Boosting Catalytic Combustion of Ethanol by Tuning Morphologies and Exposed Crystal Facets of α-Mn2O3
Abstract
:1. Introduction
2. Results and Discussion
2.1. Crystal Phase Structure and Morphology of Catalysts
2.2. Surface Area and Surface Chemical Properties
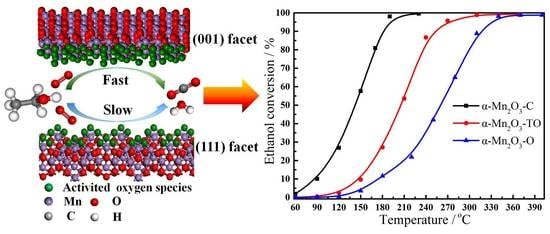
2.3. Catalytic Performance of Different Shaped α-Mn2O3 Catalysts
2.4. Effects of SV, Ethanol, and Water Vapor Concentration
2.5. The Stability of α-Mn2O3-C Catalyst
3. Materials and Methods
3.1. Catalyst Synthesis
3.2. Catalyst Characterization
3.3. Temperature-Programmed Surface Reactions (TPSR) and Mixed-Gas without Oxygen
3.4. Catalytic Performance Evaluation
3.5. Kinetic Analysis for Ethanol Total Oxidation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Si, W.Z.; Wang, Y.; Peng, Y.; Li, X.; Li, K.Z.; Li, J.H. A high-efficiency gamma-MnO2-like catalyst in toluene combustion. Chem. Commun. 2015, 51, 14977–14980. [Google Scholar] [CrossRef] [PubMed]
- Benrabaa, R.; Benadda, A.; Hammiche-Bellal, Y.; Boukhlouf, H.; Trentesaux, M.; Rubbens, A.; Vannier, R.N.; Lofberg, A. Characterization and catalytic properties of Ni-Fe spinel catalysts for total oxidation of ethanol. ChemistrySelect 2019, 4, 6415–6420. [Google Scholar] [CrossRef]
- Litt, G.; Almquist, C. An investigation of CuO/Fe2O3 catalysts for the gas-phase oxidation of ethanol. Appl. Catal. B Environ. 2009, 90, 10–17. [Google Scholar] [CrossRef]
- Rintramee, K.; Fottinger, K.; Rupprechter, G.; Wittayakun, J. Ethanol adsorption and oxidation on bimetallic catalysts containing platinum and base metal oxide supported on MCM-41. Appl. Catal. B Environ. 2012, 115, 225–235. [Google Scholar] [CrossRef]
- Aguero, F.N.; Barbero, B.P.; Gambaro, L.; Cadús, L.E. Catalytic combustion of volatile organic compounds in binary mixtures over MnOx/Al2O3 catalyst. Appl. Catal. B Environ. 2009, 91, 108–112. [Google Scholar] [CrossRef]
- Avgouropoulos, G.; Oikonomopoulos, E.; Kanistras, D.; Ioannides, T. Complete oxidation of ethanol over alkali-promoted Pt/Al2O3 catalysts. Appl. Catal. B Environ. 2006, 65, 62–69. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, Y.Y.; Li, X.Y.; Wu, X.; Li, Z. The double peaks and symmetric path phenomena in the catalytic activity of Pd/Al2O3-TiO2 catalysts with different TiO2 contents. J. Solid State Chem. 2018, 262, 335–342. [Google Scholar] [CrossRef]
- Gan, Y.H.; Tong, Y.; Jiang, Z.W.; Chen, X.W.; Li, H.G.; Jiang, X. Electro-spraying and catalytic combustion characteristics of ethanol in meso-scale combustors with steel and platinum meshes. Energy Convers. Manag. 2018, 164, 410–416. [Google Scholar] [CrossRef]
- Santos, V.P.; Carabineiro, S.A.C.; Tavares, P.B.; Pereira, M.F.R.; Órfão, J.J.M.; Figueiredo, J.L. Oxidation of CO, ethanol and toluene over TiO2 supported noble metal catalysts. Appl. Catal. B Environ. 2010, 99, 198–205. [Google Scholar] [CrossRef]
- Tan, T.H.; Scott, J.; Ng, Y.H.; Taylor, R.A.; Aguey-Zinsou, K.F.; Amal, R. Understanding plasmon and band gap photoexcitation effects on the thermal-catalytic oxidation of ethanol by TiO2-supported gold. ACS Catal. 2016, 6, 1870–1879. [Google Scholar] [CrossRef]
