Preparation and Properties of the Urea-Formaldehyde Res-In/Reactive Halloysite Nanocomposites Adhesive with Low-Formaldehyde Emission and Good Water Resistance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
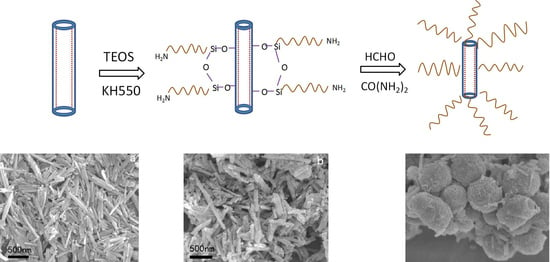
2.2. Preparation of Reactive HNTs
2.3. Preparation of UF/TH-HNTs Nanocomposites
2.4. Preparation and Testing of Plywood
2.5. Characterization
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Najjar, R.; Katourani, S.A.; Hosseini, M.G. Self-healing and corrosion protection performance of organic polysulfide@urea-formaldehyde resin core-shell nanoparticles in epoxy/PANI/ZnO nanocomposite coatings on anodized aluminum alloy. Prog. Org. Coat. 2018, 124, 110–121. [Google Scholar] [CrossRef]
- Antunes, A.; Paiva, N.; Ferra, J.; Martins, J.; Carvalho, L.; Barros-Timmons, A.; Magalhães, F.D. Highly flexible glycol-urea-formaldehyde resins. Eur. Polym. J. 2018, 105, 167–176. [Google Scholar] [CrossRef]
- Lv, R.; Peng, J.; Chen, S.; Hu, Y.; Wang, M.; Lin, J.; Zhou, X.; Zheng, X. A highly linear humidity sensor based on quartz crystal microbalance coated with urea formaldehyde resin/nano silica composite films. Sens. Actuators B Chem. 2017, 250, 721–725. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Y.; Wu, Y.; He, Z.; Wan, H. ‘Greener’ adhesives composed of urea-formaldehyde resin and cottonseed meal for wood-based composites. J. Clean. Prod. 2018, 187, 361–371. [Google Scholar] [CrossRef]
- Papadopoulou, E.; Kountouras, S.; Nikolaidou, Z.; Chrissafis, K.; Michailof, C.; Kalogiannis, K.; Lappas, A.A. Urea-formaldehyde (UF) resins prepared by means of the aqueous phase of the catalytic pyrolysis of European beech wood. COST Action FP. Biochim. Biophys. Acta. 2016, 1192, 234. [Google Scholar] [CrossRef]
- Dorieh, A.; Mahmoodi, N.O.; Mamaghani, M.; Pizzi, A.; Mohammadi, Z.; Moslemi, A. New insight into the use of latent catalysts for the synthesis of urea formaldehyde adhesives and the mechanical properties of medium density fiberboards bonded with them. Eur. Polym. J. 2019, 112, 195–205. [Google Scholar] [CrossRef]
- Cademartori, P.H.G.d.; Artner, M.A.; Alves de Freitas, R.; Magalhães, W.L.E. Alumina nanoparticles as formaldehyde scavenger for urea-formaldehyde resin: Rheological and in-situ cure performance. Compos. B. Eng. 2019, 176, 107281. [Google Scholar] [CrossRef]
- Park, B.D.; Kang, C.; Yong Park, J. Effects of formaldehyde to urea mole ratio on thermal curing behavior of urea-formaldehyde resin and properties of particleboard. J. Appl. Polym. Sci. 2006, 101, 1787–1792. [Google Scholar] [CrossRef]
- Park, B.D.; Kim, J.W. Dynamic mechanical analysis of urea–formaldehyde resin adhesives with different formaldehyde-to-urea molar ratios. J. Appl. Polym. Sci. 2010, 108, 2045–2051. [Google Scholar] [CrossRef]
- Réh, R.; Krišťák, Ľ.; Sedliačik, J.; Bekhta, P.; Božiková, M.; Kunecová, D.; Vozárová, V.; Tudor, E.M.; Antov, P.; Savov, V. Utilization of birch bark as an eco-friendly filler in urea-formaldehyde adhesives for plywood manufacturing. Polymers 2021, 13, 511. [Google Scholar] [CrossRef]
- Boran, S.; Mustafa, U.; Ondaral, S.; Gümüşkaya, E. The efficiency of tannin as a formaldehyde scavenger chemical in medium density fiberboard. Compos. B. 2012, 5, 2487–2491. [Google Scholar] [CrossRef]
