2547–2553. [Google Scholar] [CrossRef]
- Rattana, T.; Chaiyakun, S.; Witit-anun, N.; Nuntawong, N.; Chindaudom, P.; Oaew, S.; Kedkeaw, C.; Limsuwan, P. Preparation and characterization of graphene oxide nanosheets. Procedia Eng. 2012, 32, 759–764. [Google Scholar] [CrossRef] [Green Version]
- Cobos, M.; Fernández, M.J.; Fernández, M.D. Graphene based poly(Vinyl alcohol) nanocomposites prepared by in situ green reduction of graphene oxide by ascorbic acid: Influence of graphene content and glycerol plasticizer on properties. Nanomaterials 2018, 8, 1013. [Google Scholar] [CrossRef] [Green Version]
- Oldiges, K.; Diddens, D.; Ebrahiminia, M.; Hooper, J.B.; Cekic-Laskovic, I.; Heuer, A.; Bedrov, D.; Winter, M.; Brunklaus, G. Understanding transport mechanisms in ionic liquid/carbonate solvent electrolyte blends. Phys. Chem. Chem. Phys. 2018, 20, 16579–16591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Díaz, D.; Delgado-Notario, J.A.; Clericò, V.; Diez, E.; Merchán, M.D.; Velázquez, M.M. Towards Understanding the Raman Spectrum of Graphene Oxide: The Effect of the Chemical Composition. Coatings 2020, 10, 524. [Google Scholar] [CrossRef]
- Guigo, N.; Sbirrazzuoli, N. Recent Advances, Techniques and Applications. In Handbook of Thermal Analysis and Calorimetry; Vyazovkin, S., Schick, N.C., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2018; pp. 399–429. [Google Scholar]
- Ferreira, W.H.; Andrade, C.T. The role of graphene on thermally induced shape memory properties of poly(lactic acid) extruded composites. J. Therm. Anal. Calorim. 2020. [Google Scholar] [CrossRef]
- Llanes, L.C.; Clasen, S.H.; Pires, A.T.N.; Gross, I.P. Mechanical and thermal properties of poly(lactic acid) plasticized with dibutyl maleate and fumarate isomers: Promising alternatives as biodegradable plasticizers. Eur. Pol. J. 2021, 142, 110112. [Google Scholar] [CrossRef]
- Kwon, O.M.; Watanabe, H.; Ahn, K.H.; Lee, S.J. Interplay Between Structure and Property of Graphene Nanoplatelet Networks Formed by an Electric Field in a Poly(Lactic Acid) Matrix. J. Rheol. 2017, 61, 291–303. [Google Scholar] [CrossRef]
- Park, I.H.; Lee, J.Y.; Ahn, S.J.; Choi, H.J. Melt Rheology and Mechanical Characteristics of Poly(Lactic Acid)/Alkylated Graphene Oxide Nanocomposites. Polymers 2020, 12, 2402. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.Y.; Zhang, K.; Ren, J.; Zhan, H. Melt rheology of polylactide/poly(butylene adipate-co-terephthalate) blends. Carb. Pol. 2008, 74, 79–85. [Google Scholar] [CrossRef]
- Backes, E.H.; Pires, L.d.N.; Costa, L.C.; Passador, F.R.; Pessan, L.A. Analysis of the Degradation During Melt Processing of PLA/Biosilicate® Composites. J. Compos. Sci. 2019, 3, 52. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Li, C.; Wang, T.; Li, X.; Li, X. Polylactic Acid–Graphene Oxide-based Materials for Loading and Sustained Release of Poorly Soluble Pesticides. Langmuir 2020, 36, 12336–12345. [Google Scholar] [CrossRef]
- Ahmad, A.F.; Aziz, S.A.; Abbas, Z.; Obaiys, S.J.; Matori, K.A.; Zaid, M.H.M.; Raad, H.K.; Aliyu, U.S. Chemically Reduced Graphene Oxide-Reinforced Poly(Lactic Acid)/Poly(Ethylene Glycol) Nanocomposites: Preparation, Characterization, and Applications in Electromagnetic Interference Shielding. Polymers 2019, 11, 661. [Google Scholar] [CrossRef] [Green Version]
- Fu, Z.J.; Huang, H.F.; Yu, L.S.; Sun, Y.F. DSC Studies on the Dyeing Properties of PLA Fiber. Adv. Mater. Res. 2013, 750–752, 1393–1396. [Google Scholar] [CrossRef]
- Cristea, M.; Ionita, D.; Iftime, M.M. Dynamic Mechanical Analysis Investigations of PLA-Based Renewable Materials: How Are They Useful? Materials 2020, 13, 5302. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, G.; Gupta, A.; Pugazhenthi, G.; Katiyar, V. Facile dispersion of exfoliated graphene/PLA nanocomposites via in situ polycondensation with a melt extrusion process and its rheological studies. J. Appl. Polym. Sci. 2018, 135, 46476. [Google Scholar] [CrossRef] [Green Version]










