Isocyanate-Free Polyurethane Coatings and Adhesives from Mono- and Di-Saccharides
Abstract
:1. Introduction
2. Materials and Methods
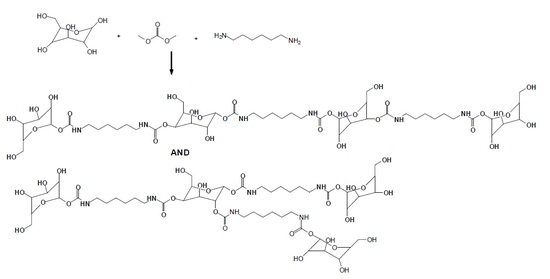
2.1. Preparation of Isocyanate-Free Polyurethanes
2.2. MALDI-TOF Analysis
2.3. FTIR
2.4. Cross-Polarisation Magic Angle Spinning Nuclear Magnetic Resonance (CP-MAS 13C NMR) Spectra
2.5. Thermomechanical Analysis (TMA)
2.6. Wood Joints Bonding
2.7. Surface Coatings Applications
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Peng, Y.; Zheng, Z.; Sun, P.; Wang, X.; Zhang, T. Synthesis and characterization of polyphenol-based polyurethane. New J. Chem. 2013, 37, 729–734. [Google Scholar] [CrossRef]
- Ge, J.-J.; Sakai, K. Compressive Properties and Biodegradabilities of Polyurethane Foams Derived from Condensed Tannins. Mokuzai Gakkaishi 1993, 39, 801–806. [Google Scholar]
- Ge, J.-J.; Sakai, K. Decomposition of polyurethane foams derived from condensed tannin II: Hydrolysis and aminolysis of polyurethane foams. J. Wood Sci. 1998, 44, 103–105. [Google Scholar] [CrossRef]
- Ge, J.-J.; Shi, X.; Cai, M.; Wu, R.; Wang, M. A novel biodegradable antimicrobial PU foam from wattle tannin. J. Appl. Polym. Sci. 2003, 90, 2756–2763. [Google Scholar] [CrossRef]
- Faruk, O.; Sain, M. Continuous Extrusion Foaming of Lignin Enhanced Thermoplastic Polyurethane (TPU). J. Biobased Mater. Bioenergy 2013, 7, 309–314. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Hatakeyama, T. Advances of Polyurethane Foams Derived from Lignin. J. Renew. Mater. 2013, 1, 113–123. [Google Scholar] [CrossRef]
- Liu, J.; Liu, H.-F.; Deng, L.; Liao, B.; Guo, Q.-X. Improving aging resistance and mechanical properties of waterborne polyurethanes modified by lignin amines. J. Appl. Polym. Sci. 2013, 130, 1736–1742. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, S.; Hu, W.-P.; Xie, Y.-J. Fractionation of the Biopolyols from Lignocellulosic Biomass for the Production of Rigid Foams. Bioenergy Res. 2013, 6, 896–902. [Google Scholar] [CrossRef]
- Whelan, J.M., Jr.; Hill, M.; Cotter, R.J. Multiple Cyclic Carbonate Polymers. U.S. Patent 3,072,613, 8 January 1963. [Google Scholar]
- Rokicki, G.; Piotrowska, A. A New route to polyurethanes from ethylene carbonate, diamines and diols. Polymer 2002, 43, 2927–2935. [Google Scholar] [CrossRef]
- Kihara, N.; Endo, T. Synthesis and properties of poly(hydroxyurethane)s. J. Polym. Sci. Part A 1993, 31, 2765–2773. [Google Scholar] [CrossRef]
- Kihara, N.; Kushida, Y.; Endo, T. Optically active poly(hydroxyurethane)s derived from cyclic carbonate and L-lysine derivatives. J. Polym. Sci. Part A 1996, 34, 2173–2179. [Google Scholar] [CrossRef]