- Branka, P.; Samaržija-Jovanović, S.; Jovanović, V.; Dekić, B.; Marković, G.; Marinović-Cincović, M. Effect of γ-irradiation on the hydrolytic stability and thermo-oxidative behavior of bio/inorganic modified urea–formaldehyde resins. Compos. B. 2015, 69, 397–405. [Google Scholar]
- Yuan, J.; Zhao, X.; Ye, L. Structure and properties of urea-formaldehyde resin/polyurethane blend prepared via in-situ polymerization. Rsc Adv. 2015, 5, 53700. [Google Scholar] [CrossRef]
- Ryu, D.Y.; Shimohara, T.; Nakabayashi, K.; Miyawaki, J.; Park, J.-I.; Yoon, S.-H. Urea/nitric acid co-impregnated pitch-based activated carbon fiber for the effective removal of formaldehyde. J. Ind. Eng. Chem. 2019, 80, 98–105. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, L.; Zhao, X. Reactive toughening of intrinsic flame retardant urea-formaldehyde foam with polyether amine: Structure and elastic deformation mechanism. Compos. B. Eng. 2019, 176, 107264. [Google Scholar] [CrossRef]
- Jeong, B.; Park, B.D.; Causin, V. Influence of synthesis method and melamine content of urea-melamine-formaldehyde resins to their features in cohesion, interphase, and adhesion performance. J. Ind. Eng. Chem. 2019, 79, 87–96. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.H.; Jeong, S.G. Formaldehyde emissions from particle board made with phenol–urea–formaldehyde resin prepared by different synthesis methods. J. Adhes. Sci. Technol. 2015, 29, 2090–2103. [Google Scholar] [CrossRef]
- Gao, S.; Cheng, Z.; Zhou, X.; Liu, Y.; Chen, R.; Wang, J.; Wang, C.; Chu, F.; Xu, F.; Zhang, D. Unexpected role of amphiphilic lignosulfonate to improve the storage stability of urea formaldehyde resin and its application as adhesives. Int. J. Biol. Macromol. 2020, 161, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Ye, L.; Zhao, X. Construction of robust siloxane coating for urea-formaldehyde foam and durable hydrophobic mechanism. Compos. Part A Appl. Sci. 2019, 122, 96–106. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, J.; Yi, X.; Zhao, X.; Liu, B.; Liu, X. Nitrogen-doped hierarchical porous carbon derived from ZIF-8 supported on carbon aerogels with advanced performance for supercapacitor. Appl. Surf. Sci. 2019, 507, 145166. [Google Scholar] [CrossRef]
- Liu, J.; Yue, K.; Xu, L.; Wu, J. Bonding performance of melamine-urea–formaldehyde and phenol-resorcinol–formaldehyde adhesive glulams at elevated temperatures. Int. J. Adhes. Adhes. 2020, 98, 102500. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, X.; Zhang, J.; Yi, X. A Z-scheme polyimide/AgBr@Ag aerogel with excellent photocatalytic performance for the degradation of oxytetracycline. Chem. Asian. J. 2019, 14, 422–430. [Google Scholar] [CrossRef]
- Shang, M.; Zhang, X.; Zhang, J.; Sun, J.; Zhao, X.; Yu, S.; Liu, X.; Liu, B.; Yi, X. Nitrogen-doped carbon composite derived from ZIF-8/Polyaniline@cellulose-derived carbon aerogel for high-performance symmetric supercapacitors. Carbohydr. Polym. 2021, 262, 117966. [Google Scholar] [CrossRef]
- Aydin, I.; Demirkir, C.; Colak, S.; Colakoglu, G. Utilization of bark flours as additive in plywood manufacturing. Eur. J. Wood Prod. 2016, 75, 1–7. [Google Scholar] [CrossRef]
- Pavlidou, S.; Papaspyrides, C. A review on polymer-layered silicate nanocomposites. Prog. Polym. Sci. 2008, 33, 1119–1198. [Google Scholar] [CrossRef]
