Advances in Biodegradable Nano-Sized Polymer-Based Ocular Drug Delivery
Abstract
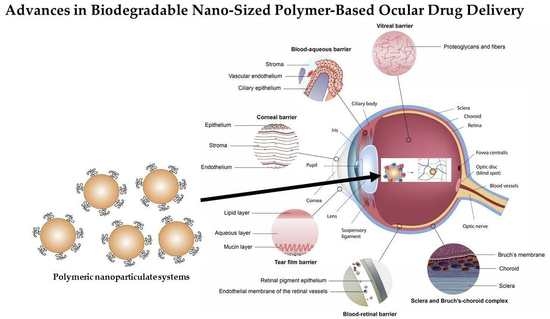
:1. Introduction
2. Ocular Physiological Defense Mechanisms for Drug Delivery
2.1. Cornea
2.2. Conjunctiva
2.3. Sclera
2.4. Blood-Ocular Barriers
2.5. Posterior Segment
3. Current Limitations of Conventional Delivery Systems Employed for Ocular Therapeutics
3.1. Eye Drops (Solutions and Suspensions)
3.2. Ointments
3.3. Intravitreal Injections
3.4. Intraocular Implants
3.5. Contact Lenses
3.6. Emulsions
4. Biodegradable Polymers in Ocular Drug Delivery
4.1. Natural Polymers
4.1.1. Chitosan
4.1.2. Hyaluronic Acid
4.1.3. Sodium Alginate
4.1.4. Carboxymethylcellulose Sodium
4.2. Synthetic Polymers
4.2.1. Poly(lactic-co-glycolic acid)
4.2.2. Poly(ɛ-caprolactone)
4.2.3. Poly(ethylene glycol)
5. Nanotechnology Employed in Ocular Drug Delivery
5.1. Nanogels
5.2. Nanoparticles
5.3. Nanosuspensions
5.4. Nanomicelles
5.5. Nanofibers
5.6. Nanoliposomes
5.7. Nanowires
6. Future Developments in Ocular Drug Delivery Using Biodegradable Polymers and Nanotechnology
7. Conclusions
Funding
Conflicts of Interest
References
- Imam, A.S.S.; Bukhari, A.S.N.; Ahmad, J.; Ali, A. Formulation and Optimization of Levofloxacin Loaded Chitosan Nanoparticle for Ocular Delivery: In Vitro Characterization, Ocular Tolerance and Antibacterial Activity. Int. J. Biol. Macromol. 2018, 108, 650–659. [Google Scholar]
- Gomes-Ballesteros, M.; Lopez-Cano, J.; Bravo-Osuna, I.; Herrero-Vanrell, R.; Molina-Martinez, I.T. Osmoprotectants and Hybrid Liposome/HPMC Systems as Potential Glaucoma Treatment. Polymers 2019, 11, 929. [Google Scholar] [CrossRef]
- Brown, D.; Heier, J.S.; Boyer, D.S.; Fruend, K.B.; Kaiser, P.; Kim, J.E.; Sarraf, D. Current Best Clinical Practices–Management of Neovascular AMD. J. Vitreoretinal Dis. 2017, 9, 294–297. [Google Scholar] [CrossRef]
- Vu, H.T.V.; Keefe, J.E.; McCarty, C.A.; Taylor, H.R. Impact of unilateral and bilateral vision loss on quality of life. Br. J. Ophthalmol. 2005, 89, 360–363. [Google Scholar] [CrossRef] [Green Version]
- Ludwig, A. The Use of Mucoadhesive Polymers in Ocular Drug Delivery. Adv. Drug Deliv. Rev. 2005, 57, 1595–1639. [Google Scholar] [CrossRef]
- Xu, Q.; Kambhampati, S.P.; Kanna, R.M. Nanotechnology Approaches for Ocular Drug Delivery. Middle East Afr. J. Ophthalmol. 2013, 20, 26–37. [Google Scholar]
- Sack, R.A.; Nune, I.; Beaton, A.; Morris, C. Host-Defense Mechanism of the Ocular Surfaces. Biosci. Rep. 2001, 21, 463–480. [Google Scholar] [CrossRef] [Green Version]
- Barar, J.; Aghanejad, A.; Fathi, M.; Omidi, Y. Advanced Drug Delivery and Targeting Technologies for the Ocular Diseases. Bioimpacts 2016, 6, 49–67. [Google Scholar] [CrossRef]
- Gupta, H.; Velpandian, T.; Jain, S. Ion and pH-Activated Novel in Situ System for Sustained Ocular Drug Delivery. J. Drug Target. 2010, 18, 499–505. [Google Scholar] [CrossRef]
- Rawas-Qalaji, M.; Williams, C. Advances in Ocular Drug Delivery. Curr. Eye Res. 2012, 37, 345–356. [Google Scholar] [CrossRef]
- Taylor, A.W. Ocular Immune Privilege. Eye 2009, 23, 1885–1889. [Google Scholar] [CrossRef]
- Streilein, J.W. Ocular Immune Privilege: The Eye Takes a Dim but Practical View of Immunity and Inflammation. J. Leukoc. Biol. 2003, 74, 179–185. [Google Scholar] [CrossRef]
- Huang, D.; Chen, D.; Rupenthal, I. Overcoming Ocular Drug Delivery Barriers Through the Use of Physical Forces. Adv. Drug Deliv. Rev. 2019, 139, 157. [Google Scholar] [CrossRef]