- Tomita, H.; Sanda, F.; Endo, T. Structural analysis of polyhydroxyurethane obtained by polyaddition of bifunctional five-membered cyclic carbonate and diamine based on the model reaction. J. Polym. Sci. Part A 2001, 39, 851–859. [Google Scholar] [CrossRef]
- Tomita, H.; Sanda, F.; Endo, T. Polyaddition behavior of bis(five- and six-membered cyclic carbonate)s with diamine. J. Polym. Sci. Part A 2001, 39, 860–867. [Google Scholar] [CrossRef]
- Tomita, H.; Sanda, F.; Endo, T. Model reaction for the synthesis of polyhydroxyurethanes from cyclic carbonates with amines: Substituent effect on the reactivity and selectivity of ring-opening direction in the reaction of five-membered cyclic carbonates with amine. J. Polym. Sci. Part A 2001, 39, 3678–3685. [Google Scholar] [CrossRef]
- Birukov, O.; Potashnikova, R.; Leykin, A.; Figovsky, O.; Shapovalov, L. Advantages in chemistry and technology of non-isocyanate polyurethane. J. Sci. Israel-Technol. Adv. 2009, 11, 160–167. [Google Scholar]
- Figovsky, O.; Shapovalov, L. Features of reaction amino-cyclocarbonate for production of new type polyurethanes. Macromol. Symp. 2002, 187, 325–332. [Google Scholar] [CrossRef]
- Camara, F.; Benyahya, S.; Besse, V.; Boutevin, G.; Auvergne, R.; Boutevin, B.; Caillol, S. Reactivity of secondary amines for the synthesis of nonisocyanate polyurethanes. Eur. Polym. J. 2014, 55, 17–26. [Google Scholar] [CrossRef]
- Blattmann, H.; Fleischer, M.; Bähr, M.; Mülhaupt, R. Isocyanate- and phosgene-free routes to polyfunctional cyclic carbonates and green polyurethanes by fixation of carbon dioxide. Macromol. Rapid Commun. 2014, 35, 1238–1254. [Google Scholar] [CrossRef] [PubMed]
- Boyer, A.; Cloutet, E.; Tassaing, T.; Gadenne, B.; Alfos, C.; Cramail, H. Solubility in CO2 and carbonation studies of epoxidized fatty acid diesters: Towards novel precursors for polyurethane synthesis. Green Chem. 2010, 12, 2205–2213. [Google Scholar] [CrossRef]
- Kim, M.-R.; Kim, H.-S.; Ha, C.-S.; Park, D.-W.; Lee, J.-K. Syntheses and thermal properties of poly(hydroxy)urethanes by polyaddition reaction of bis(cyclic carbonate) and diamines. J. Appl. Polym. Sci. 2001, 81, 2735–2743. [Google Scholar] [CrossRef]
- Ochiai, B.; Inoue, S.; Endo, T. Salt effect on polyaddition of bifunctional cyclic carbonate and diamine. J. Polym. Sci. Part A 2005, 43, 6282–6286. [Google Scholar] [CrossRef]
- Ubaghs, L.; Fricke, N.; Keul, H.; Höcker, H. Polyurethanes with pendant hydroxyl groups: Synthesis and Characterization. Macromol. Rapid Commun. 2004, 25, 517–521. [Google Scholar] [CrossRef]
- Fleischer, M.; Blattmann, H.; Mülhaupt, R. Glycerol-, pentaerythritol- and trimethylolpropane-based polyurethanes and their cellulose carbonate composites prepared via the non-isocyanate route with catalytic carbon dioxide fixation. Green Chem. 2013, 15, 934–942. [Google Scholar] [CrossRef]
