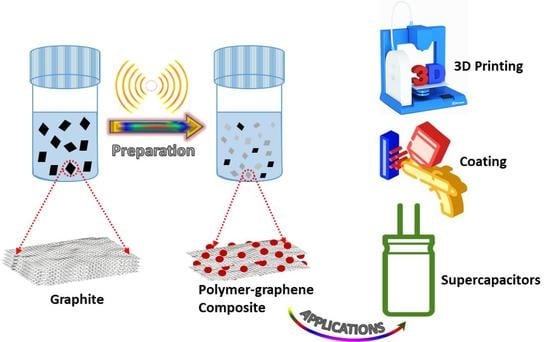
Recent Studies on Dispersion of Graphene–Polymer Composites
Abstract
:1. Introduction
1.1. Chemical Vapor Deposition
1.2. Pyrolysis
1.3. Self-Assembly
1.4. Thermal Decomposition of Silicon Carbide
1.5. Chemical Exfoliation
1.6. Mechanical Exfoliation
2. Stable Graphene Dispersions Using Solvents
3. Stable Graphene Dispersions Using Surfactants
| S. No | Dispersion Method | Graphene Source | Surfactant | Graphene Concentration | References |
|---|---|---|---|---|---|
| 1. | Sonication | Graphite | Sodium cholate | 0.15 mg/mL | [83] |
| 2. | Sonication | Graphite | Tween 80 | 0.12 mg/mL | [83] |
| 3. | Sonication | Graphite | Sodium cholate | 0.3 mg/mL | [85] |
| 5. | Tip Sonication | Graphite | Sodium cholate | 7 mg/mL | [65] |
| 6. | Sonication | Graphite powder | Surfactant from engine oil | 0.5 mg/mL | [87] |
| 7. | Sonication | Graphite | SDBS | 0.05 mg/mL | [88] |
| 8. | Sonication | Graphite micrograins | Sodium cholate | 0.52 mg/mL | [102] |
| 9. | Sonication | Graphite micrograins | Sodium deoxycholate | 2.58 mg/mL | [102] |
| 10. | Hydrothermal treatment | Graphite powder | CTAB | 40–60 µg/mL | [91] |
| 11. | Sonication | rGO | Sodium deoxycholate, poly vinyl pyrrolidone, Briji30 | 2.3 mg/mL | [99] |
| 12. | Sonication | Graphite | GO | >150 mg/mL | [100] |
| 13. | Sonication | GO | SDBS | 1.5 mg/mL | [103] |
| 14. | Sonication | GO | Gallic acid | 1.2–4 mg/mL | [104] |
| 15. | Tip sonication | Graphene powders | Silane-based dispersants | 10 mg/mL | [105] |
4. Stable Graphene Dispersions Using Polymers
5. Graphene–Polymer Dispersion/Composites Characterization
6. Graphene–Polymer Composites and Their Properties
7. Applications
7.1. 3D Printing
7.2. Coating
7.3. Supercapacitors
7.4. Other Applications
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Peres, N.M.R.; Ribeiro, R.M. Focus on Graphene. New J. Phys. 2009, 11, 095002. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Bolotin, K.I.; Sikes, K.J.; Jiang, Z.; Klima, M.; Fudenberg, G.; Hone, J.; Kim, P.; Stormer, H.L. Ultrahigh electron mobility in suspended graphene. Solid State Commun. 2008, 146, 351–355. [Google Scholar] [CrossRef] [Green Version]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior Thermal Conductivity of Single-Layer Graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.R.; Blake, P.; Grigorenko, A.N.; Novoselov, K.S.; Booth, T.J.; Stauber, T.; Peres, N.M.R.; Geim, A.K. Fine Structure Constant Defines Visual Transparency of Graphene. Science 2008, 320, 1308. [Google Scholar] [CrossRef] [Green Version]
- Sur, U.K. Graphene: A Rising Star on the Horizon of Materials Science. Int. J. Electrochem. 2012, 2012, 237689. [Google Scholar] [CrossRef]
- Eigler, S.; Hirsch, A. Chemistry with Graphene and Graphene Oxide—Challenges for Synthetic Chemists. Angew. Chem. Int. Ed. 2014, 53, 7720–7738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, S.; Kim, H.; Lee, Y.; Xu, X.; Park, J.-S.; Zheng, Y.; Balakrishnan, J.; Lei, T.; Ri Kim, H.; Song, Y.I.; et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotechnol. 2010, 5, 574–578. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Huang, Y.; Liang, J.; Wan, X.; Chen, Y. Graphene-based conducting inks for direct inkjet printing of flexible conductive patterns and their applications in electric circuits and chemical sensors. Nano Res. 2011, 4, 675–684. [Google Scholar] [CrossRef]
- Li, J.; Ye, F.; Vaziri, S.; Muhammed, M.; Lemme, M.C.; Östling, M. Efficient Inkjet Printing of Graphene. Adv. Mater. 2013, 25, 3985–3992. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wang, C. Graphene and the related conductive inks for flexible electronics. J. Mater. Chem. C 2016, 4, 7193–7207. [Google Scholar] [CrossRef]
- Das, S.R.; Nian, Q.; Cargill, A.A.; Hondred, J.A.; Ding, S.; Saei, M.; Cheng, G.J.; Claussen, J.C. 3D nanostructured inkjet printed graphene via UV-pulsed laser irradiation enables paper-based electronics and electrochemical devices. Nanoscale 2016, 8, 15870–15879. [Google Scholar] [CrossRef] [Green Version]
- Jariwala, D.; Sangwan, V.K.; Lauhon, L.J.; Marks, T.J.; Hersam, M.C. Carbon nanomaterials for electronics, optoelectronics, photovoltaics, and sensing. Chem. Soc. Rev. 2013, 42, 2824–2860. [Google Scholar] [CrossRef] [Green Version]
- Perumal, S.; Gangadaran, P.; Bae, Y.W.; Ahn, B.-C.; Cheong, I.W. Noncovalent Functionalized Graphene Nanocarriers from Graphite for Treating Thyroid Cancer Cells. ACS Biomater. Sci. Eng. 2021. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lin, L.; Ren, H.; Sun, X. Chapter 2—CVD Synthesis of Graphene. In Thermal Transport in Carbon-Based Nanomaterials; Zhang, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 19–56. [Google Scholar]
- Juang, Z.-Y.; Wu, C.-Y.; Lu, A.-Y.; Su, C.-Y.; Leou, K.-C.; Chen, F.-R.; Tsai, C.-H. Graphene synthesis by chemical vapor deposition and transfer by a roll-to-roll process. Carbon 2010, 48, 3169–3174. [Google Scholar] [CrossRef] [Green Version]
- Wei, D.; Liu, Y.; Wang, Y.; Zhang, H.; Huang, L.; Yu, G. Synthesis of N-Doped Graphene by Chemical Vapor Deposition and Its Electrical Properties. Nano Lett. 2009, 9, 1752–1758. [Google Scholar] [CrossRef]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.-H.; Kim, P.; Choi, J.-Y.; Hong, B.H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457, 706–710. [Google Scholar] [CrossRef]
- Lang, B. A LEED study of the deposition of carbon on platinum crystal surfaces. Surf. Sci. 1975, 53, 317–329. [Google Scholar] [CrossRef]
- Choucair, M.; Thordarson, P.; Stride, J.A. Gram-scale production of graphene based on solvothermal synthesis and sonication. Nat. Nanotechnol. 2009, 4, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Zhu, Y.; Lei, H.; Wang, C.; Zhao, Y.; Huo, E.; Lin, X.; Zhang, Q.; Qian, M.; Mateo, W.; et al. Synthesis of graphene-like carbon from biomass pyrolysis and its applications. Chem. Eng. J. 2020, 399, 125808. [Google Scholar] [CrossRef]
- Tang, L.; Li, X.; Ji, R.; Teng, K.S.; Tai, G.; Ye, J.; Wei, C.; Lau, S.P. Bottom-up synthesis of large-scale graphene oxide nanosheets. J. Mater. Chem. 2012, 22, 5676–5683. [Google Scholar] [CrossRef]
- Li, X.; Lau, S.P.; Tang, L.; Ji, R.; Yang, P. Multicolour light emission from chlorine-doped graphene quantum dots. J. Mater. Chem. C 2013, 1, 7308–7313. [Google Scholar] [CrossRef]
- Zhang, W.; Cui, J.; Tao, C.-A.; Wu, Y.; Li, Z.; Ma, L.; Wen, Y.; Li, G. A Strategy for Producing Pure Single-Layer Graphene Sheets Based on a Confined Self-Assembly Approach. Angew. Chem. Int. Ed. 2009, 48, 5864–5868. [Google Scholar] [CrossRef]
- Wu, X.; Hu, Y.; Ruan, M.; Madiomanana, N.K.; Hankinson, J.; Sprinkle, M.; Berger, C.; Heer, W.A.D. Half integer quantum Hall effect in high mobility single layer epitaxial graphene. Appl. Phys. Lett. 2009, 95, 223108. [Google Scholar] [CrossRef] [Green Version]
- Sutter, P. How silicon leaves the scene. Nat. Mater. 2009, 8, 171–172. [Google Scholar] [CrossRef] [Green Version]