- Ramsey, E.; Del Amo, E.M.; Toropainen, E.; Tengvall-Unadike, U.; Ranta, V.P.; Urtti, A.; Ruponen, M. Corneal and conjunctival drug permeability: Systematic comparison and pharmacokinetic impact in the eye. Eur. J. Pharm. Sci. 2018, 119, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Akpek, E.K.; Gottsch, J.D. Immune Defence at the Ocular Surface. Eye 2003, 17, 949–956. [Google Scholar] [CrossRef]
- Mandal, A.; Bisht, R.; Rupenthal, I.D.; Mitra, A.K. Polymeric Micelles for Ocular Drug Delivery: From Structural Frameworks to Recent Preclinical Studies. J. Control. Release 2017, 248, 96–116. [Google Scholar] [CrossRef]
- Kuno, K.; Fujii, S. Recent Advances in Ocular Drug Delivery Systems. Polymers 2011, 3, 193–221. [Google Scholar] [CrossRef]
- Geroski, D.H.; Edelhauser, H.F. Drug Delivery for Posterior Segment Eye Disease. Investig. Ophthalmol. Vis. Sci. 2000, 41, 961–964. [Google Scholar]
- Mousavikhamene, Z.; Abdekhodaie, M.J.; Ahmadieh, H. Facilitation of Transscleral Drug Delivery by Drug Loaded Magnetic Nanoparticles. Mater. Sci. Eng. 2017, 79, 812–820. [Google Scholar] [CrossRef]
- Eljarrat-Binstock, E.; Pe’er, J.; Domb, A.J. New Techniques for Drug Delivery to the Posterior Eye Segment. Pharm. Res. 2010, 27, 530–543. [Google Scholar] [CrossRef]
- Gaudana, R.; Ananthula, H.K.; Parenky, A.; Mitra, A.K. Ocular Drug Delivery. Am. Assoc. Pharm. Sci. J. 2010, 12, 348–360. [Google Scholar] [CrossRef]
- Kaji, H.; Nagia, N.; Nishizawa, M.; Abe, T. Drug Delivery Devices for Retinal Diseases. Adv. Drug Deliv. Rev. 2018, 128, 148–157. [Google Scholar] [CrossRef]
- Bisht, R.; Mandal, A.; Jaiswal, J.K.; Repenthal, I.D. Nanocarrier Mediated Retinal Drug Delivery: Overcoming Ocular Barriers to Treat Posterior Eye Diseases. Wires Nanomed. Nanotechnol. 2018, 10, e1473. [Google Scholar] [CrossRef]
- Asiedu, K.; Abu, S.L. The Impact of Topical Intraocular Pressure Lowering Medications on the Ocular Surface of Glaucoma Patients: A Review. J. Curr. Ophthalmol. 2019, 31, 8–15. [Google Scholar] [CrossRef]
- Subrizi, A.; del Amo, E.M.; Korzhikov-Vliakh, V.; Tennilova, T.; Ruponen, M.; Urtti, A. Design Principles of Ocular Drug Delivery Systems: Importance of Drug Payload, Release Rate, and Material Properties. Drug Discov. Today 2019. [Google Scholar] [CrossRef]
- Mohammed, N.; Rejinold, N.S.; Mangalathilam, S.; Biswas, R.; Nair, S.V.; Jayakumar, R. Fluconazole Loaded Chitin Nanogels as a Topical Ocular Drug Delivery Agent for Corneal Fungal Infections. J. Biomed. Nanotechnol. 2013, 9, 1521–1531. [Google Scholar] [CrossRef]
- Nordstrom, B.L.; Friedman, D.S.; Mozaffari, E.; Quigley, H.A.; Walker, A.M. Persistence and Adherence with Topical Glaucoma Therapy. Am. J. Ophthalmol. 2005, 140, 598. [Google Scholar] [CrossRef]
- Newman-Casey, P.A.; Robin, A.L.; Blachley, T.; Farris, K.; Heisler, M.; Resnicow, K.; Lee, P.P. The Most Common Barriers to Glaucoma Medication Adherence. Ophthalmology 2015, 122, 1308–1316. [Google Scholar] [CrossRef] [Green Version]
- Bao, Q.; Jog, R.; Shen, J.; Newman, B.; Wang, Y.; Choi, S.; Burgess, D.J. Physicochemical Attributes and Dissolution Testing of Ophthalmic Ointments. Int. J. Pharm. 2017, 523, 310–319. [Google Scholar] [CrossRef]
- Bode, C.; Kranz, H.; Siepmann, F.; Siepmann, J. In-situ Forming PLGA Implants for Intraocular Dexamethasone Delivery. Int. J. Pharm. 2018, 548, 337–348. [Google Scholar] [CrossRef]
- Del Amo, E.M.; Del Amo, E.M.; Rimpelä, A.K.; Heikkinen, E.; Kari, O.K.; Ramsay, E.; Lajunen, T.; Schmitt, M.; Pelkonen, L.; Bhattacharya, M.; et al. Pharmacokinetic Aspects of Retinal Drug Delivery. Prog. Retin. Eye Res. 2017, 57, 134–185. [Google Scholar] [CrossRef]