- Besse, V.; Auvergne, R.; Carlotti, S.; Boutevin, G.; Otazaghine, B.; Caillol, S.; Pascault, J.-P.; Boutevin, B. Synthesis of isosorbide based polyurethanes: An isocyanate free method. React. Funct. Polym. 2013, 73, 588–594. [Google Scholar] [CrossRef]
- Cornille, A.; Auvergne, R.; Figovsky, O.; Boutevin, B.; Caillol, S. A perspective approach to sustainable routes for non-isocyanate polyurethanes. Eur. Polym. J. 2017, 87, 532–552. [Google Scholar] [CrossRef]
- Nohra, B.; Candy, L.; Blanco, J.-F.; Guerin, C.; Raoul, Y.; Mouloungui, Z. From Petrochemical Polyurethanes to Biobased Polyhydroxyurethanes. Macromolecules 2013, 46, 3771–3792. [Google Scholar] [CrossRef] [Green Version]
- Annunziata, L.; Diallo, A.K.; Fouquay, S.; Michaud, G.; Simon, F.; Brusson, J.-M.; Carpentier, J.F.; Guillaume, S.M. α,ω-Di(glycerol carbonate) telechelic polyesters and polyolefins as precursors to polyhydroxyurethanes: An isocyanate-free approach. Green Chem. 2014, 16, 1947–1956. [Google Scholar] [CrossRef]
- Tundo, P.; Selva, M. The Chemistry of Dimethyl Carbonate. Acc. Chem. Res. 2002, 35, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Thébault, M.; Pizzi, A.; Dumarçay, S.; Gerardin, P.; Fredon, E.; Delmotte, L. Polyurethanes from hydrolysable tannins obtained without using isocyanates. Ind. Crops Prod. 2014, 59, 329–336. [Google Scholar] [CrossRef]
- Thébault, M.; Pizzi, A.; Essawy, H.; Baroum, A.; van Assche, G. Isocyanate free condensed tannin-based polyurethanes. Eur. Polym. J. 2015, 67, 513–523. [Google Scholar] [CrossRef]
- Thébault, M.; Pizzi, A.; Santiago-Medina, F.J.; Al-Marzouki, F.M.; Abdalla, S. Isocyanate-free polyurethanes by coreaction of condensed tannins with aminated tannins. J. Renew. Mater. 2017, 5, 21–29. [Google Scholar] [CrossRef]
- Pizzi, A. On the correlation of some theoretical and experimental parameters in polycondensation cross-460 linked networks. J. Appl. Polym. Sci. 1997, 63, 603–617. [Google Scholar] [CrossRef]
- Synthetic Resin Adhesives (Phenolic and Aminoplastic) for Wood. Specification for Close-Contact Adhesives; British Standard BS 1204; BSI: London, UK, 1993.
- AFNOR (Association Française de Normalisation). Norme Francaise NF EN ISO 2409—Peintures et Vernis—Essai de Quadrillage; AFNOR: La plaine Saint Denis, France, April 2013. [Google Scholar]
- Pretsch, E.; Simon, W.; Clerc, T.; Biemann, K. Tables of Spectral Data for Structure Determination of Organic Compounds- 13C-NMR, 1H-NMR, IR, MS, UV/VIS, 2nd ed.; Springer-Verlag: Berlin, Germany, 1989. [Google Scholar]
- Wehrli, F.W.; Wirlin, T. Interpretation of 13C NMR Spectra; Heyden: London, UK, 1978. [Google Scholar]
- Tryznowski, M.; Aleksandra Świderska, A.; Żołek-Tryznowska, Z.; Gołofit, T.; Paweł, G. Parzuchowski, P.G. Facile route to multigram synthesis of environmentally friendly non-isocyanate polyurethanes. Polymer 2015, 80, 28–236. [Google Scholar] [CrossRef]