- Alexander-Webber, J.A.; Baker, A.M.R.; Janssen, T.J.B.M.; Tzalenchuk, A.; Lara-Avila, S.; Kubatkin, S.; Yakimova, R.; Piot, B.A.; Maude, D.K.; Nicholas, R.J. Phase Space for the Breakdown of the Quantum Hall Effect in Epitaxial Graphene. Phys. Rev. Lett. 2013, 111, 096601. [Google Scholar] [CrossRef] [Green Version]
- Jobst, J.; Waldmann, D.; Speck, F.; Hirner, R.; Maude, D.K.; Seyller, T.; Weber, H.B. Quantum oscillations and quantum Hall effect in epitaxial graphene. Phys. Rev. B 2010, 81, 195434. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.H.; Yu, T.; Shen, Z.X. Two-dimensional carbon nanostructures: Fundamental properties, synthesis, characterization, and potential applications. J. Appl. Phys. 2010, 108, 071301. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Pham, V.H.; Cuong, T.V.; Nguyen-Phan, T.-D.; Pham, H.D.; Kim, E.J.; Hur, S.H.; Shin, E.W.; Kim, S.; Chung, J.S. One-step synthesis of superior dispersion of chemically converted graphene in organic solvents. Chem. Commun. 2010, 46, 4375–4377. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.-J.; Kim, K.K.; Benayad, A.; Yoon, S.-M.; Park, H.K.; Jung, I.-S.; Jin, M.H.; Jeong, H.-K.; Kim, J.M.; Choi, J.-Y.; et al. Efficient Reduction of Graphite Oxide by Sodium Borohydride and Its Effect on Electrical Conductance. Adv. Funct. Mater. 2009, 19, 1987–1992. [Google Scholar] [CrossRef]
- Zhu, C.; Guo, S.; Fang, Y.; Dong, S. Reducing Sugar: New Functional Molecules for the Green Synthesis of Graphene Nanosheets. ACS Nano 2010, 4, 2429–2437. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Yang, J.; Park, J.; Gou, X.; Wang, B.; Liu, H.; Yao, J. Facile Synthesis and Characterization of Graphene Nanosheets. J. Phys. Chem. C 2008, 112, 8192–8195. [Google Scholar] [CrossRef]
- Ci, L.; Song, L.; Jariwala, D.; Elías, A.L.; Gao, W.; Terrones, M.; Ajayan, P.M. Graphene Shape Control by Multistage Cutting and Transfer. Adv. Mater. 2009, 21, 4487–4491. [Google Scholar] [CrossRef]
- Liang, X.; Chang, A.S.P.; Zhang, Y.; Harteneck, B.D.; Choo, H.; Olynick, D.L.; Cabrini, S. Electrostatic Force Assisted Exfoliation of Prepatterned Few-Layer Graphenes into Device Sites. Nano Lett. 2009, 9, 467–472. [Google Scholar] [CrossRef]
- Yi, M.; Shen, Z. A review on mechanical exfoliation for the scalable production of graphene. J. Mater. Chem. A 2015, 3, 11700–11715. [Google Scholar] [CrossRef]
- Shojaeiarani, J.; Bajwa, D.; Holt, G. Sonication amplitude and processing time influence the cellulose nanocrystals morphology and dispersion. Nanocomposites 2020, 6, 41–46. [Google Scholar] [CrossRef] [Green Version]
- Taurozzi, J.S.; Hackley, V.A.; Wiesner, M.R. Ultrasonic dispersion of nanoparticles for environmental, health and safety assessment—issues and recommendations. Nanotoxicology 2011, 5, 711–729. [Google Scholar] [CrossRef]
- Warner, J.H.; Schäffel, F.; Bachmatiuk, A.; Rümmeli, M.H. (Eds.) Chapter 4—Methods for Obtaining Graphene. In Graphene; Elsevier: Amsterdam, The Netherlands, 2013; pp. 129–228. [Google Scholar]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb Carbon: A Review of Graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Lee, X.J.; Hiew, B.Y.Z.; Lai, K.C.; Lee, L.Y.; Gan, S.; Thangalazhy-Gopakumar, S.; Rigby, S. Review on graphene and its derivatives: Synthesis methods and potential industrial implementation. J. Taiwan Inst. Chem. Eng. 2019, 98, 163–180. [Google Scholar] [CrossRef]
- Lee, H.C.; Liu, W.-W.; Chai, S.-P.; Mohamed, A.R.; Aziz, A.; Khe, C.-S.; Hidayah, N.M.S.; Hashim, U. Review of the synthesis, transfer, characterization and growth mechanisms of single and multilayer graphene. RSC Adv. 2017, 7, 15644–15693. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Sahoo, S.; Wang, N.; Huczko, A. Graphene research and their outputs: Status and prospect. J. Sci. Adv. Mater. Devices 2020, 5, 10–29. [Google Scholar] [CrossRef]
- Catania, F.; Marras, E.; Giorcelli, M.; Jagdale, P.; Lavagna, L.; Tagliaferro, A.; Bartoli, M. A Review on Recent Advancements of Graphene and Graphene-Related Materials in Biological Applications. Appl. Sci. 2021, 11, 614. [Google Scholar] [CrossRef]
- Fang, B.; Chang, D.; Xu, Z.; Gao, C. A Review on Graphene Fibers: Expectations, Advances, and Prospects. Adv. Mater. 2020, 32, 1902664. [Google Scholar] [CrossRef]
- Idowu, A.; Boesl, B.; Agarwal, A. 3D graphene foam-reinforced polymer composites—A review. Carbon 2018, 135, 52–71. [Google Scholar] [CrossRef]
- Hareesha, M.; Yogesha, B.; Naik, L.L.; Saravanabavan, D. Development on graphene based polymer composite materials and their applications—A recent review. AIP Conf. Proc. 2021, 2316, 030016. [Google Scholar] [CrossRef]
- Govindaraj, P.; Fox, B.; Aitchison, P.; Hameed, N. A Review on Graphene Polymer Nanocomposites in Harsh Operating Conditions. Ind. Eng. Chem. Res. 2019, 58, 17106–17129. [Google Scholar] [CrossRef]
- Chen, W.; Weimin, H.; Li, D.; Chen, S.; Dai, Z. A critical review on the development and performance of polymer/graphene nanocomposites. Sci. Eng. Compos. Mater. 2018, 25, 1059–1073. [Google Scholar] [CrossRef]
- Noh, Y.J.; Joh, H.-I.; Yu, J.; Hwang, S.H.; Lee, S.; Lee, C.H.; Kim, S.Y.; Youn, J.R. Ultra-high dispersion of graphene in polymer composite via solvent freefabrication and functionalization. Sci. Rep. 2015, 5, 9141. [Google Scholar] [CrossRef] [Green Version]
- Wu, B.; Yang, X. Molecular Simulation of Electrolyte-Induced Interfacial Interaction between SDS/Graphene Assemblies. J. Phys. Chem. C 2013, 117, 23216–23223. [Google Scholar] [CrossRef]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’Ko, Y.K.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamilton, C.E.; Lomeda, J.R.; Sun, Z.; Tour, J.M.; Barron, A.R. High-Yield Organic Dispersions of Unfunctionalized Graphene. Nano Lett. 2009, 9, 3460–3462. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, A.; Khan, U.; Nirmalraj, P.N.; Boland, J.; Coleman, J.N. Graphene Dispersion and Exfoliation in Low Boiling Point Solvents. J. Phys. Chem. C 2011, 115, 5422–5428. [Google Scholar] [CrossRef]
- Wang, W.; Gai, Y.; Song, N.; Xiao, D.; Tan, H.; Zhao, Y. Highly Efficient Production of Graphene by an Ultrasound Coupled with a Shear Mixer in Supercritical CO2. Ind. Eng. Chem. Res. 2018, 57, 16701–16708. [Google Scholar] [CrossRef]
- Zhang, B.; Ning, W.; Zhang, J.; Qiao, X.; Zhang, J.; He, J.; Liu, C.-Y. Stable dispersions of reduced graphene oxide in ionic liquids. J. Mater. Chem. 2010, 20, 5401–5403. [Google Scholar] [CrossRef]
- Bordes, E.; Morcos, B.; Bourgogne, D.; Andanson, J.-M.; Bussière, P.-O.; Santini, C.C.; Benayad, A.; Costa Gomes, M.; Pádua, A.A.H. Dispersion and Stabilization of Exfoliated Graphene in Ionic Liquids. Front. Chem. 2019, 7, 223. [Google Scholar] [CrossRef]
- Villar-Rodil, S.; Paredes, J.I.; Martínez-Alonso, A.; Tascón, J.M.D. Preparation of graphene dispersions and graphene-polymer composites in organic media. J. Mater. Chem. 2009, 19, 3591–3593. [Google Scholar] [CrossRef]
- Hernandez, Y.; Lotya, M.; Rickard, D.; Bergin, S.D.; Coleman, J.N. Measurement of Multicomponent Solubility Parameters for Graphene Facilitates Solvent Discovery. Langmuir 2010, 26, 3208–3213. [Google Scholar] [CrossRef]
