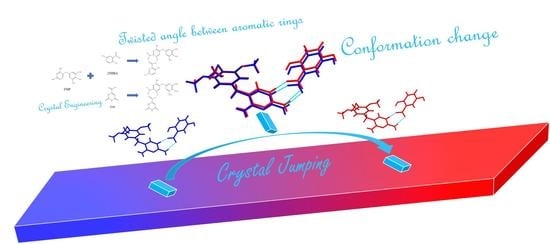
An Attempt to Design Thermosalient Crystals by Co-Crystallization: The Twisted Angle between Aromatic Rings
Abstract
:1. Introduction
2. Experimental Part
2.1. Single Crystal Preparation
2.2. Characterization
2.3. Theoretical Calculation
3. Results and Discussion
3.1. Observing TS Effect
3.2. Differential Scanning Calorimetry and Thermogravimetric Analysis
3.3. Variable-Temperature Powder X-ray Diffraction (VT-PXRD)
3.4. Thermal Expansion Coefficients
3.5. Molecular Conformation and Packing Comparison
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naumov, P.; Karothu, D.P.; Ahmed, E.; Catalano, L.; Commins, P.; Mahmoud Halabi, J.; Al-Handawi, M.B.; Li, L. The Rise of the Dynamic Crystals. J. Am. Chem. Soc. 2020, 142, 13256–13272. [Google Scholar] [CrossRef]
- Karamertzanis, P.G.; Price, S.L. Energy Minimization of Crystal Structures Containing Flexible Molecules. J. Chem. Theory Comput. 2006, 2, 1184–1199. [Google Scholar] [CrossRef]
- Rath, B.B.; Vittal, J.J. Photoreactive Crystals Exhibiting [2 + 2] Photocycloaddition Reaction and Dynamic Effects. Acc. Chem. Res. 2022, 55, 1445–1455. [Google Scholar] [CrossRef] [PubMed]
- Abendroth, J.M.; Bushuyev, O.S.; Weiss, P.S.; Barrett, C.J. Controlling Motion at the Nanoscale: Rise of the Molecular Machines. ACS Nano 2015, 9, 7746–7768. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Commins, P.; Al-Handawi, M.B.; Karothu, D.P.; Halabi, J.M.; Schramm, S.; Weston, J.; Rezgui, R.; Naumov, P. Martensitic Organic Crystals as Soft Actuators. Chem. Sci. 2019, 10, 7327–7332. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wang, J.; Guo, X.; Yang, S.; Ozen, M.O.; Chen, P.; Liu, X.; Du, W.; Xiao, F.; Demirci, U.; et al. Multi-Stimuli-Responsive Programmable Biomimetic Actuator. Nat Commun 2019, 10, 4087. [Google Scholar] [CrossRef]
- Dattler, D.; Fuks, G.; Heiser, J.; Moulin, E.; Perrot, A.; Yao, X.; Giuseppone, N. Design of Collective Motions from Synthetic Molecular Switches, Rotors, and Motors. Chem. Rev. 2020, 120, 310–433. [Google Scholar] [CrossRef]
- Naumov, P.; Chizhik, S.; Panda, M.K.; Nath, N.K.; Boldyreva, E. Mechanically Responsive Molecular Crystals. Chem. Rev. 2015, 115, 12440–12490. [Google Scholar] [CrossRef]
- Desta, I.T.; Chizhik, S.A.; Sidelnikov, A.A.; Karothu, D.P.; Boldyreva, E.V.; Naumov, P. Mechanically Responsive Crystals: Analysis of Macroscopic Strain Reveals “Hidden” Processes. J. Phys. Chem. A 2020, 124, 300–310. [Google Scholar] [CrossRef]
- Karothu, D.P.; Mahmoud Halabi, J.; Li, L.; Colin-Molina, A.; Rodríguez-Molina, B.; Naumov, P. Global Performance Indices for Dynamic Crystals as Organic Thermal Actuators. Adv. Mater. 2020, 32, 1906216. [Google Scholar] [CrossRef]
- Hean, D.; Alde, L.G.; Wolf, M.O. Photosalient and Thermosalient Crystalline Hemithioindigo-Anthracene Based Isomeric Photoswitches. J. Mater. Chem. C 2021, 9, 6789–6795. [Google Scholar] [CrossRef]
- Duan, Y.; Semin, S.; Tinnemans, P.; Xu, J.; Rasing, T. Fully Controllable Structural Phase Transition in Thermomechanical Molecular Crystals with a Very Small Thermal Hysteresis. Small 2021, 17, 2006757. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, H.; Konomura, S. Thermosalience Coupled to Abrupt Spin Crossover with Dynamic Ligand Motion in an Iron(II) Molecular Crystal. CrystEngComm 2022, 24, 4224–4234. [Google Scholar] [CrossRef]