- Wahlberg, A.; Pettersson, L.J.; Bruce, K.; Andersson, M.; Jansson, K. Preparation, evaluation and characterization of copper catalysts for ethanol fuelled diesel engines. Appl. Catal. B Environ. 1999, 23, 271–281. [Google Scholar] [CrossRef]
- Peluso, M.A.; Pronsato, E.; Sambeth, J.E.; Thomas, H.J.; Busca, G. Catalytic combustion of ethanol on pure and alumina supported K-Mn oxides: An IR and flow reactor study. Appl. Catal. B Environ. 2008, 78, 73–79. [Google Scholar] [CrossRef]
- Hammiche-Bellal, Y.; Benadda, A.; Meddour-Boukhobza, L.; Barama, S.; Djadoun, A.; Barama, A. Preparation and catalytic activity in ethanol combustion reaction of cobalt-iron spinel catalysts. Catal. Commun. 2013, 42, 62–67. [Google Scholar] [CrossRef]
- Rao, T.; Shen, M.; Jia, L.; Hao, J.; Wang, J. Oxidation of ethanol over Mn–Ce–O and Mn–Ce–Zr–O complex compounds synthesized by sol–gel method. Catal. Commun. 2007, 8, 1743–1747. [Google Scholar] [CrossRef]
- Morales, M.R.; Barbero, B.P.; Cadús, L. Total oxidation of ethanol and propane over Mn-Cu mixed oxide catalysts. Appl. Catal. B Environ. 2006, 67, 229–236. [Google Scholar] [CrossRef]
- Hu, L.; Peng, Q.; Li, Y. Selective synthesis of Co3O4 nanocrystal with different shape and crystal plane effect on catalytic property for methane combustion. J. Am. Chem. Soc. 2008, 130, 16136–16137. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shen, W.J. Morphology-dependent nanocatalysts: Rod-shaped oxides. Chem. Soc. Rev. 2014, 43, 1543–1574. [Google Scholar] [CrossRef]
- Wang, F.; Dai, H.X.; Deng, J.G.; Bai, G.M.; Ji, K.M.; Liu, Y.X. Manganese oxides with rod-, wire-, tube-, and flower-Like morphologies: Highly effective catalysts for the removal of toluene. Environ. Sci. Technol. 2012, 46, 4034–4041. [Google Scholar] [CrossRef]
- Aneggi, E.; Wiater, D.; de Leitenburg, C.; Llorca, J.; Trovarelli, A. Shape-Dependent activity of ceria in soot combustion. ACS Catal. 2014, 4, 172–181. [Google Scholar] [CrossRef]
- Dai, Y.T.; Men, Y.; Wang, J.G.; Liu, S.; Li, S.; Li, Y.Y.; Wang, K. Tailoring the morphology and crystal facet of Mn3O4 for highly efficient catalytic combustion of ethanol. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 121276. [Google Scholar] [CrossRef]
- Xie, X.; Yong, L.; Liu, Z.Q.; Haruta, M.; Shen, W. Low-temperature oxidation of CO catalysed by Co3O4 nanorods. Nature 2009, 458, 746–749. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.Z.; Yu, Z.N.; Gao, F.; Wang, J.J.; Pang, H.A.; Lu, Q.Y. Low-symmetry iron oxide nanocrystals bound by high-index facets. Angew. Chem. Int. Ed. 2010, 49, 6328–6332. [Google Scholar] [CrossRef] [PubMed]
- Nolan, M.; Parker, S.C.; Watson, G.W. The electronic structure of oxygen vacancy defects at the low index surfaces of ceria. Surf. Sci. 2005, 595, 223–232. [Google Scholar] [CrossRef]
- Qu, Z.P.; Bu, Y.B.; Qin, Y.; Wang, Y.; Fu, Q. The improved reactivity of manganese catalysts by Ag in catalytic oxidation of toluene. Appl. Catal. B Environ. 2013, 132, 353–362. [Google Scholar] [CrossRef]
- Santos, V.P.; Pereira, M.F.R.; Orfao, J.J.M.; Figueiredo, J.L. The role of lattice oxygen on the activity of manganese oxides towards the oxidation of volatile organic compounds. Appl. Catal. B Environ. 2010, 99, 353–363. [Google Scholar] [CrossRef]