- Yasin, M.N.; Svirskis, D.; Seyfoddin, A.; Rupenthal, I.D. Implants for Drug Delivery to the Posterior Segment of the Eye: A Focus on Stimuli-Responsive and Tunable Release Systems. J. Control. Release 2014, 196, 208–221. [Google Scholar] [CrossRef]
- Torres-Luna, C.; Hu, N.; Koolivand, A.; Fan, X.; Zhu, Y.; Domsy, R.; Yang, J.; Yang, A.; Wang, N.S. Effect of a Cationic Surfactant on Microemulsion Globules and Drug Release from Hydrogel Contact Lenses. Pharmaceutics 2019, 11, 262. [Google Scholar] [CrossRef]
- Xinming, L.; Yingde, C.; Lloyd, A.W.; Mikhalovsky, S.V.; Sandeman, S.R.; Howel, C.A.; Liewen, L. Polymeric Hydrogels for Novel Contact Lens-Based Ophthalmic Drug Delivery Systems: A Review. Contact Lens Anterior Eye 2008, 31, 57–64. [Google Scholar] [CrossRef]
- Paradiso, P.; Serro, A.P.; Saramango, B.; Colaco, R.; Chauhan, A. Controlled Release of Antibiotics from Vitamin E-Loaded Silicone-Hydrogel Contact Lenses. J. Pharm. Sci. 2016, 105, 1164–1172. [Google Scholar] [CrossRef]
- Patel, A.; Cholkar, K.; Agrahari, V.; Mitra, A.K. Ocular Drug Delivery Systems: An Overview. World J. Pharmacol. 2013, 2, 47–64. [Google Scholar] [CrossRef]
- Tamilvanan, S.; Benita, S. The Potential of Lipid Emulsion for Ocular Delivery of Lipophilic Drugs. Eur. J. Pharm. Biopharm. 2004, 58, 357–368. [Google Scholar] [CrossRef]
- Peterson, G.I. Biodegradable Shape Memory Polymers in Medicine. Adv. Healthc. Mater. 2017, 6, 1700694. [Google Scholar] [CrossRef]
- Di Colo, G.; Zambito, Y.; Zaino, C.; Sansò, M. Selected Polysaccharides at Comparison for their Mucoadhesiveness and Effect on Precorneal Residence of Different Drugs in the Rabbit Model. Drug Dev. Ind. Pharm. 2009, 35, 941–949. [Google Scholar] [CrossRef]
- Kimura, H.; Ogura, Y. Biodegradable polymers for ocular drug delivery. Ophthalmologica 2001, 215, 143–155. [Google Scholar] [CrossRef]
- Almeida, H.; Amaral, M.H.; Lobão, P.; Lobo, J.M.S. In Situ Gelling Systems: A Strategy to Improve the Bioavailability of Ophthalmic Pharmaceutical Formulations. Drug Discov. Today 2014, 19, 400–412. [Google Scholar] [CrossRef]
- Geethalakshma, A.; Karki, R.; Sagi, P.; Jha, S.K.; Venkatesh, D.P. Temperature Triggered in Situ Gelling System for Betaxolol in Glaucoma. J. Appl. Pharm. Sci. 2013, 3, 153–159. [Google Scholar] [CrossRef]
- Bawa, P.; Pillay, V.; Choonara, Y.E.; du Toit, L.C. Stimuli-Responsive Polymers and Their Applications in Drug Delivery. Biomed. Mater. 2009, 4, 022001. [Google Scholar] [CrossRef]
- Du Toit, L.C.; Carmichael, T.; Govender, T.; Kumar, P.; Choonara, Y.E.; Pillay, V. In Vitro, In Vivo and in Silico Evaluation of the Bioresponsive Behavior of an Intelligent Intraocular Implant. Pharm. Res. 2014, 31, 607–634. [Google Scholar] [CrossRef]
- Irimia, T.; Ghica, M.V.; Popa, L.; Anuta, V.; Arsene, A.L.; Dinu-Pirvu, C.E. Strategies for Improving Ocular Drug Bioavailability and Corneal Wound Healing with Chitosan-Based Delivery Systems. Polymers 2018, 10, 1221. [Google Scholar] [CrossRef]
- Quinones, J.P.; Peniche, H.; Peniche, C. Chitosan based self-assembled nanoparticles in drug delivery. Polymers 2018, 10, 235. [Google Scholar] [CrossRef]
- Zhao, R.; Li, J.; Wang, J.; Yin, Z.; Zhu, Y.; Liu, W. Development of Timolol-Loaded Galactosylated Chitosan Nanoparticles and Evaluation of Their Potential for Ocular Drug Delivery. AAPS Pharm Sci. Technol. 2017, 18, 997–1008. [Google Scholar] [CrossRef]