- Qin, J.; Wang, X.; Jiang, Q.; Cao, M. Optimizing Dispersion, Exfoliation, Synthesis, and Device Fabrication of Inorganic Nanomaterials Using Hansen Solubility Parameters. ChemPhysChem 2019, 20, 1069–1097. [Google Scholar] [CrossRef]
- Yi, M.; Shen, Z.; Ma, S.; Zhang, X. A mixed-solvent strategy for facile and green preparation of graphene by liquid-phase exfoliation of graphite. J. Nanopart. Res. 2012, 14, 1003. [Google Scholar] [CrossRef]
- Fedi, F.; Miglietta, M.L.; Polichetti, T.; Ricciardella, F.; Massera, E.; Ninno, D.; Di Francia, G. A study on the physicochemical properties of hydroalcoholic solutions to improve the direct exfoliation of natural graphite down to few-layers graphene. Mater. Res. Express 2015, 2, 035601. [Google Scholar] [CrossRef]
- Yi, M.; Shen, Z.; Zhang, X.; Ma, S. Achieving concentrated graphene dispersions in water/acetone mixtures by the strategy of tailoring Hansen solubility parameters. J. Phys. D Appl. Phys. 2012, 46, 025301. [Google Scholar] [CrossRef]
- Xu, Y.; Cao, H.; Xue, Y.; Li, B.; Cai, W. Liquid-Phase Exfoliation of Graphene: An Overview on Exfoliation Media, Techniques, and Challenges. Nanomaterials 2018, 8, 942. [Google Scholar] [CrossRef] [Green Version]
- Narayan, R.; Kim, S.O. Surfactant mediated liquid phase exfoliation of graphene. Nano Converg. 2015, 2, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, U.; Porwal, H.; O’Neill, A.; Nawaz, K.; May, P.; Coleman, J.N. Solvent-Exfoliated Graphene at Extremely High Concentration. Langmuir 2011, 27, 9077–9082. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.-H.; Zhao, H.-R.; Yu, H.-B. A water-based green approach to large-scale production of aqueous compatible graphene nanoplatelets. Sci. Rep. 2018, 8, 5567. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Fang, M.; Wu, F.; Wu, H.; Wang, L.; Chen, G. Preparation of graphene by exfoliation of graphite using wet ball milling. J. Mater. Chem. 2010, 20, 5817–5819. [Google Scholar] [CrossRef]
- Dong, L.; Chen, Z.; Zhao, X.; Ma, J.; Lin, S.; Li, M.; Bao, Y.; Chu, L.; Leng, K.; Lu, H.; et al. A non-dispersion strategy for large-scale production of ultra-high concentration graphene slurries in water. Nat. Commun. 2018, 9, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ainee Fatimah, A.; Fatin Humaizah Abd, M.; Hur Munawar Kabir, M.; Irman Abdul, R.; Faizal, M.; Hua, C.C.; Suria, R.; Shahidan, R. Graphene Colloidal Dispersion in Various Organic Solvents. Malays. J. Anal. Sci. 2013, 17, 475–480. [Google Scholar]
- Konios, D.; Stylianakis, M.M.; Stratakis, E.; Kymakis, E. Dispersion behaviour of graphene oxide and reduced graphene oxide. J. Colloid Interface Sci. 2014, 430, 108–112. [Google Scholar] [CrossRef]
- Hatakeyama, K.; Koinuma, M.; Shimizu, Y.; Hakuta, Y. A Two-Step Method for Stable and Impurity-Free Graphene Oxide Dispersion in Various Organic Solvents without a Stabilizer or Chemical Modification. Bull. Chem. Soc. Jpn. 2019, 92, 511–520. [Google Scholar] [CrossRef]
- Mypati, S.; Sellathurai, A.; Kontopoulou, M.; Docoslis, A.; Barz, D.P.J. High concentration graphene nanoplatelet dispersions in water stabilized by graphene oxide. Carbon 2021, 174, 581–593. [Google Scholar] [CrossRef]
- Khan, U.; O’Neill, A.; Lotya, M.; De, S.; Coleman, J.N. High-Concentration Solvent Exfoliation of Graphene. Small 2010, 6, 864–871. [Google Scholar] [CrossRef]
- Shabafrooz, V.; Bandla, S.; Hanan, J.C. Graphene dispersion in a surfactant-free, polar solvent. J. Mater. Sci. 2018, 53, 559–572. [Google Scholar] [CrossRef]
- Zhao, W.; Wu, F.; Wu, H.; Chen, G. Preparation of Colloidal Dispersions of Graphene Sheets in Organic Solvents by Using Ball Milling. J. Nanomater. 2010, 2010, 528235. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Shen, Z.; Yi, M.; Zhang, X.; Ma, S. A green, rapid and size-controlled production of high-quality graphene sheets by hydrodynamic forces. RSC Adv. 2014, 4, 36464–36470. [Google Scholar] [CrossRef]
- Zhang, X.; Coleman, A.C.; Katsonis, N.; Browne, W.R.; van Wees, B.J.; Feringa, B.L. Dispersion of graphene in ethanol using a simple solvent exchange method. Chem. Commun. 2010, 46, 7539–7541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; He, W.; Jing, X. Preparation of a Stable Graphene Dispersion with High Concentration by Ultrasound. J. Phys. Chem. B 2010, 114, 10368–10373. [Google Scholar] [CrossRef]
- Wang, S.; Yi, M.; Shen, Z. The effect of surfactants and their concentration on the liquid exfoliation of graphene. RSC Adv. 2016, 6, 56705–56710. [Google Scholar] [CrossRef] [Green Version]
- Perumal, S.; Lee, H.M.; Cheong, I.W. Dispersion Behavior of Graphene with Different Solvents and Surfactants. J. Adhe. Interface 2019, 20, 53–60. [Google Scholar]
- Lotya, M.; King, P.J.; Khan, U.; De, S.; Coleman, J.N. High-Concentration, Surfactant-Stabilized Graphene Dispersions. ACS Nano 2010, 4, 3155–3162. [Google Scholar] [CrossRef]
- Ibrahim, A.; Ridha, S.; Amer, A.; Shahari, R.; Ganat, T. Influence of Degree of Dispersion of Noncovalent Functionalized Graphene Nanoplatelets on Rheological Behaviour of Aqueous Drilling Fluids. Int. J. Chem. Eng. 2019, 2019, 8107168. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, X.; Li, H.; Xing, X.; Jin, L.E.; Cao, Q.; Li, P. Direct exfoliation of graphite into graphene in aqueous solution using a novel surfactant obtained from used engine oil. J. Mater. Sci. 2018, 53, 2484–2496. [Google Scholar] [CrossRef]
- Lotya, M.; Hernandez, Y.; King, P.J.; Smith, R.J.; Nicolosi, V.; Karlsson, L.S.; Blighe, F.M.; De, S.; Wang, Z.; McGovern, I.T.; et al. Liquid Phase Production of Graphene by Exfoliation of Graphite in Surfactant/Water Solutions. J. Am. Chem. Soc. 2009, 131, 3611–3620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Large, M.J.; Ogilvie, S.P.; Amorim Graf, A.; Lynch, P.J.; O’Mara, M.A.; Waters, T.; Jurewicz, I.; Salvage, J.P.; Dalton, A.B. Large-Scale Surfactant Exfoliation of Graphene and Conductivity-Optimized Graphite Enabling Wireless Connectivity. Adv. Mater. Technol. 2020, 5, 2000284. [Google Scholar] [CrossRef]
- Liscio, A.; Kouroupis-Agalou, K.; Kovtun, A.; Gebremedhn, E.; El Garah, M.; Rekab, W.; Orgiu, E.; Giorgini, L.; Samorì, P.; Beljonne, D.; et al. Exfoliation of Few-Layer Graphene in Volatile Solvents Using Aromatic Perylene Diimide Derivatives as Surfactants. ChemPlusChem 2017, 82, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Vacacela Gomez, C.; Tene, T.; Guevara, M.; Tubon Usca, G.; Colcha, D.; Brito, H.; Molina, R.; Bellucci, S.; Tavolaro, A. Preparation of Few-Layer Graphene Dispersions from Hydrothermally Expanded Graphite. Appl. Sci. 2019, 9, 2539. [Google Scholar] [CrossRef] [Green Version]
- Noroozi, M.; Zakaria, A.; Radiman, S.; Abdul Wahab, Z. Environmental Synthesis of Few Layers Graphene Sheets Using Ultrasonic Exfoliation with Enhanced Electrical and Thermal Properties. PLoS ONE 2016, 11, e0152699. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zhang, Z.; He, C.; Dai, L.; Liu, J.; Wang, L. Rationally Designed Surfactants for Few-Layered Graphene Exfoliation: Ionic Groups Attached to Electron-Deficient π-Conjugated Unit through Alkyl Spacers. ACS Nano 2014, 8, 6663–6670. [Google Scholar] [CrossRef]