- Cornille, A.; Serres, J.; Michaud, G.; Simon, F.; Fouquay, S.; Boutevin, B.; Caillol, S. Syntheses of epoxyurethane polymers from isocyanate free oligo-polyhydroxyurethane. Eur. Pol. J. 2016, 75, 175–189. [Google Scholar] [CrossRef]
- Besse, V.; Foyer, G.; Auvergne, R.; Caillol, S.; Boutevin, B. Access to nonisocyanate poly(thio)urethanes: A comparative study. J. Polym. Sci. Part A 2013, 51, 3284–3296. [Google Scholar] [CrossRef]
- Carré, C.; Bonneta, L.; Avérous, L. Original biobased nonisocyanate polyurethanes: solvent- and catalyst-free synthesis, thermal properties and rheological behaviour. RSC Adv. 2014, 4, 54018–54025. [Google Scholar] [CrossRef]
- Rodriguez-Valverde, M.A.; Cabrerizo-Vilchez, M.A.; Rosales-Lopez, P.; Paez-Duenas, A.; Hidalgo-Alvarez, R. Contact angle measurements on two (wood and stone) non-ideal surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2002, 206, 485–495. [Google Scholar] [CrossRef]
- Kamoun, C.; Pizzi, A.; Garcia, R. The effect of humidity on cross-linked and entanglement networking of formaldehyde-based wood adhesives. Holz Roh Werkst. 1998, 56, 235–243. [Google Scholar] [CrossRef]
- Shi, S.Q.; Gardner, D.J. Dynamic adhesive wettability of wood. Wood Fiber Sci. 2001, 33, 58–68. [Google Scholar]
- Magami, S.M. Monitoring the degree of curing in coatings using contact angle and surface reflectance measurements. Surf. Coat. Int. 2014, 97, 250–253. [Google Scholar]
- Saha, S.; Kocaefe, D.; Krause, C.; Larouche, T. Effect of titania and zinc oxide particles on acrylic polyurethane coating performance. Prog. Org. Coat. 2011, 70, 170–177. [Google Scholar] [CrossRef]
- Pánek, M.; Oberhofnerová, E.; Zeidler, A.; Šedivka, P. Efficacy of Hydrophobic Coatings in Protecting Oak Wood Surfaces during Accelerated Weathering. Coatings 2017, 7, 172. [Google Scholar] [CrossRef]
- Pizzi, A.; Zhao, C.; Kamoun, C.; Heinrich, H. TTT and CHT curing diagrams of water-borne polycondensation resins on lignocellulosic substrates. J. Appl. Polym. Sci. 2001, 80, 2128–2139. [Google Scholar] [CrossRef]
- Enns, B.; Gillham, J.K. Effect of the extent of cure on the modulus, glass transition, water absorption, and density of an amine-cured epoxy. J. Appl. Polym. Sci. 1983, 28, 2831–2846. [Google Scholar] [CrossRef]
























| 116.9 Da = dimethyl carbonate + Na+ 176.7 Da = glucose (calc. 180) deprotonated × 2 200 Da = glucose deprotonated + 23 Da (Na+) 263.8 Da (calc. 261 Da) (with Na+) protonated  322.8 Da without Na+  378.8 Da (calc. 380) without Na+  440.9 Da (Calc. 439) without Na+  464 Da = without Na+  522 Da = 464 + 1 × DMC without Na+  546 Da = 522 + 23 (Na+)580 Da = without Na+  and/or  601 Da = 580 + 23 (Na+) deprotonated = (603 Da calculated) 632 Da (calc. 