- Zhu, X.; Su, M.; Tang, S.; Wang, L.; Liang, X.; Meng, F.; Hong, Y.; Xu, Z. Synthesis of Thiolated Chitosan and Preparation of Nanoparticles with Sodium Alginate for Ocular Drug Delivery. Mol. Vis. 2012, 18, 1973–1982. [Google Scholar]
- Bhatta, R.S.; Chandasana, H.; Chonker, Y.S.; Rathi, C.; Kumar, D.; Mitra, K.; Shukla, P.K. Mucoadhesive Nanoparticles for Prolonged Ocular Delivery of Natamycin: In Vitro and Pharmacokinetics Studies. Int. J. Pharm. 2012, 432, 105–112. [Google Scholar] [CrossRef]
- Cheung, R.C.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef]
- Yeh, T.H.; Hsu, L.W.; Tseng, M.T.; Lee, P.L.; Sonjae, K.; Ho, Y.C.; Sung, H.W. Mechanism and Consequence of Chitosan-Mediated Reversible Epithelial Tight Junction Opening. Biomaterials 2011, 32, 6164–6173. [Google Scholar] [CrossRef]
- Silva, M.M.; Calado, R.; Marto, J.; Bettencourt, A.; Almeida, A.J.; Goncalves, L.M.D. Chitosan Nanoparticles as a Mucoadhesive Drug Delivery System for Ocular Administration. Mar. Drugs 2017, 15, 370. [Google Scholar] [CrossRef]
- Fischak, C.; Klaus, R.; Werkmeister, R.M.; Hohenadl, C.; Prinz, M.; Schmettere, L.; Garhofer, G. Effect of Topically Administered Chitosan-N-acetylcysteine on Cornal Wound Healing in a Rabbit Model. J. Ophthalmol. 2017, 2017, 1–6. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Guo, S.; Zhao, K. Biomedical Applications of Chitosan and Its Derivative Nanoparticles. Polymers 2018, 10, 462. [Google Scholar] [CrossRef]
- Lui, Y.; Wang, Y.; Yang, J.; Zhang, H.; Gan, L. Cationized Hyaluronic Acid Coated Spanlastics for Cyclosporine a Ocular Delivery: Prolonged Ocular Retention, Enhanced Corneal Permeation and Improved Tear Production. Int. J. Pharm. 2019, 565, 133–142. [Google Scholar]
- De la Fuente, M.; Seijo, B.; Alonso, M.J. Novel Hyaluronic Acid-Chitosan Nanoparticles for Ocular Gene Therapy. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2016–2024. [Google Scholar] [CrossRef] [Green Version]
- Bongiovi, F. Hyaluronic Acid-Based Micelles as Ocular Platform to modulate the loading, release and corneal permeation of corticosteroids. Macromol. Biosci. 2017, 17, 1700261. [Google Scholar] [CrossRef]
- Salzillo, R.; Schiraldi, C.; Corsuto, L.; D’Agostino, A.; Filosa, R.; De Rosa, M.; La Gatta, A. Optimization of Hyaluronan-Based Eye Drop Formulations. Carbohydr. Polym. 2016, 53, 275–283. [Google Scholar] [CrossRef]
- Graca, A.; Gonçalves, L.M.; Raposo, S.; Ribeiro, H.M.; Marto, J. Useful in Vitro Techniques to Evaluate the Mucoadhesive Properties of Hyaluronic Acid-Based Ocular Delivery Systems. Pharmaceutocs 2018, 10, 110. [Google Scholar] [CrossRef]
- Egbu, R.; Brocchini, S.; Khaw, P.T.; Awwad, S. Antibody Loaded Collapsible Hyaluronic Acid Hydrogels for Intraocular Delivery. Eur. J. Pharm. Biopharm. 2018, 124, 95–103. [Google Scholar] [CrossRef]
- Widjaja, L.K.; Bora, M.; Chan, P.N.P.H.; Lipik, V.; Wong, T.T.L.; Venkatraman, S.S. Hyaluronic Acid-Based Nanocomposites Hydrogels for Ocular Drug Delivery Applications. J. Biomed. Mater. Res. 2013, 102, 3056–3065. [Google Scholar] [CrossRef]
- Szekalska, M.; Pucilowska, A.; Szymanska, E.; Ciosek, P.; Winnicka, K. Alginate: Current Use and Future Perspectives in Pharmaceutical and Biomedical Applications. Int. J. Polym. Sci. 2016, 2016, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Ma, Q.; Su, J.; Wang, C.; Kankala, R.K.; Zeng, M.; Lin, H.; Zhou, S.F. Dual-Responsive Alginate Hydrogels for Controlled Release of Therapeutics. Molecules 2019, 24, 2089. [Google Scholar] [CrossRef]