- Li, L.; Zhou, M.; Jin, L.; Mo, Y.; Xu, E.; Chen, H.; Liu, L.; Wang, M.; Chen, X.; Zhu, H. Green Preparation of Aqueous Graphene Dispersion and Study on Its Dispersion Stability. Materials 2020, 13, 4069. [Google Scholar] [CrossRef] [PubMed]
- Vega-Rios, A.; Rentería-Baltiérrez, F.Y.; Hernández-Escobar, C.A.; Zaragoza-Contreras, E.A. A new route toward graphene nanosheet/polyaniline composites using a reactive surfactant as polyaniline precursor. Synth. Met. 2013, 184, 52–60. [Google Scholar] [CrossRef]
- Shin, Y.; Vranic, S.; Just-Baringo, X.; Gali, S.M.; Kisby, T.; Chen, Y.; Gkoutzidou, A.; Prestat, E.; Beljonne, D.; Larrosa, I.; et al. Stable, concentrated, biocompatible, and defect-free graphene dispersions with positive charge. Nanoscale 2020, 12, 12383–12394. [Google Scholar] [CrossRef]
- Feng, B.-B.; Wang, Z.-H.; Suo, W.-H.; Wang, Y.; Wen, J.-C.; Li, Y.-F.; Suo, H.-L.; Liu, M.; Ma, L. Performance of graphene dispersion by using mixed surfactants. Mater. Res. Express 2020, 7, 095009. [Google Scholar] [CrossRef]
- Song, Y.; Lee, H.; Ko, J.; Ryu, J.; Kim, M.; Sohn, D. Preparation and Characterization of Surfactant-Exfoliated Graphene. Bull. Korean Chem. Soc. 2014, 35, 2009–2012. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Merino, M.J.; Paredes, J.I.; Villar-Rodil, S.; Guardia, L.; Solís-Fernández, P.; Salinas-Torres, D.; Cazorla-Amorós, D.; Morallón, E.; Martínez-Alonso, A.; Tascón, J.M.D. Investigating the influence of surfactants on the stabilization of aqueous reduced graphene oxide dispersions and the characteristics of their composite films. Carbon 2012, 50, 3184–3194. [Google Scholar] [CrossRef]
- Luo, J.; Yang, L.; Sun, D.; Gao, Z.; Jiao, K.; Zhang, J. Graphene Oxide “Surfactant”-Directed Tunable Concentration of Graphene Dispersion. Small 2020, 16, 2003426. [Google Scholar] [CrossRef] [PubMed]
- Kazi, S.N.; Badarudin, A.; Zubir, M.N.M.; Ming, H.N.; Misran, M.; Sadeghinezhad, E.; Mehrali, M.; Syuhada, N.I. Investigation on the use of graphene oxide as novel surfactant to stabilize weakly charged graphene nanoplatelets. Nanoscale Res. Lett. 2015, 10, 212. [Google Scholar] [CrossRef] [Green Version]
- Ramalingam, P.; Pusuluri, S.T.; Periasamy, S.; Veerabahu, R.; Kulandaivel, J. Role of deoxy group on the high concentration of graphene in surfactant/water media. RSC Adv. 2013, 3, 2369–2378. [Google Scholar] [CrossRef]
- Uddin, M.E.; Kuila, T.; Nayak, G.C.; Kim, N.H.; Ku, B.-C.; Lee, J.H. Effects of various surfactants on the dispersion stability and electrical conductivity of surface modified graphene. J. Alloys Compd. 2013, 562, 134–142. [Google Scholar] [CrossRef]
- Li, J.; Xiao, G.; Chen, C.; Li, R.; Yan, D. Superior dispersions of reduced graphene oxide synthesized by using gallic acid as a reductant and stabilizer. J. Mater. Chem. A 2013, 1, 1481–1487. [Google Scholar] [CrossRef]
- Cui, J.; Zhou, S. High-Concentration Self-Cross-Linkable Graphene Dispersion. Chem. Mater. 2018, 30, 4935–4942. [Google Scholar] [CrossRef]
- Wajid, A.S.; Das, S.; Irin, F.; Ahmed, H.S.T.; Shelburne, J.L.; Parviz, D.; Fullerton, R.J.; Jankowski, A.F.; Hedden, R.C.; Green, M.J. Polymer-stabilized graphene dispersions at high concentrations in organic solvents for composite production. Carbon 2012, 50, 526–534. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Q.; Huang, Y.; Ma, Y.; Yin, S.; Zhang, X.; Sun, W.; Chen, Y. Organic Photovoltaic Devices Based on a Novel Acceptor Material: Graphene. Adv. Mater. 2008, 20, 3924–3930. [Google Scholar] [CrossRef]
- Mohan, V.B.; Lau, K.-t.; Hui, D.; Bhattacharyya, D. Graphene-based materials and their composites: A review on production, applications and product limitations. Compos. Part B Eng. 2018, 142, 200–220. [Google Scholar] [CrossRef]
- Itapu, B.; Jayatissa, A.H. A Review in Graphene/Polymer Composites. Chem. Sci. Int. J. 2018, 23, 1–16. [Google Scholar] [CrossRef]
- Zhang, S.-p.; Song, H.-o. Preparation of dispersible graphene oxide as a filler to increase the thermal stability of a flame retarding polymer. Carbon 2013, 56, 394. [Google Scholar] [CrossRef]
- Sun, Y.; Shi, G. Graphene/polymer composites for energy applications. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 231–253. [Google Scholar] [CrossRef]
- Tripathi, S.N.; Rao, G.S.S.; Mathur, A.B.; Jasra, R. Polyolefin/graphene nanocomposites: A review. RSC Adv. 2017, 7, 23615–23632. [Google Scholar] [CrossRef] [Green Version]
- Perumal, S.; Park, K.T.; Lee, H.M.; Cheong, I.W. PVP-b-PEO block copolymers for stable aqueous and ethanolic graphene dispersions. J. Colloid Interface Sci. 2016, 464, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Perumal, S.; Lee, H.M.; Cheong, I.W. A study of adhesion forces between vinyl monomers and graphene surfaces for non-covalent functionalization of graphene. Carbon 2016, 107, 74–76. [Google Scholar] [CrossRef]
- Perumal, S.; Raji, A.; Cheong, I.W. Interaction of Zwitterionic and Ionic Monomers with Graphene Surfaces. Langmuir 2018, 34, 6737–6747. [Google Scholar] [CrossRef]
- Yam, C.-M.; Xiao, Z.; Gu, J.; Boutet, S.; Cai, C. Modification of Silicon AFM Cantilever Tips with an Oligo(ethylene glycol) Derivative for Resisting Proteins and Maintaining a Small Tip Size for High-Resolution Imaging. J. Am. Chem. Soc. 2003, 125, 7498–7499. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lee, H.M.; Choi, S.W.; Cheong, I.W. A study on amphiphilic fluorinated block copolymer in graphite exfoliation using supercritical CO2 for stable graphene dispersion. J. Colloid Interface Sci. 2018, 510, 162–171. [Google Scholar] [CrossRef]
- Lee, H.M.; Perumal, S.; Cheong, I.W. Amphiphilic Fluorinated Block Copolymer Synthesized by RAFT Polymerization for Graphene Dispersions. Polymers 2016, 8, 101. [Google Scholar] [CrossRef] [Green Version]
- Perumal, S.; Lee, H.M.; Cheong, I.W. High-concentration graphene dispersion stabilized by block copolymers in ethanol. J. Colloid Interface Sci. 2017, 497, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Park, K.T.; Perumal, S.; Lee, H.M.; Kim, Y.H.; Cheong, I.W. Solvent-Polymer Interactions for Stable Non-Aqueous Graphene Dispersions in the Presence of PVK-b-PVP Block Copolymer. J. Adhes. Interface 2017, 18, 109–117. [Google Scholar] [CrossRef]
- Perumal, S.; Atchudan, R.; Edison, T.N.J.I.; Shim, J.-J.; Lee, Y.R. Exfoliation and Noncovalent Functionalization of Graphene Surface with Poly-N-Vinyl-2-Pyrrolidone by In Situ Polymerization. Molecules 2021, 26, 1534. [Google Scholar] [CrossRef]
- Wajid, A.S.; Das, S.; Irin, F.; Ahmed, H.S.; Shelburne, J.L.; Parviz, D.; Fullerton, R.J.; Jankowski, A.; Hedden, R.; Green, M.J. Polymer-stabilized graphene dispersions at high concentrations in organic solvents for nanocomposite production. arXiv 2011, arXiv:1107.1519. [Google Scholar]
- Ou, E.; Xie, Y.; Peng, C.; Song, Y.; Peng, H.; Xiong, Y.; Xu, W. High concentration and stable few-layer graphene dispersions prepared by the exfoliation of graphite in different organic solvents. RSC Adv. 2013, 3, 9490–9499. [Google Scholar] [CrossRef]