- Takazawa, K.; Inoue, J.; Matsushita, Y. Repeatable Actuations of Organic Single Crystal Fibers Driven by Thermosalient-Phase-Transition-Induced Buckling. Small 2022, 18, 2204500. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jing, B.; Chang, Z.; Gong, J. Desolvation Induced Crystal Jumping: Reversible Hydration and Dehydration of a Spironolactone–Saccharin Cocrystal with Water as the Jumping-Mate. CrystEngComm 2021, 23, 6838–6842. [Google Scholar] [CrossRef]
- Sahoo, S.C.; Panda, M.K.; Nath, N.K.; Naumov, P. Biomimetic Crystalline Actuators: Structure–Kinematic Aspects of the Self-Actuation and Motility of Thermosalient Crystals. J. Am. Chem. Soc. 2013, 135, 12241–12251. [Google Scholar] [CrossRef]
- Klaser, T.; Popović, J.; Fernandes, J.; Tarantino, S.; Zema, M.; Skoko, Ž. Does Thermosalient Effect Have to Concur with a Polymorphic Phase Transition? The Case of Methscopolamine Bromide. Crystals 2018, 8, 301. [Google Scholar] [CrossRef]
- Colin-Molina, A.; Karothu, D.P.; Jellen, M.J.; Toscano, R.A.; Garcia-Garibay, M.A.; Naumov, P.; Rodríguez-Molina, B. Thermosalient Amphidynamic Molecular Machines: Motion at the Molecular and Macroscopic Scales. Matter 2019, 1, 1033–1046. [Google Scholar] [CrossRef]
- Seki, T.; Mashimo, T.; Ito, H. Anisotropic Strain Release in a Thermosalient Crystal: Correlation between the Microscopic Orientation of Molecular Rearrangements and the Macroscopic Mechanical Motion. Chem. Sci. 2019, 10, 4185–4191. [Google Scholar] [CrossRef]
- Tamboli, M.I.; Karothu, D.P.; Shashidhar, M.S.; Gonnade, R.G.; Naumov, P. Effect of Crystal Packing on the Thermosalient Effect of the Pincer-Type Diester Naphthalene-2,3-Diyl-Bis(4-Fluorobenzoate): A New Class II Thermosalient Solid. Chem. Eur. J. 2018, 24, 4133–4139. [Google Scholar] [CrossRef]
- Sahoo, S.C.; Sinha, S.B.; Kiran, M.S.R.N.; Ramamurty, U.; Dericioglu, A.F.; Reddy, C.M.; Naumov, P. Kinematic and Mechanical Profile of the Self-Actuation of Thermosalient Crystal Twins of 1,2,4,5-Tetrabromobenzene: A Molecular Crystalline Analogue of a Bimetallic Strip. J. Am. Chem. Soc. 2013, 135, 13843–13850. [Google Scholar] [CrossRef] [PubMed]
- Skoko, Ž.; Zamir, S.; Naumov, P.; Bernstein, J. The Thermosalient Phenomenon. “Jumping Crystals” and Crystal Chemistry of the Anticholinergic Agent Oxitropium Bromide. J. Am. Chem. Soc. 2010, 132, 14191–14202. [Google Scholar] [CrossRef] [PubMed]
- Takeda, T.; Ozawa, M.; Akutagawa, T. Jumping Crystal of a Hydrogen-Bonded Organic Framework Induced by the Collective Molecular Motion of a Twisted π System. Angew. Chem. Int. Ed. 2019, 58, 10345–10352. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, Y.; Itoh, Y.; Aida, T. Jumping Crystals of Pyrene Tweezers: Crystal-to-Crystal Transition Involving π / π -to-CH/ π Assembly Mode Switching. Chem. Asian J. 2017, 12, 811–815. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Yamamoto, S.; Seki, T.; Ito, H.; Garcia-Garibay, M.A. Anisotropic Thermal Expansion as the Source of Macroscopic and Molecular Scale Motion in Phosphorescent Amphidynamic Crystals. Angew. Chem. Int. Ed. 2019, 58, 18003–18010. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Mashimo, T.; Ito, H. Crystal Jumping of Simple Hydrocarbons: Cooling-Induced Salient Effect of Bis-, Tri-, and Tetraphenylethene through Anisotropic Lattice Dimension Changes without Thermal Phase Transitions. Chem. Lett. 2020, 49, 174–177. [Google Scholar] [CrossRef]
- Kato, K.; Seki, T.; Ito, H. (9-Isocyanoanthracene)Gold(I) Complexes Exhibiting Two Modes of Crystal Jumps by Different Structure Change Mechanisms. Inorg. Chem. 2021, 60, 10849–10856. [Google Scholar] [CrossRef]