629) with + 23 Da (Na+)  and/or 632.9 (calc. 635) without Na+  667 Da (calc. 675) with + 23 Da (Na+)  689 Da = 667 + Na+. Thus a case of a structure with 2 × Na+ 733 Da (730 calculated) without Na+  879 Da, without Na+ (calc. 878 Da) a PUR tetramer  903 Da = 879 Da + Na+ (+23 Da) (Calculated 902 Da) a PUR tetramer 925 Da = 879 Da + 2× Na+ (calculated 926 Da) a PUR tetramer 1225.6 Da (1225.2 calculated), without Na+ (6 urethane linkages) PUR tetramer  And  |
| Peaks (cm−1) | Assignment |
|---|---|
| 1051, 1087, 1150 | C–O–C stretching vibration |
| 1193 | C–O stretching vibration |
| 1325 | –C–N stretching vibration |
| 1394.5 | –CH3 Surface bending vibration |
| 1573 | N–H bending vibration, urethane bridge |
| 1642 | C=O (carbonyl) stretching vibration |
| 1718 | C=O stretching vibration of urethane bridge |
| 1744 | C=O stretching of carbonate |
| 2857, 2932 | C–H stretching vibration |
| 3100–3200 | N–H, O–H stretching vibration |
| Resin | Dry strength (MPa) | 24 h Cold Water Soak Strength (MPa) | 2 h Boiled Strength (MPa) |
|---|---|---|---|
| A2 | 3.15 ± 0.05 | 3.62 ± 0.02 | 3.38 ± 0.04 |
| B2 | 2.76 ± 0.09 | 1.32 ± 0.08 | 1.24 ± 0.04 |
| Wood | A2 | B2 | |
|---|---|---|---|
| Time (Minutes) | Theta (Degrees) | Theta (Degrees) | Theta (Degrees) |
| 0 | 49.1 ± 1.24 | 64.4 ± 1.41 | 40.0 ± 2.17 |
| 1 | 36.1 ± 0.43 | 63.2 ± 1.30 | 20.6 ± 3.22 |
| 2 | 31.0 ± 1.68 | 61.6 ± 1.64 | 11.2 ± 0.10 |
| 3 | 28.2 ± 2.06 | 61.0 ± 1.19 | 8.8 ± 0.0 |
| 4 | 22.6 ± 1.66 | 59.9 ± 1.36 | 5.3 ± 0.39 |
| 5 | 19.5 ± 3.24 | 58.3 ± 1.35 | 3.6 ± 0.0 |
| 6 | 13.6 ± 0.81 | 57.2 ± 1.34 | - |
| 7 | - | 56.9 ± 0.97 | - |
| 8 | - | 54.7 ± 1.95 | - |
| 9 | - | 50.6 ± 1.02 | - |
| 10 | - | 49.2 ± 0.97 | - |
| A2 | B2 | |
|---|---|---|
| Time (Minutes) | Theta (Degrees) | Theta (Degrees) |
| 0 | 80.6 ± 1.82 | 80.3 ± 0.65 |
| 1 | 76.3 ± 2.56 | 79.0 ± 1.40 |
| 2 | 74.5 ± 2.18 | 77.2 ± 2.15 |
| 3 | 73.4 ± 2.54 | 75.4 ± 2.04 |
| 4 | 72.1 ± 2.05 | 73.4 ± 0.38 |
| 5 | 69.0 ± 2.31 | 73.5 ± 0.78 |
| 6 | 67.7 ± 2.04 | 71.4 ± 1.70 |
| 7 | 65.9 ± 1.44 | 68.8 ± 1.70 |
| 8 | 64.8 ± 2.22 | 67.9 ± 2.81 |
| 9 | 62.9 ± 1.19 | 66.3 ± 2.69 |
| 10 | 62.3 ± 1.37 | 64.7 ± 1.70 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xi, X.; Pizzi, A.; Delmotte, L. Isocyanate-Free Polyurethane Coatings and Adhesives from Mono- and Di-Saccharides. Polymers 2018, 10, 402. https://doi.org/10.3390/polym10040402
Xi X, Pizzi A, Delmotte L. Isocyanate-Free Polyurethane Coatings and Adhesives from Mono- and Di-Saccharides. Polymers. 2018; 10(4):402. https://doi.org/10.3390/polym10040402
Chicago/Turabian StyleXi, Xuedong, Antonio Pizzi, and Luc Delmotte. 2018. "Isocyanate-Free Polyurethane Coatings and Adhesives from Mono- and Di-Saccharides" Polymers 10, no. 4: 402. https://doi.org/10.3390/polym10040402