- Vaculikova, L.; Valovicova, V.; Plevova, A.E.; Napruszewska, B.D.; Duraczynska, D.; Karcz, R.; Serwicka, E.M. Synthesis, characterization and catalytic activity of cryptomelane/montmorillonite composites. Appl. Clay Sci. 2021, 202, 8065–8068. [Google Scholar] [CrossRef]
- Tang, W.X.; Wu, X.F.; Li, D.Y.; Wang, Z.; Liu, G.; Liu, H.D.; Chen, Y.F. Oxalate route for promoting activity of manganese oxide catalysts in total VOCs’ oxidation: Effect of calcination temperature and preparation method. J. Mater. Chem. A 2014, 2, 2544–2554. [Google Scholar] [CrossRef]
- Bai, B.Y.; Li, J.H.; Hao, J.M. 1D-MnO2, 2D-MnO2 and 3D-MnO2 for low-temperature oxidation of ethanol. Appl. Catal. B Environ. 2015, 164, 241–250. [Google Scholar] [CrossRef]
- Li, X.; Zheng, J.K.; Liu, S.; Zhu, T.L. A novel wormhole-like mesoporous hybrid MnCoOx catalyst for improved ethanol catalytic oxidation. J. Colloid. Interface Sci. 2019, 555, 667–675. [Google Scholar] [CrossRef]
- Lahousse, C.; Bernier, A.; Grange, P.; Delmon, B.; Papaefthimiou, P.; Ioannides, T.; Verykios, X. Evaluation of γ-MnO2 as a VOC removal catalyst: Comparison with a noble metal catalyst. J. Catal. 1998, 178, 214–225. [Google Scholar] [CrossRef]
- Liu, Y.X.; Dai, H.X.; Deng, J.G.; Du, Y.C.; Li, X.W.; Zhao, Z.X.; Wang, Y.; Gao, B.Z.; Yang, H.G.; Guo, G.S. In situ poly(methyl methacrylate)-templating generation and excellent catalytic performance of MnOx/3DOM LaMnO3 for the combustion of toluene and methanol. Appl. Catal. B Environ. 2013, 140, 493–505. [Google Scholar] [CrossRef]
- Cheng, L.; Men, Y.; Wang, J.; Wang, H.; An, W.; Wang, Y.; Duan, Z.; Liu, J. Crystal facet-dependent reactivity of α-Mn2O3 microcrystalline catalyst for soot combustion. Appl. Catal. B Environ. 2017, 204, 374–384. [Google Scholar] [CrossRef]
- Ji, F.; Men, Y.; Wang, J.G.; Sun, Y.L.; Wang, Z.D.; Zhao, B.; Tao, X.T.; Xu, G.J. Promoting diesel soot combustion efficiency by tailoring the shapes and crystal facets of nanoscale Mn3O4. Appl. Catal. B Environ. 2019, 242, 227–237. [Google Scholar] [CrossRef]
- Xu, J.; Deng, Y.Q.; Luo, Y.; Mao, W.; Yang, X.J.; Han, Y.F. Operando Raman spectroscopy and kinetic study of low-temperature CO oxidation on an α-Mn2O3 nanocatalyst. J. Catal. 2013, 300, 225–234. [Google Scholar] [CrossRef]
- Ghiassee, M.; Rezaei, M.; Meshkani, F.; Mobini, S. Preparation of the Mn/Co mixed oxide catalysts for low-temperature CO oxidation reaction. Environ. Sci. Pollut. Res. 2021, 28, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, P.; Rezaei, M.; Meshkani, F. Ultrasound-assisted hydrothermal method for the preparation of the M-Fe2O3-CuO (M: Mn, Ag, Co) mixed oxides nanocatalysts for low-temperature CO oxidation. Ultrason. Sonochem. 2019, 57, 212–222. [Google Scholar] [CrossRef]
- Kim, S.C.; Shim, W.G. Catalytic combustion of VOCs over a series of manganese oxide catalysts. Appl. Catal. B Environ. 2010, 98, 180–185. [Google Scholar] [CrossRef]
- Mitran, G.; Chen, S.J.; Seo, D.K. Role of oxygen vacancies and Mn4+/Mn3+ ratio in oxidation and dry reforming over cobalt-manganese spinel oxides. Mol. Catal. 2020, 483, 110704. [Google Scholar] [CrossRef]