| Size | 40 µm |
| Thickness | 1–2 nm |
| Oxygen Content (XPS) | 30% |
| BET | 400 m2/g |
| Number of Layers | 1–2 |
| GO | IL | |
|---|---|---|
| PLP | 0 wt.% | 5 wt.% |
| PGO | 1 wt.% | 0 wt.% |
| PGL | 1 wt.% | 5 wt.% |
| Zone 1 | Zone 2 | Zone 3 | Zone 4 | Die | |
|---|---|---|---|---|---|
| High | 160 °C | 185 °C | 200 °C | 200 °C | 190 °C |
| Low | 125 °C | 145 °C | 150 °C | 150 °C | 140 °C |
| Temperature Profile | Feeder (rpm) | Extruder (rpm) | Pressure (bar) | Torque (N·m) | SME (kJ/kg) |
|---|---|---|---|---|---|
| Low | 10 | 130 | 7.5 | 2.9 | 0.368 |
| High | 10 | 130 | 5 | 1.7 | 0.241 |
| PLA | PLP | PGO | PGL | |
|---|---|---|---|---|
| SME (kJ/kg) | 0.368 | 0.112 | 0.300 | 0.096 |
| PLA | Assignment | IL | Assignment | GO | Assignment |
|---|---|---|---|---|---|
| 2947 | -C-H3 [46,47] | 2964 | C-H [50,51] CH3 [50,51] | 3500, 3000 | O-H [55,56,57] |
| 2882 | -C-H [46] -C-H3 [47] | 2877 | CH3 [49] | 1719 | C=C [56] C=O [55,56,57] |
| 2297 | CH3 [46,47] | 1470 | CH2 [50] CH3 [51,52] | 1617 | C=O [55,56,57] C=C [56] |
| 1747 | C=O [46,47,48,49] | 871 | P-F ring [50] | 1037 | C-OH C-O [57,58] |
| 1452 | CH3 [46,47,49] | 814 | PF6 [51,53] C-H [53] | 980 | COOH C-O [58] |
| 1360 | -C-H3 [47,49] C H-CH3 [46] | 559 | F-P-F PF6 [54] | ||
| 1180 | C-O-C [47,48,49] -C-O [46] | ||||
| 1130 | C-O-C [47] ras CH3 [46] | ||||
| 1090 | C-O-C [46,47] | ||||
| 1045 | C-C [46] C-O-C [47,48,49] | ||||
| 760 | αCH3 [47] C=O [46] | ||||
| 695 | C=O [46] |
| PLA | Assignment | IL | Assignment | GO | Assignment |
| 711 | C=O [59] | 307 | CH3-N-CH [53] | 1350 | D band [60] |
| 760 | C=O [59] | 471 | PF6 [53] | 1585 | G band [60] |
| 1092 | C-O-C [59] | 743 | PF6 [53] | 2690 | 2D band [60] |
| 1125 | CH3 [59] | 1462 | CH3 [53] | ||
| 1179 | C-O [59] | 2984 | CH3 [53] | ||
| 1386 | CH3 [59] | ||||
| 1450 | C-H [59] | ||||
| 1773 | C=O [59] |
| Weight Loss (%) | PLA | PLP | PGO | PGL |
|---|---|---|---|---|
| 5 | 334 °C | 349 °C | 334 °C | 318 °C |
| 50 | 365 °C | 381 °C | 367 °C | 365 °C |
| 95 | 382 °C | 409 °C | 383 °C | 383 °C |
| PLA | PLP | PGO | PGL | |
|---|---|---|---|---|
| Tg | 57.3 °C | 55.8 °C | 56.4 °C | 57.7 °C |
| ΔHrel | 0.51 J/g | 0.53 J/g | 0.18 J/g | 0.14 J/g |
| E’ (MPa) | E’’ (MPa) | tan δ | |
|---|---|---|---|
| PLA | 55.1 ± 0.2 | 54.9 ± 0.7 | 58.5 ± 0.2 |
| PLP | 46.1 ± 0.3 | 46.5 ± 0.5 | 55.5 ± 0.6 |
| PGO | 55.3 ± 0.2 | 55.8 ± 0.4 | 61.3 ± 0.3 |
| PGL | 42.2 ± 0.1 | 42.5 ± 0.6 | 49.5 ± 0.4 |
| Temperature (°C) | PLA | PLP | PGO | PGL |
|---|---|---|---|---|
| 110 | 0.01–100% | 0.01–100% | 0.01–100% | 0.01–100% |
| 130 | 0.01–100% | 0.01–100% | 0.01–100% | 0.1–100% |
| 150 | 0.01–100% | 0.01–100% | 0.05–100% | 0.1–100% |
| 170 | 0.01–100% | 0.1–100% | 0.1–100% | 0.2–100% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Rodríguez, C.; Avilés, M.-D.; Pamies, R.; Carrión-Vilches, F.-J.; Sanes, J.; Bermúdez, M.-D. Extruded PLA Nanocomposites Modified by Graphene Oxide and Ionic Liquid. Polymers 2021, 13, 655. https://doi.org/10.3390/polym13040655
Sánchez-Rodríguez C, Avilés M-D, Pamies R, Carrión-Vilches F-J, Sanes J, Bermúdez M-D. Extruded PLA Nanocomposites Modified by Graphene Oxide and Ionic Liquid. Polymers. 2021; 13(4):655. https://doi.org/10.3390/polym13040655
Chicago/Turabian StyleSánchez-Rodríguez, Cristian, María-Dolores Avilés, Ramón Pamies, Francisco-José Carrión-Vilches, José Sanes, and María-Dolores Bermúdez. 2021. "Extruded PLA Nanocomposites Modified by Graphene Oxide and Ionic Liquid" Polymers 13, no. 4: 655. https://doi.org/10.3390/polym13040655