- Xu, L.; McGraw, J.-W.; Gao, F.; Grundy, M.; Ye, Z.; Gu, Z.; Shepherd, J.L. Production of High-Concentration Graphene Dispersions in Low-Boiling-Point Organic Solvents by Liquid-Phase Noncovalent Exfoliation of Graphite with a Hyperbranched Polyethylene and Formation of Graphene/Ethylene Copolymer Composites. J. Phys. Chem. C 2013, 117, 10730–10742. [Google Scholar] [CrossRef]
- Hamdi, S.S.; Al-Kayiem, H.H.; Muhsan, A.S.; Magaril, E. Experimental dataset on the dispersion stability of natural polymer non-covalently functionalized graphene nanoplatelets in high salinity brines. Data Brief 2020, 31, 105702. [Google Scholar] [CrossRef]
- Mohammadsalih, Z.G.; Inkson, B.J.; Chen, B. The effect of dispersion condition on the structure and properties of polystyrene/graphene oxide nanocomposites. Polym. Compos. 2021, 42, 320–328. [Google Scholar] [CrossRef]
- Gudarzi, M.M.; Sharif, F. Molecular level dispersion of graphene in polymer matrices using colloidal polymer and graphene. J. Colloid Interface Sci. 2012, 366, 44–50. [Google Scholar] [CrossRef]
- Jo, K.; Lee, T.; Choi, H.J.; Park, J.H.; Lee, D.J.; Lee, D.W.; Kim, B.S. Stable Aqueous Dispersion of Reduced Graphene Nanosheets via Non-Covalent Functionalization with Conducting Polymers and Application in Transparent Electrodes. Langmuir 2011, 27, 2014–2018. [Google Scholar] [CrossRef] [PubMed]
- Nuvoli, D.; Alzari, V.; Sanna, R.; Scognamillo, S.; Piccinini, M.; Peponi, L.; Kenny, J.M.; Mariani, A. The production of concentrated dispersions of few-layer graphene by the direct exfoliation of graphite in organosilanes. Nanoscale Res. Lett. 2012, 7, 674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yup, L.J.; Insik, I. Enhanced Solvent Exfoliation of Graphite to Graphene Dispersion in the Presence of Polymer Additive. Chem. Lett. 2011, 40, 567–569. [Google Scholar] [CrossRef]
- Carrasco, P.M.; Montes, S.; García, I.; Borghei, M.; Jiang, H.; Odriozola, I.; Cabañero, G.; Ruiz, V. High-concentration aqueous dispersions of graphene produced by exfoliation of graphite using cellulose nanocrystals. Carbon 2014, 70, 157–163. [Google Scholar] [CrossRef]
- Bhawal, P.; Ganguly, S.; Chaki, T.K.; Das, N.C. Synthesis and characterization of graphene oxide filled ethylene methyl acrylate hybrid nanocomposites. RSC Adv. 2016, 6, 20781–20790. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Kudin, K.N.; Ozbas, B.; Schniepp, H.C.; Prud’homme, R.K.; Aksay, I.A.; Car, R. Raman Spectra of Graphite Oxide and Functionalized Graphene Sheets. Nano Lett. 2008, 8, 36–41. [Google Scholar] [CrossRef]
- No, Y.-S.; Choi, H.K.; Kim, J.-S.; Kim, H.; Yu, Y.-J.; Choi, C.-G.; Choi, J.S. Layer number identification of CVD-grown multilayer graphene using Si peak analysis. Sci. Rep. 2018, 8, 571. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Coh, S.; Tan, L.Z.; Regan, W.; Yuk, J.M.; Chatterjee, E.; Crommie, M.F.; Cohen, M.L.; Louie, S.G.; Zettl, A. Raman Spectroscopy Study of Rotated Double-Layer Graphene: Misorientation-Angle Dependence of Electronic Structure. Phys. Rev. Lett. 2012, 108, 246103. [Google Scholar] [CrossRef] [Green Version]
- Perumal, S.; Atchudan, R.; Cheong, I.W. Poly[2-(methacryloyloxy)ethyl phosphorylcholine]-Stabilized graphene-iron oxide composites for water splitting. Int. J. Hydrogen Energy 2021, 46, 10850–10861. [Google Scholar] [CrossRef]
- Wilczewski, S.; Skórczewska, K.; Tomaszewska, J.; Lewandowski, K.; Szulc, J.; Runka, T. Manufacturing homogenous PVC/graphene nanocomposites using a novel dispersion agent. Polym. Test. 2020, 91, 106868. [Google Scholar] [CrossRef]
- Farivar, F.; Lay Yap, P.; Karunagaran, R.U.; Losic, D. Thermogravimetric Analysis (TGA) of Graphene Materials: Effect of Particle Size of Graphene, Graphene Oxide and Graphite on Thermal Parameters. C 2021, 7, 41. [Google Scholar]
- Liu, X.; Shao, X.Y.; Fang, G.B.; He, H.F.; Wan, Z.G. Preparation and properties of chemically reduced graphene oxide/copolymer-polyamide nanocomposites. e-Polymers 2017, 17, 3–14. [Google Scholar] [CrossRef]
- Ossonon, B.D.; Bélanger, D. Synthesis and characterization of sulfophenyl-functionalized reduced graphene oxide sheets. RSC Adv. 2017, 7, 27224–27234. [Google Scholar] [CrossRef] [Green Version]
- Pan, C.; Liu, L.; Gai, G. Recent Progress of Graphene-Containing Polymer Hydrogels: Preparations, Properties, and Applications. Macromol. Mater. Eng. 2017, 302, 1700184. [Google Scholar] [CrossRef]
- Liao, G.; Hu, J.; Chen, Z.; Zhang, R.; Wang, G.; Kuang, T. Preparation, Properties, and Applications of Graphene-Based Hydrogels. Front. Chem. 2018, 6. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Zhang, S.; Guo, L.; Li, W. Applications of graphene-based composite hydrogels: A review. RSC Adv. 2017, 7, 51008–51020. [Google Scholar] [CrossRef]
- Medina, R.P.; Nadres, E.T.; Ballesteros, F.C.; Rodrigues, D.F. Incorporation of graphene oxide into a chitosan–poly(acrylic acid) porous polymer nanocomposite for enhanced lead adsorption. Environ. Sci. Nano 2016, 3, 638–646. [Google Scholar] [CrossRef]
- Zhang, E.; Wang, T.; Lian, C.; Sun, W.; Liu, X.; Tong, Z. Robust and thermo-response graphene–PNIPAm hybrid hydrogels reinforced by hectorite clay. Carbon 2013, 62, 117–126. [Google Scholar] [CrossRef]
- Wang, J.; Xian, H.; Peng, T.; Sun, H.; Zheng, F. Three-dimensional graphene-wrapped PANI nanofiber composite as electrode material for supercapacitors. RSC Adv. 2015, 5, 13607–13612. [Google Scholar] [CrossRef]
- Xue, R.; Xin, X.; Wang, L.; Shen, J.; Ji, F.; Li, W.; Jia, C.; Xu, G. A systematic study of the effect of molecular weights of polyvinyl alcohol on polyvinyl alcohol–graphene oxide composite hydrogels. Phys. Chem. Chem. Phys. 2015, 17, 5431–5440. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Shen, J.; Ma, H.; Lu, X.; Shi, M.; Li, N.; Ye, M. Preparation and characterization of pH- and temperature-responsive hydrogels with surface-functionalized graphene oxide as the crosslinker. Soft Matter 2012, 8, 3139–3145. [Google Scholar] [CrossRef]
- Abdali, H.; Ajji, A. Functionalized Graphene/Polymer Nanofiber Composites and Their Functional Applications. In Graphene Functionalization Strategies: From Synthesis to Applications; Khan, A., Jawaid, M., Neppolian, B., Asiri, A.M., Eds.; Springer: Singapore, 2019; pp. 127–156. [Google Scholar]
- Park, G.; Kim, S.; Chae, S.; Han, H.; Le, T.-H.; Yang, K.S.; Chang, M.; Kim, H.; Yoon, H. Combining SWNT and Graphene in Polymer Nanofibers: A Route to Unique Carbon Precursors for Electrochemical Capacitor Electrodes. Langmuir 2019, 35, 3077–3086. [Google Scholar] [CrossRef]
- Meng, C.; Yu, S.-L.; Wang, H.-Q.; Cao, Y.; Tong, L.-M.; Liu, W.-T.; Shen, Y.-R. Graphene-doped polymer nanofibers for low-threshold nonlinear optical waveguiding. Light Sci. Appl. 2015, 4, e348. [Google Scholar] [CrossRef]
- Bao, Q.; Zhang, H.; Yang, J.-x.; Wang, S.; Tang, D.Y.; Jose, R.; Ramakrishna, S.; Lim, C.T.; Loh, K.P. Graphene–Polymer Nanofiber Membrane for Ultrafast Photonics. Adv. Funct. Mater. 2010, 20, 782–791. [Google Scholar] [CrossRef]