- Gandhe, A.R.; Rebello, J.S.; Figueiredo, J.L.; Fernandes, J.B. Manganese oxide OMS-2 as an effective catalyst for total oxidation of ethyl acetate. Appl. Catal. B Environ. 2007, 72, 129–135. [Google Scholar] [CrossRef]
- Peluso, M.A.; Gambaro, L.A.; Pronsato, E.; Gazzoli, D.; Thomas, H.J.; Sambeth, J.E. Synthesis and catalytic activity of manganese dioxide (type OMS-2) for the abatement of oxygenated VOCs. Catal. Today 2008, 133, 487–492. [Google Scholar] [CrossRef]
- Saputra, E.; Muhammad, S.; Sun, H.; Ang, H.M.; Tadé, M.; Wang, S. Shape-controlled activation of peroxymonosulfate by single crystal α-Mn2O3 for catalytic phenol degradation in aqueous solution. Appl. Catal. B Environ. 2014, 154–155, 246–251. [Google Scholar] [CrossRef]
- Li, S.; Men, Y.; Liu, S.; Wang, J.G. Boosting the efficiencies of ethanol total combustion by Cs incorporation into rod-shaped alpha-MnO2 catalysts. Colloids Surf. A Physicochem. Eng. Asp. 2022, 650, 129607. [Google Scholar] [CrossRef]
- Feng, B.B.; Qin, C.L.; Shi, Y.; Zhang, L.D. Catalytic combustion study of ethanol over manganese oxides with different morphologies. Energy Fuels 2022, 36, 9221–9229. [Google Scholar] [CrossRef]
- Li, W.N.; Zhang, L.; Sithambaram, S.; Yuan, J.; Shen, X.F.; Aindow, M.; Suib, S.L. Shape evolution of single-crystalline Mn2O3 using a solvothermal approach. J. Phys. Chem. C 2007, 111, 14694–14697. [Google Scholar] [CrossRef]
- Lei, S.; Tang, K.; Zhen, F.; Liu, Q.; Zheng, H. Preparation of α-Mn2O3 and MnO from thermal decomposition of MnCO3 and control of morphology. Mater. Lett. 2006, 60, 53–56. [Google Scholar] [CrossRef]
- Wang, D.; Dai, H. Low-Temperature synthesis of single-crystal germanium nanowires by chemical vapor deposition. Angew. Chem. Int. Ed. 2002, 41, 4977–4980. [Google Scholar] [CrossRef]
- Han, N.; Zhou, Z.Y.; Sun, S.G.; Ding, Y.; Wang, Z.L. Synthesis of tetrahexahedral platinum nanocrystals with high-index facets and high electro-oxidation activity. Science 2007, 316, 732–735. [Google Scholar]
- Chen, Z.W.; Jiao, Z.; Pan, D.Y.; Li, Z.; Wu, M.H.; Shek, C.H.; Wu, C.M.L.; Lai, J.K.L. Recent advances in manganese oxide nanocrystals: Fabrication, characterization, and microstructure. Chem. Rev. 2012, 112, 3833–3855. [Google Scholar] [CrossRef]
- Zhang, L.; Qin, Z.; Liu, Z.; Hou, X.; Yi, L. Novel Mn3O4 micro-octahedra: Promising cataluminescence sensing material for acetone. Chem. Mater. 2009, 41, 5066–5071. [Google Scholar] [CrossRef]
- Gac, W. The influence of silver on the structural, redox and catalytic properties of the cryptomelane-type manganese oxides in the low-temperature CO oxidation reaction. Appl. Catal. B Environ. 2007, 75, 107–117. [Google Scholar] [CrossRef]
- Hua, J.L.; Gong, S.H.; Tana; Zhang, X.J.; Wei, L.; Wen, J.S. Morphological impact of manganese–cerium oxides on ethanol oxidation. Catal. Sci. Technol. 2011, 1, 1677–1682. [Google Scholar]
- Kapteijn, F.; Singoredjo, L.; Andreini, A.; Moulijn, J.A. Activity and selectivity of pure manganese oxides in the selective catalytic reduction of nitric oxide with ammonia. Appl. Catal. B Environ. 1994, 3, 173–189. [Google Scholar] [CrossRef]