- Miura, Y.; Takeda, T.; Yoshioka, N.; Akutagawa, T. Thermosalient Effect of 5-Fluorobenzoyl-4-(4-Methoxyphenyl)Ethynyl-1-Methylimidazole without Phase Transition. Crystal Growth Design 2022, 22, 5904–5911. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A Program for Hirshfeld Surface Analysis, Visualization and Quantitative Analysis of Molecular Crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- Omoto, K.; Nakae, T.; Nishio, M.; Yamanoi, Y.; Kasai, H.; Nishibori, E.; Mashimo, T.; Seki, T.; Ito, H.; Nakamura, K.; et al. Thermosalience in Macrocycle-Based Soft Crystals via Anisotropic Deformation of Disilanyl Architecture. J. Am. Chem. Soc. 2020, 142, 12651–12657. [Google Scholar] [CrossRef]
- Rath, B.B.; Gallo, G.; Dinnebier, R.E.; Vittal, J.J. Reversible Thermosalience in a One-Dimensional Coordination Polymer Preceded by Anisotropic Thermal Expansion and the Shape Memory Effect. J. Am. Chem. Soc. 2021, 143, 2088–2096. [Google Scholar] [CrossRef] [PubMed]
- Decremps, F.; Fischer, M.; Polian, A.; Itié, J.P.; Sieskind, M. Ionic Layered PbFCl-Type Compounds under High Pressure. Phys. Rev. B 1999, 59, 4011–4022. [Google Scholar] [CrossRef]
- Ardit, M.; Cruciani, G.; Dondi, M.; Garbarino, G.L.; Nestola, F. Phase Transitions during Compression of Thaumasite, Ca3Si(OH)6(CO3)(SO4)·12H2O: A High-Pressure Synchrotron Powder X-Ray Diffraction Study. Mineral. Mag. 2014, 78, 1193–1208. [Google Scholar] [CrossRef]
- Arkhipov, S.G.; Losev, E.A.; Nguyen, T.T.; Rychkov, D.A.; Boldyreva, E.V. A Large Anisotropic Plasticity of L -Leucinium Hydrogen Maleate Preserved at Cryogenic Temperatures. Acta Crystallogr. B Struct. Sci. Cryst. Eng. Mater. 2019, 75, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Cliffe, M.J.; Goodwin, A.L. PASCal: A Principal Axis Strain Calculator for Thermal Expansion and Compressibility Determination. J. Appl. Crystallogr. 2012, 45, 1321–1329. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld Surface Analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Mitchell, A.S.; Spackman, M.A. Hirshfeld Surfaces: A New Tool for Visualising and Exploring Molecular Crystals. Chem. Eur. J. 1998, 4, 2136–2141. [Google Scholar] [CrossRef]
- Mackenzie, C.F.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer Model Energies and Energy Frameworks: Extension to Metal Coordination Compounds, Organic Salts, Solvates and Open-Shell Systems. IUCrJ 2017, 4, 575–587. [Google Scholar] [CrossRef]











| Direction | |||||
|---|---|---|---|---|---|
| Axes | α (MK−1) | σα (MK−1) | a | b | c |
| X1 | −19.0021 | 1.0841 | −0.9994 | 0.0000 | −0.0349 |
| X2 | 7.3277 | 0.1673 | 0.0000 | 1.0000 | 0.0000 |
| X3 | 82.9283 | 1.4665 | 0.2691 | 0.0000 | 0.9631 |
| V | 71.4168 | 1.5963 |
| Direction | |||||
|---|---|---|---|---|---|
| Axes | α (MK−1) | σα (MK−1) | a | b | c |
| X1 | −168.6973 | 19.7555 | −0.2108 | 0.9757 | −0.0596 |
| X2 | 35.3957 | 7.1966 | 0.4194 | 0.5539 | 0.7192 |
| X3 | 334.5309 | 52.3126 | −0.8506 | −0.5225 | 0.0585 |
| V | 243.9352 | 42.2856 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.; Xiao, Y.; Qi, L.; Bai, Y.; Sun, Y.; Ye, Y.; Xie, C. An Attempt to Design Thermosalient Crystals by Co-Crystallization: The Twisted Angle between Aromatic Rings. Crystals 2023, 13, 701. https://doi.org/10.3390/cryst13040701
Hu X, Xiao Y, Qi L, Bai Y, Sun Y, Ye Y, Xie C. An Attempt to Design Thermosalient Crystals by Co-Crystallization: The Twisted Angle between Aromatic Rings. Crystals. 2023; 13(4):701. https://doi.org/10.3390/cryst13040701
Chicago/Turabian StyleHu, Xingchen, Yuntian Xiao, Luguang Qi, Yunhe Bai, Ying Sun, Yang Ye, and Chuang Xie. 2023. "An Attempt to Design Thermosalient Crystals by Co-Crystallization: The Twisted Angle between Aromatic Rings" Crystals 13, no. 4: 701. https://doi.org/10.3390/cryst13040701