- Jang, W.; Yun, J.; Seo, Y.; Byun, H.; Hou, J.; Kim, J.-H. Mixed Dye Removal Efficiency of Electrospun Polyacrylonitrile–Graphene Oxide Composite Membranes. Polymers 2020, 12, 2009. [Google Scholar] [CrossRef]
- Boland, C.S.; Khan, U.; Binions, M.; Barwich, S.; Boland, J.B.; Weaire, D.; Coleman, J.N. Graphene-coated polymer foams as tuneable impact sensors. Nanoscale 2018, 10, 5366–5375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, Z.; Weng, C.; Liu, L.; Hou, Y.; Zhao, X.; Kuang, J.; Shi, J.; Wei, Y.; Lou, J.; Zhang, Z. Multifunctional Polymer-Based Graphene Foams with Buckled Structure and Negative Poisson’s Ratio. Sci. Rep. 2016, 6, 32989. [Google Scholar] [CrossRef] [PubMed]
- Teodorescu, F.; Quéniat, G.; Foulon, C.; Lecoeur, M.; Barras, A.; Boulahneche, S.; Medjram, M.S.; Hubert, T.; Abderrahmani, A.; Boukherroub, R.; et al. Transdermal skin patch based on reduced graphene oxide: A new approach for photothermal triggered permeation of ondansetron across porcine skin. J. Control. Release 2017, 245, 137–146. [Google Scholar] [CrossRef] [Green Version]
- Pagneux, Q.; Ye, R.; Chengnan, L.; Barras, A.; Hennuyer, N.; Staels, B.; Caina, D.; Osses, J.I.A.; Abderrahmani, A.; Plaisance, V.; et al. Electrothermal patches driving the transdermal delivery of insulin. Nanoscale Horiz. 2020, 5, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Konwar, A.; Kandimalla, R.; Kalita, S.; Chowdhury, D. Approach To Fabricate a Compact Cotton Patch without Weaving: A Smart Bandage Material. ACS Sustain. Chem. Eng. 2018, 6, 5806–5817. [Google Scholar] [CrossRef]
- Tang, L.-C.; Wan, Y.-J.; Yan, D.; Pei, Y.-B.; Zhao, L.; Li, Y.-B.; Wu, L.-B.; Jiang, J.-X.; Lai, G.-Q. The effect of graphene dispersion on the mechanical properties of graphene/epoxy composites. Carbon 2013, 60, 16–27. [Google Scholar] [CrossRef]
- Loeffen, A.; Cree, D.; Sabzevari, M.; Wilson, L. Effect of Graphene Oxide as a Reinforcement in a Bio-Epoxy Composite. J. Compos. Sci. 2021, 5, 91. [Google Scholar] [CrossRef]
- Bai, Q.-Q.; Wei, X.; Yang, J.-H.; Zhang, N.; Huang, T.; Wang, Y.; Zhou, Z.-W. Dispersion and network formation of graphene platelets in polystyrene composites and the resultant conductive properties. Compos. Part A Appl. Sci. Manuf. 2017, 96, 89–98. [Google Scholar] [CrossRef]
- Poutrel, Q.-A.; Wang, Z.; Wang, D.; Soutis, C.; Gresil, M. Effect of pre and Post-Dispersion on Electro-Thermo-Mechanical Properties of a Graphene Enhanced Epoxy. Appl. Compos. Mater. 2017, 24, 313–336. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Atif, R.; Vo, T.; Inam, F. Graphene Nanoplatelets in Epoxy System: Dispersion, Reaggregation, and Mechanical Properties of Nanocomposites. J. Nanomater. 2015, 2015, 561742. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Kim, S.W.; Yun, H.; Kim, B.J. Impact of size control of graphene oxide nanosheets for enhancing electrical and mechanical properties of carbon nanotube–polymer composites. RSC Adv. 2017, 7, 30221–30228. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Wang, C.; Tang, J.; Zhang, J.; Shang, Q.; Hu, Y.; Wang, H.; Wu, Q.; Zhou, Y.; Lei, W.; et al. High-Performance Biobased Unsaturated Polyester Nanocomposites with Very Low Loadings of Graphene. Polymers 2018, 10, 1288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.; Lan, C.; Zhang, H.; Guan, J.; Zhang, F.; Fei, B.; Zhang, J. Study on Graphene/CNC-Coated Bamboo Pulp Fabric Preparation of Fabrics with Thermal Conductivity. Polymers 2019, 11, 1265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Zhai, S.; Chen, Y.; Xu, Z. Anisotropic Cellulose Nanofibers/Polyvinyl Alcohol/Graphene Aerogels Fabricated by Directional Freeze-drying as Effective Oil Adsorbents. Polymers 2019, 11, 712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarhini, A.; Tehrani-Bagha, A.; Kazan, M.; Grady, B. The effect of graphene flake size on the properties of graphene-based polymer composite films. J. Appl. Polym. Sci. 2021, 138, 49821. [Google Scholar] [CrossRef]
- Yoon, O.J.; Jung, C.Y.; Sohn, I.Y.; Kim, H.J.; Hong, B.; Jhon, M.S.; Lee, N.-E. Nanocomposite nanofibers of poly(d, l-lactic-co-glycolic acid) and graphene oxide nanosheets. Compos. Part A Appl. Sci. Manuf. 2011, 42, 1978–1984. [Google Scholar] [CrossRef]
- Ansari, M.O.; Khan, M.M.; Ansari, S.A.; Amal, I.; Lee, J.; Cho, M.H. pTSA doped conducting graphene/polyaniline nanocomposite fibers: Thermoelectric behavior and electrode analysis. Chem. Eng. J. 2014, 242, 155–161. [Google Scholar] [CrossRef]
- Li, S.; Shu, K.; Zhao, C.; Wang, C.; Guo, Z.; Wallace, G.; Liu, H.K. One-Step Synthesis of Graphene/Polypyrrole Nanofiber Composites as Cathode Material for a Biocompatible Zinc/Polymer Battery. ACS Appl. Mater. Interfaces 2014, 6, 16679–16686. [Google Scholar] [CrossRef] [Green Version]
- Ding, X.; Zhao, Y.; Hu, C.; Hu, Y.; Dong, Z.; Chen, N.; Zhang, Z.; Qu, L. Spinning fabrication of graphene/polypyrrole composite fibers for all-solid-state, flexible fibriform supercapacitors. J. Mater. Chem. A 2014, 2, 12355–12360. [Google Scholar] [CrossRef]
- Liu, H.; Hou, L.; Peng, W.; Zhang, Q.; Zhang, X. Fabrication and characterization of polyamide 6-functionalized graphene nanocomposite fiber. J. Mater. Sci. 2012, 47, 8052–8060. [Google Scholar] [CrossRef]
- Jakus, A.E.; Secor, E.B.; Rutz, A.L.; Jordan, S.W.; Hersam, M.C.; Shah, R.N. Three-Dimensional Printing of High-Content Graphene Scaffolds for Electronic and Biomedical Applications. ACS Nano 2015, 9, 4636–4648. [Google Scholar] [CrossRef]
- Liu, Y.; Weng, B.; Razal, J.M.; Xu, Q.; Zhao, C.; Hou, Y.; Seyedin, S.; Jalili, R.; Wallace, G.G.; Chen, J. High-Performance Flexible All-Solid-State Supercapacitor from Large Free-Standing Graphene-PEDOT/PSS Films. Sci. Rep. 2015, 5, 17045. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Lv, R.; Bai, S. Recent advances on 3D printing graphene-based composites. Nano Mater. Sci. 2019, 1, 101–115. [Google Scholar] [CrossRef]
- Ambrosi, A.; Pumera, M. 3D-printing technologies for electrochemical applications. Chem. Soc. Rev. 2016, 45, 2740–2755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, M.; Pinho, I.S.; Covas, J.A.; Alves, N.M.; Paiva, M.C. 3D printing of graphene-based polymeric nanocomposites for biomedical applications. Funct. Compos. Mater. 2021, 2, 8. [Google Scholar] [CrossRef]
- Angulo-Pineda, C.; Srirussamee, K.; Palma, P.; Fuenzalida, V.M.; Cartmell, S.H.; Palza, H. Electroactive 3D Printed Scaffolds Based on Percolated Composites of Polycaprolactone with Thermally Reduced Graphene Oxide for Antibacterial and Tissue Engineering Applications. Nanomaterials 2020, 10, 428. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wang, C.; Fu, L.; Ye, S.; Wang, M.; Zhou, Y. Fabrication and Application of Novel Porous Scaffold in Situ-Loaded Graphene Oxide and Osteogenic Peptide by Cryogenic 3D Printing for Repairing Critical-Sized Bone Defect. Molecules 2019, 24, 1669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unagolla, J.M.; Jayasuriya, A.C. Enhanced cell functions on graphene oxide incorporated 3D printed polycaprolactone scaffolds. Mater. Sci. Eng. C 2019, 102, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rajzer, I.; Kurowska, A.; Jabłoński, A.; Kwiatkowski, R.; Piekarczyk, W.; Hajduga, M.B.; Kopeć, J.; Sidzina, M.; Menaszek, E. Scaffolds modified with graphene as future implants for nasal cartilage. J. Mater. Sci. 2020, 55, 4030–4042. [Google Scholar] [CrossRef]