- Shelley, H.; Rodrigues-Galarza, R.M.; Duran, S.H.; Abarca, E.M.; Babu, R.J. In-situ Gel Formulation for Enhanced Ocular Delivery of Nepafenac. J. Pharm. Sci. 2018, 107, 3089–3097. [Google Scholar] [CrossRef]
- Costa, J.R.; Silva, N.C.; Sarmento, B.; Pintado, M. Potential Chitosan-Coated Alginate Nanoparticles for Ocular Delivery of Daptomycin. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1255–1262. [Google Scholar] [CrossRef]
- Al-Kinani, A.A.; Zidan, G.; Elsaid, N.; Seyfoddin, A.; Alani, A.W.G.; Alany, R.G. Ophthalmic Gels: Past, Present and Future. Adv. Drug Deliv. Rev. 2018, 126, 113–126. [Google Scholar] [CrossRef]
- Jain, D.; Carvalho, E.; Banerjee, R. Biodegradable Hybrid Polymeric Membranes for Ocular Drug Delivery. Acta Biomater. 2010, 6, 1370–1379. [Google Scholar] [CrossRef]
- Mir, M.; Ahmed, N.; Rehman, A.U. Recent Applications of PLGA Based Nanostructures in Drug Delivery. Colloids Surf. B Biointerfaces 2017, 159, 217–231. [Google Scholar] [CrossRef]
- Vasconcelos, A.; Vega, E.; Perez, Y.; Gomara, M.J.; Garcia, M.L.; Haro, I. Conjugation of Cell-Penetrating Peptides with Poly (lactic-co-glycolic acid)-polyethylene Glycol Nanoparticles Improves Ocular Drug Delivery. Int. J. Nanomed. 2015, 10, 609–631. [Google Scholar]
- Tahara, K.; Karasawa, K.; Onodera, R.; Takeuchi, H. Feasibility of Drug Delivery to the Eye’s Posterior Segment by Topical Instillation of PLGA Nanoparticles. Asian J. Pharm. Sci. 2017, 12, 394–399. [Google Scholar] [CrossRef]
- Lee, C.H.; Li, Y.J.; Huang, C.C.; Lai, J.Y. Poly(ɛ-caprolactone) Nanocapsule Carriers with Sustained Drug Release: Single Dose for Long-Term Glaucoma Treatment. Nanoscale 2017, 9, 11754–11764. [Google Scholar] [CrossRef]
- Cao, Y.; Samy, K.E.; Bernards, D.A.; Desai, T.A. Recent Advances in Intraocular Sustained-Release Drug Delivery Devices. Drug Discov. Today 2019. [Google Scholar] [CrossRef]
- Sun, S.; Li, J.; Li, X.; Lan, B.; Zhou, S.; Meng, Y.; Cheng, L. Episcleral Drug Film for Better-Targeted Ocular Drug Delivery and Controlled Release Using Multi-Layered Poly-ɛ-caprolactone. Acta Biomater. 2016, 37, 143–154. [Google Scholar] [CrossRef]
- Sinha, V.R.; Bansal, K.; Kaushik, R.; Kumria, R.; Trehan, A. Poly-ɛ-caprolactone Microspheres and Nanospheres: An Overview. Int. J. Pharm. 2004, 278, 1–23. [Google Scholar] [CrossRef]
- Da Silva, G.R.; Lima, T.H.; Orefice, R.L.; Silva-Cunha, A.; Zhao, M.; Behar-Cohen, F. In Vitro and in Vivo Ocular Biocompatibility of Electrospun poly(ɛ-caprolactone) Nanofibers. Eur. J. Pharm. Sci. 2015, 73, 9–19. [Google Scholar] [CrossRef]
- Shi, S.; Zhang, Z.; Luo, Z.; Yu, J.; Liang, R.; Li, X.; Chen, H. Chitosan Grafted Methoxy (ethylene glycol)-poly(ɛ-caprolactone) Nanosuspension for Ocular Drug Delivery of Hydrophobic Diclofenac. Sci. Rep. 2015, 5, 11337. [Google Scholar] [CrossRef]
- Meng, T.; Kulkarni, V.; Simmers, R.; Brar, V.; Xu, Q. Therapeutic implications of nanomedicine for ocular drug delivery. Drug Discov. Today 2019. [Google Scholar] [CrossRef]
- Kakkar, S.; Karuppayil, S.M.; Raut, J.S.; Giasanti, F.; Papucci, L.; Nicola, S.; Kaur, I.P. Lipid-Polyethylene Glycol Based Nano-Ocular Formulation of Ketoconazole. Int. J. Pharm. 2015, 495, 276–289. [Google Scholar] [CrossRef]
- Du Toit, L.C.; Govender, T.; Carmichael, T.; Kumar, P.; Choonara, Y.E.; Pillay, V. Design of an Anti-Inflammatory Composite Nanosystem and Evaluation of Its Potential for Ocular Drug Delivery. J. Pharm. Sci. 2013, 102, 2780–2805. [Google Scholar] [CrossRef]