- Han, Y.F.; Chen, F.; Zhong, Z.; Ramesh, K.; Widjaja, E. Controlled synthesis, characterization, and catalytic properties of Mn2O3 and Mn3O4 nanoparticles supported on mesoporous silica SBA-15. J. Phys. Chem. B 2006, 110, 24450–24456. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.R.; Barbero, B.P.; Cadús, L. Combustion of volatile organic compounds on manganese iron or nickel mixed oxide catalysts. Appl. Catal. B Environ. 2007, 74, 1–10. [Google Scholar] [CrossRef]
- Trawczyński, J.; Bielak, B.; Miśta, W. Oxidation of ethanol over supported manganese catalysts—Effect of the carrier. Appl. Catal. B Environ. 2005, 55, 277–285. [Google Scholar] [CrossRef]
- Strohmeier, B.R.; Hercules, D.M. Surface spectroscopic characterization of manganese/aluminum oxide catalysts. J. Phys. Chem. 1984, 88, 4922–4929. [Google Scholar] [CrossRef]
- Cheng, C.M.; Huang, Y.; Wang, N.; Jiang, T.; Hu, S.; Zheng, B.Z.; Yuan, H.Y.; Xiao, D. Facile fabrication of Mn2O3 nanoparticle-assembled hierarchical hollow spheres and their sensing for hydrogen peroxide. ACS Appl. Mater. Interfaces. 2015, 7, 9526–9533. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, X.Y.; Dai, Q.G.; Li, D. Effect of Ce and La on the structure and activity of MnOx catalyst in catalytic combustion of chlorobenzene. Appl. Catal. B Environ. 2012, 111, 141–149. [Google Scholar] [CrossRef]
- Piumetti, M.; Bensaid, S.; Fino, D.; Russo, N. Nanostructured ceria-zirconia catalysts for CO oxidation: Study on surface properties and reactivity. Appl. Catal. B Environ. 2016, 197, 35–46. [Google Scholar] [CrossRef]
- Poolwong, J.; Del Gobbo, S.; D’Elia, V. Transesterification of dimethyl carbonate with glycerol by perovskite-based mixed metal oxide nanoparticles for the atom-efficient production of glycerol carbonate. J. Ind. Eng. Chem. 2021, 104, 43–60. [Google Scholar] [CrossRef]
- Rousseau, S.; Loridant, S.; Delichere, P.; Boreave, A.; Deloume, J.P.; Vernoux, P. La(1−x)SrxCo1−y FeyO3 perovskites prepared by sol–gel method: Characterization and relationships with catalytic properties for total oxidation of toluene. Appl. Catal. B Environ. 2009, 88, 438–447. [Google Scholar] [CrossRef]
- Over, H.; Seitsonen, A.P. Oxidation of metal surfaces. Science 2002, 297, 2003–2005. [Google Scholar] [CrossRef]
- Widmann, D.; Behm, R.J. Active oxygen on a Au/TiO2 catalyst: Formation, stability, and CO oxidation activity. Angew. Chem. Int. Ed. 2011, 50, 10241–10245. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.H.; Li, L.M.; Yang, X.S.; Guo, Z.L.; Jing, F.L.; Chu, W. High-performance CoxM3-xAlOy (M = Ni, Mn) catalysts derived from microwave -assisted synthesis of hydrotalcite precursors for methane catalytic combustion. Catal. Today 2020, 347, 23–30. [Google Scholar] [CrossRef]
- Kessaratikoon, T.; Saengsaen, S.; Del Gobbo, S.; D’Elia, V.; Sooknoi, T. High surface area ZnO-Nanorods catalyze the clean thermal methane oxidation to CO2. Catalysts 2022, 12, 1533. [Google Scholar] [CrossRef]
- Kucharczyk, B.; Okal, J.; Tylus, W.; Winiarski, J.; Szczygiel, B. The effect of the calcination temperature of LaFeO3 precursors on the properties and catalytic activity of perovskite in methane oxidation. Ceram. Int. 2019, 45, 2779–2788. [Google Scholar] [CrossRef]