- Moslemi, A.; Zolfagharlou koohi, M.; Behzad, T.; Pizzi, A. Addition of cellulose nanofibers extracted from rice straw to urea formaldehyde resin; effect on the adhesive characteristics and medium density fiberboard properties. Int. J. Adhes. Adhes. 2020, 99, 102582. [Google Scholar] [CrossRef]
- Ma, D.; Wei, J.; Zhao, Y.; Chen, Y.; Tang, S. The removal ofura nium using novel temperature sensitive urea-formaldehyde resin: Adsorption and fast regeneration. Sci. Total Environ. 2020, 735, 139399. [Google Scholar] [CrossRef] [PubMed]
- Ghahri, S.; Mohebby, B. Soybean as adhesive for wood composites: Applications and properties. In Soybean—The Basis of Yield, Bio-Mass and Productivity; InTech Publisher: London, UK, 2017. [Google Scholar]
- Amine, M.; Hamid, R.M.; Pizzi, A. Improving UF particleboard adhesives water resistance by small albumin and sunflower oil additions. Holz. Roh. Werkstoff. 2013, 71, 277–279. [Google Scholar]
- Mansouri, H.R.; Pizzi, A. Urea-formaldehyde-propionaldehyde physical gelation resins for improved swelling in water. J. Appl. Polym. Sci. 2010, 102, 5131–5136. [Google Scholar] [CrossRef]
- Cremonini, C.; Pizzi, A. Field weathering of plywood panels bonded with UF adhesives and low proportions of melamine salts. Holz. Roh. Werkstoff. 1999, 57, 318. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Y.; Fang, Y.; Zhu, J.; Li, X.; Qi, J.; Wu, W. Synthesis of nitrogen-doped flower-like carbon microspheres from urea-formaldehyde resins for high-performance supercapacitor. J. Alloys Compd. 2020, 812, 152109. [Google Scholar] [CrossRef]
- Uzoma, P.C.; Liu, F.; Han, E. Multi-stimuli-triggered and self-repairable fluorocarbon organic coatings with urea-formaldehyde microcapsules filled with fluorosilane. J. Mater. Sci. Technol. 2020, 45, 70–83. [Google Scholar] [CrossRef]
- Sun, J.-T.; Li, J.-W.; Tsou, C.-H.; Pang, J.-C.; Chung, R.-J.; Chiu, C.-W. Polyurethane/nanosilver-doped halloysite nanocomposites: Thermal, mechanical properties, and antibacterial properties. Polymers 2020, 12, 2729. [Google Scholar] [CrossRef] [PubMed]
- Ranjbaran, S.; Nazerian, M.; Kermanian, H.; Koosha, M.; Garmaroody, E.R. High strength papers impregnated with urea/melamine formaldehyde resin/nanosilica nanocomposite coatings: The effects of paper type, blend ratio and nano-content. Mater. Today Commun. 2020, 25, 101300. [Google Scholar] [CrossRef]
- Zhao, X.; Yi, X.; Wang, X.; Zhang, J.; Liu, B.; Liu, X. Highly efficient visible-light-induced photoactivity of carbonized polyimide aerogel for antibiotic degradation. Nanotechnology 2020, 31, 235707. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Ye, L.; Zhang, Z.; Zhao, X. Facile construction of robust super-hydrophobic coating for urea-formaldehyde foam: Durable hydrophobicity and Self-cleaning ability. Compos. Part A Appl. Sci. 2020, 132, 105831. [Google Scholar] [CrossRef]
- Wibowo, E.S.; Lubis, M.A.; Park, B.D.; Kim, J.S.; Causin, V. Converting crystalline thermosetting urea–formaldehyde resins to amorphous polymer using modified nanoclay. J. Ind. Eng. Chem. 2020, 87, 78–89. [Google Scholar] [CrossRef]