- Zorzi, G.K.; Parraga, J.E.; Seijo, B.; Sanchez, A. Hybrid Nanoparticle Design Based on Cationized Gelatin and the Polyanions Dextran Sulfate and Chondroitin Sulfate for Ocular Gene Therapy. Macromol. Biosci. 2011, 11, 905–913. [Google Scholar] [CrossRef]
- Leonardi, A.; Bucolo, C.; Romano, G.L.; Platania, C.B.M.; Drago, F.; Puglisi, G.; Pignatello, R. Influence of Different Surfactants on the Technological Properties and in Vivo Ocular Tolerability of Lipid Nanoparticles. Int. J. Pharm. 2014, 470, 133–140. [Google Scholar] [CrossRef]
- Weng, Y.; Liu, J.; Jin, S.; Guo, W.; Liang, X.; Hu, Z. Nanotechnology-Based Strategies for Treatment of Ocular Diseases. Acta Pharm. Sin. B 2017, 7, 281–291. [Google Scholar] [CrossRef]
- Lorenzo-Veiga, B.; Sigurdsson, H.H.; Loftsson, T.; Alverez-Lorenzo, C. Cyclo-dextrin-amphiphilic Copolymer Supramolecular Assemblies for the Ocular Delivery of Natamycin. Nanomaterials 2019, 9, 745. [Google Scholar] [CrossRef]
- Farokhzad, O.C.; Langer, R. Impact of Nanotechnology on Drug Delivery. Am. Chem. Soc. Nano 2009, 3, 16–20. [Google Scholar]
- Soni, K.S.; Desale, S.S.; Bronich, T.K. Nanogels: An Overview of Properties, Biomedical Applications and Obstacles to Clinical Translation. J. Control. Release 2016, 240, 109–126. [Google Scholar] [CrossRef]
- Rejinold, N.S.; Nair, A.; Sabitha, M.; Chennezhi, K.P.; Tamura, H.; Nair, S.V.; Jayakumar, R. Synthesis, Characterization and in Vitro Cytocompatibility Studies of Chitin Nanogels for Biomedical Applications. Carbohydr. Polym. 2012, 87, 943–949. [Google Scholar] [CrossRef]
- Brannigan, R.P.; Khutoryanskiy, V.V. Synthesis and Evaluation of Mucoadhesive Acryloyl-Guaternized PDMAEMA Nanogels for Ocular Drug Delivery. Colloids Surf. B Biointerfaces 2017, 155, 538–543. [Google Scholar] [CrossRef]
- Sivaram, A.J.; Rajitha, P.; Maya, S.; Jayakumar, R.; Sabitha, M. Nanogels for Delivery, Imaging and Therapy. Wiley Interdiscip. Rev. 2015, 7, 509–533. [Google Scholar] [CrossRef]
- Liu, R.; Sun, L.; Fang, S.; Wang, S.; Chen, J.; Xiao, X.; Liu, C. Thermosensitive in Situ Nanogel as Ophthalmic Delivery System of Curcumin: Development, Characterization in Vitro Permeation and in Vivo Pharmacokinetic Studies. Pharm. Dev. Technol. 2016, 21, 576–582. [Google Scholar]
- Iglesias, G.R.; Reyes-Ortega, F.; Checa Fernandez, B.L.; Delgao, A.V. Hyperthermia-Triggered Gemcitabine Release from Polymer-Coated Magnetite Nanoparticles. Polymers 2018, 10, 269. [Google Scholar] [CrossRef]
- Janagam, D.R.; Wu, L.; Lowe, T.L. Nanoparticles for Drug Delivery to the Anterior Segment of the Eye. Adv. Drug Deliv. Rev. 2017, 122, 31–64. [Google Scholar] [CrossRef]
- Abrego, G.; Alvarado, H.; Souto, E.B.; Guevara, B.; Bellowa, L.H.; Parra, A.; Calpena, A.; Garcia, M.L. Biopharmaceutical Profile of Pranoprofen-Loaded PLGA Nanoparticles Containing Hydrogels for Ocular Administration. Eur. J. Pharm. Biopharm. 2015, 95, 261–270. [Google Scholar] [CrossRef]
- Battaglia, L.; Serpe, L.; Foglietta, F.; Muntoni, E.; Gallarate, M.; Del Pozo, R.A.; Solinis, M.A. Application of Lipid Nanoparticles to Ocular Drug Delivery. Expert Opin. Drug Deliv. 2016, 13, 1743–1757. [Google Scholar] [CrossRef]
- Bachu, R.D.; Chowdhury, P.; Al-Saedi, Z.H.F.; Karla, P.K.; Boddu, S.H.S. Ocular Drug Delivery Barriers—Role of Nanocarriers in the Treatment of Anterior Segment Ocular Diseases. Pharmaceutics 2018, 10, 28. [Google Scholar] [CrossRef]
- Das, S.; Suresh, P.K. Nanosuspensions: A New Vehicle for the Improvement of the Delivery of Drugs to the Ocular Surface. Application to Amphotericin, B. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 242–247. [Google Scholar] [CrossRef]