- Belaid, H.; Nagarajan, S.; Teyssier, C.; Barou, C.; Barés, J.; Balme, S.; Garay, H.; Huon, V.; Cornu, D.; Cavaillès, V.; et al. Development of new biocompatible 3D printed graphene oxide-based scaffolds. Mater. Sci. Eng. C 2020, 110, 110595. [Google Scholar] [CrossRef]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef]
- Wei, X.; Li, D.; Jiang, W.; Gu, Z.; Wang, X.; Zhang, Z.; Sun, Z. 3D Printable Graphene Composite. Sci. Rep. 2015, 5, 11181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, P.; Jia, J.; Peng, S.; Yang, W.; Bin, S.; Shuai, C. Graphene oxide-driven interfacial coupling in laser 3D printed PEEK/PVA scaffolds for bone regeneration. Virtual Phys. Prototyp. 2020, 15, 211–226. [Google Scholar] [CrossRef]
- Markandan, K.; Lai, C.Q. Enhanced mechanical properties of 3D printed graphene-polymer composite lattices at very low graphene concentrations. Compos. Part A Appl. Sci. Manuf. 2020, 129, 105726. [Google Scholar] [CrossRef]
- Guo, H.; Zhao, H.; Niu, H.; Ren, Y.; Fang, H.; Fang, X.; Lv, R.; Maqbool, M.; Bai, S. Highly Thermally Conductive 3D Printed Graphene Filled Polymer Composites for Scalable Thermal Management Applications. ACS Nano 2021, 15, 6917–6928. [Google Scholar] [CrossRef]
- Gnanasekaran, K.; Heijmans, T.; van Bennekom, S.; Woldhuis, H.; Wijnia, S.; de With, G.; Friedrich, H. 3D printing of CNT- and graphene-based conductive polymer nanocomposites by fused deposition modeling. Appl. Mater. Today 2017, 9, 21–28. [Google Scholar] [CrossRef]
- Li, Z.; Li, J.; Cui, J.; Qiu, H.; Yang, G.; Zheng, S.; Yang, J. Dispersion and parallel assembly of sulfonated graphene in waterborne epoxy anticorrosion coatings. J. Mater. Chem. A 2019, 7, 17937–17946. [Google Scholar] [CrossRef]
- Hussain, A.K.; Sudin, I.; Basheer, U.M.; Yusop, M.Z.M. A review on graphene-based polymer composite coatings for the corrosion protection of metals. Corros. Rev. 2019, 37, 343–363. [Google Scholar] [CrossRef]
- Ollik, K.; Lieder, M. Review of the Application of Graphene-Based Coatings as Anticorrosion Layers. Coatings 2020, 10, 883. [Google Scholar] [CrossRef]
- Abakah, R.R.; Huang, F.; Hu, Q.; Wang, Y.; Jing, L. Comparative Study of Corrosion Properties of Different Graphene Nanoplate/Epoxy Composite Coatings for Enhanced Surface Barrier Protection. Coatings 2021, 11, 285. [Google Scholar] [CrossRef]
- Ehsani, A.; Heidari, A.A.; Sajedi, M. Graphene and Graphene/Polymer Composites as the Most Efficient Protective Coatings for Steel, Aluminum and Copper in Corrosive Media: A Review of Recent Studies. Chem. Rec. 2020, 20, 467–493. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, Z.; Zhang, W.; Yi, M.; Ma, H.; Liu, L.; Liu, L.; Zhao, Y. Graphene Coating for Enhancing the Atom Oxygen Erosion Resistance of Kapton. Coatings 2020, 10, 644. [Google Scholar] [CrossRef]
- Rezvani Moghaddam, A.; Ranjbar, Z. Chapter 5—Dispersion and dispersion stability of graphene in aqueous media for waterborne coating application. In Handbook of Waterborne Coatings; Zarras, P., Soucek, M.D., Tiwari, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 103–123. [Google Scholar]
- Masood, M.T.; Papadopoulou, E.L.; Heredia-Guerrero, J.A.; Bayer, I.S.; Athanassiou, A.; Ceseracciu, L. Graphene and polytetrafluoroethylene synergistically improve the tribological properties and adhesion of nylon 66 coatings. Carbon 2017, 123, 26–33. [Google Scholar] [CrossRef]
- Wang, S.; Hu, Z.; Shi, J.; Chen, G.; Zhang, Q.; Weng, Z.; Wu, K.; Lu, M. Green synthesis of graphene with the assistance of modified lignin and its application in anticorrosive waterborne epoxy coatings. Appl. Surf. Sci. 2019, 484, 759–770. [Google Scholar] [CrossRef]
- Irfan, M.; Bhat, S.I.; Ahmad, S. Waterborne reduced graphene oxide dispersed bio-polyesteramide nanocomposites: An approach towards eco-friendly anticorrosive coatings. New J. Chem. 2019, 43, 4706–4720. [Google Scholar] [CrossRef]
- Li, J.; Jiang, Z.; Gan, L.; Qiu, H.; Yang, G.; Yang, J. Functionalized graphene/polymer composite coatings for autonomous early-warning of steel corrosion. Compos. Commun. 2018, 9, 6–10. [Google Scholar] [CrossRef]
- Dutta, D.; Ganda, A.N.F.; Chih, J.-K.; Huang, C.-C.; Tseng, C.-J.; Su, C.-Y. Revisiting graphene–polymer nanocomposite for enhancing anticorrosion performance: A new insight into interface chemistry and diffusion model. Nanoscale 2018, 10, 12612–12624. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Panigrahi, A.; Singh, S.K.; Pradhan, S.K. Enhanced corrosion resistance and mechanical properties of nanostructured graphene-polymer composite coating on copper by electrophoretic deposition. J. Coat. Technol. Res. 2018, 15, 583–592. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, H.; Wei, M.; Zheng, Z.; Vu, D.D.; Bui, T.T.X.; Huang, X. Preparation, characterization, and properties of graphene oxide/urushiol-formaldehyde polymer composite coating. J. Coat. Technol. Res. 2018, 15, 1343–1356. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y. Graphene and Polymer Composites for Supercapacitor Applications: A Review. Nanoscale Res. Lett. 2017, 12, 387. [Google Scholar] [CrossRef] [PubMed]
- Snook, G.A.; Kao, P.; Best, A.S. Conducting-polymer-based supercapacitor devices and electrodes. J. Power Sources 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Ke, Q.; Wang, J. Graphene-based materials for supercapacitor electrodes—A review. J. Mater. 2016, 2, 37–54. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.B.; Lee, J.-M. Graphene for supercapacitor applications. J. Mater. Chem. A 2013, 1, 14814–14843. [Google Scholar] [CrossRef]
- Skrypnychuk, V.; Boulanger, N.; Nordenström, A.; Talyzin, A. Aqueous Activated Graphene Dispersions for Deposition of High-Surface Area Supercapacitor Electrodes. J. Phys. Chem. Lett. 2020, 11, 3032–3038. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, H.; Eredia, M.; Qiu, H.; Baaziz, W.; Ersen, O.; Ciesielski, A.; Bonn, M.; Wang, H.I.; Samorì, P. Water-Dispersed High-Quality Graphene: A Green Solution for Efficient Energy Storage Applications. ACS Nano 2019, 13, 9431–9441. [Google Scholar] [CrossRef] [Green Version]
- Jeong, H.T.; Kim, S.H.; Ahn, W.J.; Choi, J.Y.; Park, H.Y.; Kim, C.H.; Kim, Y.R. Reduced Graphene Oxide /Polyaniline Composite Material for Supercapacitor Electrode. J. Korean Appl. Sci. 2018, 35, 1088–1095. [Google Scholar] [CrossRef]
- Islam, M.M.; Chidembo, A.T.; Aboutalebi, S.H.; Cardillo, D.; Liu, H.K.; Konstantinov, K.; Dou, S.X. Liquid Crystalline Graphene Oxide/PEDOT:PSS Self-Assembled 3D Architecture for Binder-Free Supercapacitor Electrodes. Front. Energy Res. 2014, 2. [Google Scholar] [CrossRef] [Green Version]