- Akbari, V.; Jouyandeh, M.; Paran, S.M.R.; Ganjali, M.R.; Abdollahi, H.; Vahabi, H.; Ahmadi, Z.; Formela, K.; Esmaeili, A.; Mohaddespour, A.; et al. Effect of surface treatment of halloysite nanotubes (HNTs) on the kinetics of epoxy resin cure with amines. Polymers 2020, 12, 930. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.; Wu, D.; Wan, H.; Jia, L.; Chen, G.; Li, J.; Cao, Y.; Liu, X.; Ma, R. Facile synthesis and characterization of halloysite@W18O49 nanocomposite with enhanced photocatalytic properties. Appl. Clay. Sci. 2019, 183, 105319. [Google Scholar] [CrossRef]
- Deng, L.; Yuan, P.; Liu, D.; Du, P.; Zhou, J.; Wei, Y.; Song, Y. Effects of calcination and acid treatment on improving benzene adsorption performance of halloysite. Appl. Clay. Sci. 2019, 181, 105240. [Google Scholar] [CrossRef]
- Huang, D.; Zhang, Z.; Zheng, Y.; Quan, Q.; Wang, W.; Wang, A. Synergistic effect of chitosan and halloysite nanotubes on improving agar film properties. Food Hydrocoll. 2020, 101, 105471. [Google Scholar] [CrossRef]
- Danyliuk, N.; Tomaszewska, J.; Tatarchuk, T. Halloysite nanotubes and halloysite-based composites for environmental and biomedical applications. J. Mol. Liq. 2020, 309, 113077. [Google Scholar] [CrossRef]
- Bertolino, V.; Cavallaro, G.; Milioto, S.; Lazzara, G. Polysaccharides/halloysite nanotubes for smart bionanocomposite materials. Carbohydr. Polym. 2020, 245, 116502. [Google Scholar] [CrossRef]
- Yah, W.O.; Xu, H.; Soejima, H.; Ma, W.; Lvov, Y.; Takahara, A. Biomimetic dopamine derivative for selective polymer modification of halloysite nanotube lumen. J. Am. Chem. Soc. 2012, 134, 12134–12137. [Google Scholar] [CrossRef]
- Kaze, C.R.; Alomayri, T.; Hasan, A.; Tome, S.; Lecomte-Nana, G.L.; Nemaleu, J.G.D.; Tchakoute, H.K.; Kamseu, E.; Melo, U.C.; Rahier, H. Reaction kinetics and rheological behaviour of meta-halloysite based geopolymer cured at room temperature: Effect of thermal activation on physicochemical and microstructural properties. Appl. Clay. Sci. 2020, 196, 105773. [Google Scholar] [CrossRef]
- Yu, W.; Xu, H.; Tan, D.; Fang, Y. Adsorption of iodate on nanosized tubular halloysite. Appl. Clay. Sci. 2020, 184, 105407. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, P. Adsorption behavior of methylene blue on halloysite nanotubes. Micropor. Mesopor. Mater. 2008, 112, 419–424. [Google Scholar] [CrossRef]
- Peng, L.; Zhao, Y.; Bing, Z.; Liu, J.; Yang, Y.; Liu, J. Study on the adsorption of neutral red from aqueous solution onto halloysite nanotubes. Water Res. 2010, 44, 1489. [Google Scholar]
- Jasinski, E.; Bounor-Legaré, V.; Taguet, A.; Beyou, E. Influence of halloysite nanotubes onto the fire properties of polymer based composites: A review. Polym. Degrad. Stab. 2021, 183, 109407. [Google Scholar] [CrossRef]
- Grylewicz, A.; Mozia, S. Polymeric mixed-matrix membranes modified with halloysite nanotubes for water and wastewater treatment: A review. Sep. Purif. Technol. 2021, 256, 117827. [Google Scholar] [CrossRef]
- Asgar, H.; Jin, J.; Miller, J.; Kuzmenko, I.; Gadikota, G. Contrasting thermally-induced structural and microstructural evolution of alumino-silicates with tubular and planar arrangements: Case study of halloysite and kaolinite. Colloid. Surface. A. 2021, 613, 126106. [Google Scholar] [CrossRef]
- Joussein, E.; Petit, S.; Delvaux, B. Behavior of halloysite clay under formamide treatment. Appl. Clay. Sci. 2007, 35, 17–24. [Google Scholar] [CrossRef]