- Sahoo, S.; Dilnawaz, F.; Krishnakumar, S. Nanotechnology in Ocular Drug Delivery. Drug Discov. Today 2008, 13, 144–151. [Google Scholar] [CrossRef]
- Pignatello, R.; Bucolo, C.; Ferrara, P.; Maltese, A.; Puleo, A.; Puglisi, G. Eudragit RS100 ® Nanosuspensions for the Ophthalmic Controlled Delivery of Ibuprofen. Eur. J. Pharm. Sci. 2002, 162, 53–61. [Google Scholar] [CrossRef]
- Vaishya, R.D.; Khurana, V.; Patel, S.; Mitra, A.K. Controlled Ocular Drug Delivery with Nanomicelles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2014, 6, 422–437. [Google Scholar] [CrossRef]
- Cagel, M.; Tesan, F.C.; Bernabeu, E.; Salgueiro, M.J.; Zubillage, M.B.; Moretton, M.A.; Chiappetta, D.A. Polymeric Mixed Micelles as Nanomedicines: Achievements and Perspectives. Eur. J. Pharm. Biopharm. 2017, 113, 211–228. [Google Scholar] [CrossRef]
- Cholkar, K.; Patel, A.; Dutt Vadlapudi, A.; Mitra, A. Novel Nanomicellar Formulation Approached for Anterior and Posterior Segment Ocular Drug Delivery. Recent Pat. Nanomed. 2012, 2, 82–95. [Google Scholar] [CrossRef]
- Vadlapudi, A.D.; Cholkar, K.; Vadlapatla, R.K.; Mitra, A.K. Aqueous Nanomicellar Formulation for Topical Delivery of Biotinylated Lipid Prodrug of Acyclovir: Formulation Development and Ocular Biocompatibility. J. Ocul. Pharmacol. Ther. 2014, 30, 49–58. [Google Scholar] [CrossRef] [Green Version]
- Cholkar, K.; Gunda, S.; Earla, R.; Pal, D.; Mitra, A.K. Nanomicellar Topical Aqueous Drop Formulation of Rapamycin for Back-of-the-Eye Delivery. AAPS Pharmscitech 2015, 16, 610–622. [Google Scholar] [CrossRef]
- Deepak, A.; Goyal, A.K.; Rath, G. Nanofiber in Transmucosal Drug Delivery. J. Drug Deliv. Sci. Technol. 2018, 43, 379–387. [Google Scholar] [CrossRef]
- Sun, K.; Yu, Z.; Cai, Z.; Yu, L.; Lv, Y. Voriconazole Composited Polyvinyl Alcohol/Hydroxypropyl-β-Cyclodextrin Nanofibers for Ophthalmic Delivery. PLoS ONE 2016, 11, e0167961. [Google Scholar] [CrossRef]
- Kovacs, A.; Demuth, B.; Masko, A.; Zelko, R. Preformulation Studies of Furosemide-Loaded Electrospun Nanofibrous System for Buccal Administration. Polymer 2017, 9, 643. [Google Scholar] [CrossRef]
- Lancina, M.G.; Singh, S.; Kompella, U.B.; Husain, S.; Yang, H. Fast Dissolving Dendrimer Nanofiber Mats as Alternative to Eye Drops for More Efficient Antiglaucoma Drug Delivery. ACS Biomater. Sci. Eng. 2017, 3, 1861–1868. [Google Scholar] [CrossRef]
- Agarwal, R.; Iezhutsa, I.; Agarwal, P.; Nasir, N.A.A.; Razali, N.; Alyautdin, R.; Ismail, N.M. Liposomes in Topical Ophthalmic Drug Delivery: An Update. Drug Deliv. 2016, 4, 1075–1091. [Google Scholar] [CrossRef]
- Wang, F.; Bao, X.; Fang, A.; Li, H.; Zhou, Y.; Liu, Y.; Jiang, C.; Wu, J.; Song, X. Nanoliposome-Encapsulated Brinzolamide-hydropropyl-β-cyclodextrin Inclusion Complex: A Potential Therapeutic Ocular Drug Delivery System. Front. Pharmacol. 2018, 9, 91. [Google Scholar] [CrossRef]
- Kaiser, J.N.; Imai, H.; Haakenson, J.K.; Brucklacher, R.M.; Fox, T.E.; Shanmugavelandy, S.S.; Unrath, K.A.; Pedersen, M.M.; Dai, P.; Freeman, W.M.; et al. Nanoliposomal Minocycline for Ocular Drug Delivery. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 130–140. [Google Scholar] [CrossRef]
- Vicario-de-la-Torre, M.; Caballo-Gonzalez, M.; Vico, E.; Morales-Fernandez, L.; Arriola-Villalobos, P.; de las Heras, B.; Benitez-del-Castillo, J.M.; Guzman, M.; Millar, T.; Herreo-Vanrell, R.; et al. Novel Nano-Liposome Formulation for Dry Eyes with Components Similar to the Preocular Tear Film. Polymers 2018, 10, 425. [Google Scholar] [CrossRef]