- Lai, E.; Yue, X.; Ning, W.e.; Huang, J.; Ling, X.; Lin, H. Three-Dimensional Graphene-Based Composite Hydrogel Materials for Flexible Supercapacitor Electrodes. Front. Chem. 2019, 7. [Google Scholar] [CrossRef] [Green Version]
- Simionescu, O.-G.; Popa, R.C.; Avram, A.; Dinescu, G. Thin films of nanocrystalline graphene/graphite: An overview of synthesis and applications. Plasma Process. Polym. 2020, 17, 1900246. [Google Scholar] [CrossRef]
- Sahu, P.K.; Pandey, R.K.; Dwivedi, R.; Mishra, V.N.; Prakash, R. Polymer/Graphene oxide nanocomposite thin film for NO2 sensor: An in situ investigation of electronic, morphological, structural, and spectroscopic properties. Sci. Rep. 2020, 10, 2981. [Google Scholar] [CrossRef] [PubMed]
- Jan, R.; Habib, A.; Akram, M.A.; Ahmad, I.; Shah, A.; Sadiq, M.; Hussain, A. Flexible, thin films of graphene–polymer composites for EMI shielding. Mater. Res. Express 2017, 4, 035605. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, A.; Cho, K.Y.; Das, T.K.; Sudarsan, V. An asymmetric electrically conducting self-aligned graphene/polymer composite thin film for efficient electromagnetic interference shielding. AIP Adv. 2017, 7, 015103. [Google Scholar] [CrossRef] [Green Version]
- Eda, G.; Chhowalla, M. Graphene-based Composite Thin Films for Electronics. Nano Lett. 2009, 9, 814–818. [Google Scholar] [CrossRef]
- Dai, X.; Wu, J.; Qian, Z.; Wang, H.; Jian, J.; Cao, Y.; Rummeli, M.H.; Yi, Q.; Liu, H.; Zou, G. Ultra-smooth glassy graphene thin films for flexible transparent circuits. Sci. Adv. 2016, 2, e1601574. [Google Scholar] [CrossRef] [Green Version]
- Zarghami Dehaghani, M.; Kaffashi, B.; Haponiuk, J.T.; Piszczyk, L. Shape memory thin films of Polyurethane: Does graphene content affect the recovery behavior of Polyurethane nanocomposites? Polym. Compos. 2020, 41, 3376–3388. [Google Scholar] [CrossRef]
- Jahandideh, H.; Nguyen, Q.A.; Tufenkji, N. Polymer-Free Emulsion-Templated Graphene-Based Sponges for Contaminant Removal. ACS Appl. Mater. Interfaces 2020, 12, 52095–52103. [Google Scholar] [CrossRef]
- Zhang, B.-X.; Hou, Z.-L.; Yan, W.; Zhao, Q.-L.; Zhan, K.-T. Multi-dimensional flexible reduced graphene oxide/polymer sponges for multiple forms of strain sensors. Carbon 2017, 125, 199–206. [Google Scholar] [CrossRef]
- Hu, Z.; Ji, X.; Li, B.; Luo, Y. A self-assembled graphene/polyurethane sponge for excellent electromagnetic interference shielding performance. RSC Adv. 2019, 9, 25829–25835. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-M.; Chang, Y.-C.; Cheng, L.-C.; Liu, C.-H.; Chang, S.C.; Hsien, T.-Y.; Wang, D.-M.; Hsieh, H.-J. Preparation of graphene-embedded hydroxypropyl cellulose/chitosan/polyethylene oxide nanofiber membranes as wound dressings with enhanced antibacterial properties. Cellulose 2020, 27, 2651–2667. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Z.; Liu, X.; Shen, X.; Zheng, Q.; Xue, Q.; Kim, J.-K. Ultralight Graphene Foam/Conductive Polymer Composites for Exceptional Electromagnetic Interference Shielding. ACS Appl. Mater. Interfaces 2017, 9, 9059–9069. [Google Scholar] [CrossRef] [PubMed]
- Le Gall, M.; Serantoni, V.; Louche, H.; Jourdan, F.; Sigaudo-Roussel, D.; Bonod, C.; Ferraro, S.; Othmen, R.; Bourrier, A.; Dahri-Correia, L.; et al. Monolayer graphene-on-polymer dressings promote healing and stabilize skin temperature on acute and chronic wound models. bioRxiv 2021. [Google Scholar] [CrossRef]
- Kusama, S.; Sato, K.; Matsui, Y.; Kimura, N.; Abe, H.; Yoshida, S.; Nishizawa, M. Transdermal electroosmotic flow generated by a porous microneedle array patch. Nat. Commun. 2021, 12, 658. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Jamaledin, R.; Chen, G.; Baghbantaraghdari, Z.; Zare, E.N.; Di Natale, C.; Onesto, V.; Vecchione, R.; Lee, J.; Tay, F.R.; et al. Stimuli-responsive transdermal microneedle patches. Mater. Today 2021. [Google Scholar] [CrossRef]
- Rajabi, M.; Roxhed, N.; Shafagh, R.Z.; Haraldson, T.; Fischer, A.C.; Wijngaart, W.V.; Stemme, G.; Niklaus, F. Flexible and Stretchable Microneedle Patches with Integrated Rigid Stainless Steel Microneedles for Transdermal Biointerfacing. PLoS ONE 2016, 11, e0166330. [Google Scholar] [CrossRef] [Green Version]











| S. No | Dispersion Method | Graphene Source | Solvent | Concentration | Reference |
|---|---|---|---|---|---|
| 1. | Sonication | Graphite | Chloroform | 3.4 µg/mL | [62] |
| 2. | Sonication | Graphite | IPA | 3.1 µg/mL | [62] |
| 3. | Sonication | Graphite | Acetone | 1.2 µg/mL | [62] |
| 4. | Sonication | Graphite | NMP | 1.2 mg/mL | [77] |
| 5. | Sonication | Graphene nanoplatelets | Ethylene glycol | 0.075 mg/mL | [78] |
| 6. | Sonication | Graphite powder | NMP | 2 to 63 mg/mL | [69] |
| 7. | Sonication | Graphite | Actone, chloroform, and isopropanol | 0.5 mg/mL | [57] |
| 8. | Ball milling | Graphite nanosheets | NMP, DMF, THF, tetramethyluren (TMU), acetone, ethanol, and formamide | 88, 88, 97, 76, 66, 10.32, and 3.67 µg/mL | [79] |
| 9. | Shear mixer (9500 rpm) | Graphite powder | IPA-water mixture | 0.27 mg/mL | [80] |
| 10. | Solvent exchange process | Graphite powder | NMP transferred to ethanol | 0.04 mg/mL | [81] |
| 11. | Tip sonication | Graphite | Water | 0.55 mg/mL | [70] |
| 12. | Sonication | GO | NMP | ~8.7 µg/mL | [74] |
| 13. | Sonication | rGO | o-dichlorobenzene and chloronapthalene | ~9 and ~8.1 µg/mL | [74] |
| 14. | Sonication | Graphite | Water | 1 mg/mL | [82] |
| 15. | Pretreatment and shear mixing | graphite | water | 50 mg/mL | [72] |
| S. No | Dispersion Method | Graphene Source | Polymer | Graphene Concentration | References |
|---|---|---|---|---|---|
| 1. | Supercritical CO2 and Sonication | Graphite | PTFEMA-b-PVP | 0.16–0.30 mg/mL | [117] |
| 2. | Sonication | Graphite | PVP-b-PEO | 2.6 mg/mL | [113] |
| 3. | Sonication | Graphite | PEO-b-PVP | 1.7 mg/mL | [119] |
| 5. | Sonication | rGO | PEO-b-PVP | 1.8 mg/mL | [119] |
| 6. | Sonication | Graphite | PTFEMA-b-PVP | 0.26–0.38 mg/mL | [117] |
| 7. | Sonication | Graphite | Organosilane | 0.66–8.0 mg/mL | [129] |
| 8. | Sonication | Graphite | Polyacryclic acid (PAA) | 0.013 mg/mL | [130] |
| 9. | Tip Sonication | Expanded Graphite | PVPyr | 0.4–0.72 mg/mL | [122] |
| 10. | Autoclave and sonication | Graphite | PVPyr | 0.1 mg/mL | [123] |
| 11. | Heating | GO | PEDOT:PSS | 1.0 mg/mL | [128] |
| 12. | Sonication | Graphite | Cellulose nanocrystal (CNCs) | 0.3–1.08 mg/mL | [131] |
| 13. | Sonication | Graphite | HBPE in THF | 0.016–0.045 mg/mL | [124] |
| 14. | Sonication | Graphite | HBPE in chloroform | 0.025–0.18 mg/mL | [124] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perumal, S.; Atchudan, R.; Cheong, I.W. Recent Studies on Dispersion of Graphene–Polymer Composites. Polymers 2021, 13, 2375. https://doi.org/10.3390/polym13142375
Perumal S, Atchudan R, Cheong IW. Recent Studies on Dispersion of Graphene–Polymer Composites. Polymers. 2021; 13(14):2375. https://doi.org/10.3390/polym13142375
Chicago/Turabian StylePerumal, Suguna, Raji Atchudan, and In Woo Cheong. 2021. "Recent Studies on Dispersion of Graphene–Polymer Composites" Polymers 13, no. 14: 2375. https://doi.org/10.3390/polym13142375