- Horváth, E.; Kristóf, J.; Kurdi, R.; Makó, É.; Khunová, V. Study of urea intercalation into halloysite by thermoanalytical and spectroscopic techniques. J. Therm. Anal. Calorim. 2011, 105, 53–59. [Google Scholar] [CrossRef]
- Okhrimenko, D.V.; Budi, A.; Ceccato, M.; Ceccato, M.; Johansson, D.B.; Lybye, D.; Bechgaard, K.; Stipp, S.L.S. Wettability and hydrolytic stability of 3-aminopropylsilane coupling agent and phenol-urea-formaldehyde binder on silicate surfaces and fibers. Polym. Degrad. Stab. 2021, 183, 109431. [Google Scholar] [CrossRef]
- Makaremi, M.; Pasbakhsh, P.; Cavallaro, G.; Lazzara, G.; Aw, Y.K.; Lee, S.M.; Milioto, S. Effect of morphology and size of halloysite nanotubes on functional pectin bionanocomposites for food packaging applications. ACS Appl. Mat. Inter. 2017, 9, 17476. [Google Scholar] [CrossRef]
- Gao, P.-P.; Zhou, Z.-H.; Yang, B.; Ji, X.; Pan, M.; Tang, J.-h.; Lin, H.; Zhong, G.-J.; Li, Z.-M. Structural regulation of poly(urea-formaldehyde) microcapsules containing lube base oil and their thermal properties. Prog. Org. Coat. 2021, 150, 105990. [Google Scholar] [CrossRef]
- Antov, P.; Savov, V.; Krišťák, Ľ.; Réh, R.; Mantanis, G.I. Eco-friendly, high-density fiberboards bonded with urea-formaldehyde and ammonium lignosulfonate. Polymers 2021, 13, 220. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Q.; Zhang, Y.; Cheng, H.; Lu, Y. Silane-Grafted Silica-Covered Kaolinite as Filler of Styrene Butadiene Rubber. Appl. Clay. Sci. 2012, 65, 134–138. [Google Scholar] [CrossRef]
- Chen, S.; Yang, Z.; Wang, F. Investigation on the properties of PMMA/reactive halloysite nanocomposites based on halloysite with double bonds. Polymers 2018, 10, 919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamarneh, A. Novel Wood Adhesives from Bio-Based Materials and Polyketones; University of Groningen: Groningen, The Netherlands, 2010. [Google Scholar]
- Wescott, J.M.; Frihart, C.R. Water Resistant Vegetable Protein Adhesive Dispersion Compositions. U.S. Patent 7,345,136, 18 May 2008. [Google Scholar]
- Hosseyni, M.; Rahimi, S.; Faezipour, M.; Faezipour, M.M. Effect of nanoclay particles on the properties of particleboards. J. Basic. Appl. Sci. Res. 2014, 4, 280–287. [Google Scholar]









| Samples | Mw | Mn | Mw/Mn |
|---|---|---|---|
| UF | 23,115 | 22,359 | 1.03 |
| UF-H-20% | 25,633 | 22,510 | 1.14 |
| UF-TH-20% | 28,294 | 22,704 | 1.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.; Chen, S.; Yi, X.; Zhao, X.; Zhang, J.; Liu, X.; Liu, B. Preparation and Properties of the Urea-Formaldehyde Res-In/Reactive Halloysite Nanocomposites Adhesive with Low-Formaldehyde Emission and Good Water Resistance. Polymers 2021, 13, 2224. https://doi.org/10.3390/polym13142224
Song J, Chen S, Yi X, Zhao X, Zhang J, Liu X, Liu B. Preparation and Properties of the Urea-Formaldehyde Res-In/Reactive Halloysite Nanocomposites Adhesive with Low-Formaldehyde Emission and Good Water Resistance. Polymers. 2021; 13(14):2224. https://doi.org/10.3390/polym13142224
Chicago/Turabian StyleSong, Jingbiao, Shiwei Chen, Xibin Yi, Xinfu Zhao, Jing Zhang, Xiaochan Liu, and Benxue Liu. 2021. "Preparation and Properties of the Urea-Formaldehyde Res-In/Reactive Halloysite Nanocomposites Adhesive with Low-Formaldehyde Emission and Good Water Resistance" Polymers 13, no. 14: 2224. https://doi.org/10.3390/polym13142224