- Pondman, K.M.; Bunt, N.D.; Maijenburh, A.W.; van Wezel, R.J.A.; Kishore, U.; Abelmann, L.; Elshof, J.E.; ten Haken, B. Magnetic Drug Delivery with FePd Nanowires. J. Magn. Magn. Mater. 2015, 380, 299–306. [Google Scholar] [CrossRef]
- Delcassian, D.; Patel, A.K.; Cortinas, A.B.; Langer, R. Drug Delivery Across the Length Scales. J. Drug Target. 2018, 1029–2330. [Google Scholar] [CrossRef]
- Christiansen, A.T.; Tao, S.L.; Smith, M.; Wnek, G.E.; Prause, J.U.; Young, M.J.; Klaasen, H.; Kaplan, H.J.; la Cour, M.; Kiilgaard, J.F. Subretinal Implantation of Electronsoun, Shirt Nanowire and Smooth Poly(ε-caprolactone) Scaffolds to the Subretinal Space of Porcine Eyes. Stem Cells Int. 2012, 2012, 454295. [Google Scholar] [CrossRef]
- Tian, R.; Sharma, A.; Nozari, A.; Subramaniam, R.; Lundstedt, T.; Sharma, S. Nanowired Drug Delivery to Enhance Neuroprotection in Spinal Cord Injury. CNS Neurol. Disord. 2012, 11, 85–95. [Google Scholar]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriques-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Mahlumba, P.; Choonara, Y.E.; Kumar, P.; Du Toit, L.C.; Pillay, V. Stimuli-Responsive Polymeric Systems for Controlled Protein and Peptide Delivery: Future Implications and for Ocular Delivery. Molecules 2016, 21, 1002. [Google Scholar] [CrossRef]






| Dosage Form | Advantages | Disadvantages | References |
|---|---|---|---|
| Eye Drops (Solutions and Suspensions) | Ease of administration. Little discomfort to the patient. Non-invasive. | Low bioavailability. Limit to dosage size. Frequent administration. Low patient adherence. | [2,20,21,25,26] |
| Ointments | Ease of administration. Decreased clearance rate following administration. High bioavailability than liquid formulations. | Blurred vision. Inaccurate dosing. Challenges in formulating. | [20,28] |
| Intravitreal Injections | Drug administered directly to posterior segment. | Invasive. Multiple risks with frequent administrations. Frequent administration. | [29,30] |
| Intraocular Implants | Extended drug release. If biodegradable polymers used, no need for removal. Able to be developed as stimuli-responsive. | Surgical implantation and removal if not biocompatible. Risks associated with insertion. Insertion uncomfortable for patients. | [29,31] |
| Contact Lenses | Increased residence time compared to other formulations. Keeps drug in contact with the surface of the eye for improved permeation. | Rapid release of drug within the first few hours. Cannot be used continuously. Risk of infection. | [32,33] |
| Emulsions | Improved bioavailability over other formulations. Improved residence time. Sustained drug release profiles. | Susceptible to flocculation and instability. Destabilized by tear fluid. | [35,36] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lynch, C.; Kondiah, P.P.D.; Choonara, Y.E.; du Toit, L.C.; Ally, N.; Pillay, V. Advances in Biodegradable Nano-Sized Polymer-Based Ocular Drug Delivery. Polymers 2019, 11, 1371. https://doi.org/10.3390/polym11081371
Lynch C, Kondiah PPD, Choonara YE, du Toit LC, Ally N, Pillay V. Advances in Biodegradable Nano-Sized Polymer-Based Ocular Drug Delivery. Polymers. 2019; 11(8):1371. https://doi.org/10.3390/polym11081371
Chicago/Turabian StyleLynch, Courtney, Pierre P. D. Kondiah, Yahya E. Choonara, Lisa C. du Toit, Naseer Ally, and Viness Pillay. 2019. "Advances in Biodegradable Nano-Sized Polymer-Based Ocular Drug Delivery" Polymers 11, no. 8: 1371. https://doi.org/10.3390/polym11081371