- Arandiyan, H.; Dai, H.; Deng, J.; Liu, Y.; Bai, B.; Wang, Y.; Li, X.; Xie, S.; Li, J. Three-dimensionally ordered macroporous La0.6Sr0.4MnO3 with high surface areas: Active catalysts for the combustion of methane. J. Catal. 2013, 307, 327–339. [Google Scholar] [CrossRef]
- An, W.; Baber, A.E.; Fang, X.; Soldemo, M.; Weissenrieder, J.; Stacchiola, D.; Ping, L. Mechanistic study of CO titration on CuxO/Cu(1 1 1) (x ≤ 2) surfaces. ChemCatChem 2014, 6, 2364–2372. [Google Scholar] [CrossRef]
- Papaefthimiou, P.; Ioannides, T.; Verykios, X.E. Performance of doped Pt/TiO2 (W6+) catalysts for combustion of volatile organic compounds (VOCs)-ScienceDirect. Appl. Catal. B Environ. 1998, 15, 75–92. [Google Scholar] [CrossRef]
- Wang, K.; Men, Y.; Liu, S.; Wang, J.G.; Li, Y.Y.; Tang, Y.H.; Li, Z.P.; An, W.; Pan, X.L.; Li, L. Decoupling the size and support/metal loadings effect of Ni/SiO2 catalysts for CO2 methanation. Fuel 2021, 304, 121388. [Google Scholar] [CrossRef]
- Li, Y.Y.; Men, Y.; Liu, S.; Wang, J.G.; Wang, K.; Tang, Y.H.; An, W.; Pan, X.L.; Li, L. Remarkably efficient and stable Ni/Y2O3 catalysts for CO2 methanation: Effect of citric acid addition. Appl. Catal. B Environ. 2021, 293, 120206. [Google Scholar] [CrossRef]











| Catalyst | SBET a (m2/g) | Vp (cm3/g) | Dp b (nm) | T (°C) | H2 Consumptions (mmol/g) | Surface Element Molar Ratio c | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Peak1 | Peak2 | Mn2O3→Mn3O4 | Mn3O4→MnO | Total | Mn4+/Mn3+ | Oads/Olatt | ||||
| α-Mn2O3-C | 30.5 | 0.212 | 22.1 | 287 | 383 | 2.5 | 3.7 | 6.2 | 1.27 | 0.53 |
| α-Mn2O3-TO | 2.5 | 0.009 | 13.5 | 409 | 458 | 3.6 | 2.5 | 6.1 | 1.15 | 0.43 |
| α-Mn2O3-O | 1.0 | 0.004 | 12.9 | 449 | 496 | 3.7 | 2.3 | 6.0 | 1.07 | 0.38 |
| Catalysts | Catalytic Activity (°C) | T (°C) | Ethanol Conversion (%) | Normalized Rate (mmol·min−1·m−2) × 104 | Ea (kJ/mol) | R2 for Ea | ||
|---|---|---|---|---|---|---|---|---|
| T10 | T50 | T90 | ||||||
| α-Mn2O3-C | 90 | 140 | 178 | 90 | 10.1 | 2.84 | 55.3 | 0.99 |
| α-Mn2O3-TO | 151 | 208 | 259 | 90 | 0.50 | 1.53 | 63.7 | 0.99 |
| α-Mn2O3-O | 179 | 261 | 314 | 90 | 0.10 | 0.84 | 68.3 | 0.98 |
| Ethanol Con. Tem (°C) | 600 ppm Ethanol SV of 96,000 mL/(g·h) | 600 ppm Ethanol SV of 192,000 mL/(g·h) | 1200 ppm Ethanol SV of 192,000 mL/(g·h) | 600 ppm Ethanol, 6 vol.% H2O, SV of 192,000 mL/(g·h) |
|---|---|---|---|---|
| T10 | 79 | 90 | 99 | 150 |
| T50 | 136 | 140 | 156 | 195 |
| T90 | 172 | 178 | 203 | 218 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Men, Y.; Ji, F.; Shi, F.; Wang, J.; Liu, S.; Magkoev, T.T.; An, W. Boosting Catalytic Combustion of Ethanol by Tuning Morphologies and Exposed Crystal Facets of α-Mn2O3. Catalysts 2023, 13, 865. https://doi.org/10.3390/catal13050865
Liu W, Men Y, Ji F, Shi F, Wang J, Liu S, Magkoev TT, An W. Boosting Catalytic Combustion of Ethanol by Tuning Morphologies and Exposed Crystal Facets of α-Mn2O3. Catalysts. 2023; 13(5):865. https://doi.org/10.3390/catal13050865
Chicago/Turabian StyleLiu, Wangwang, Yong Men, Fei Ji, Feng Shi, Jinguo Wang, Shuang Liu, Tamerlan T. Magkoev, and Wei An. 2023. "Boosting Catalytic Combustion of Ethanol by Tuning Morphologies and Exposed Crystal Facets of α-Mn2O3" Catalysts 13, no. 5: 865. https://doi.org/10.3390/catal13050865