Efficient Oxidative Dehydrogenation of Ethylbenzene over K/CeO2 with Exceptional Styrene Yield
Abstract
:Highlights
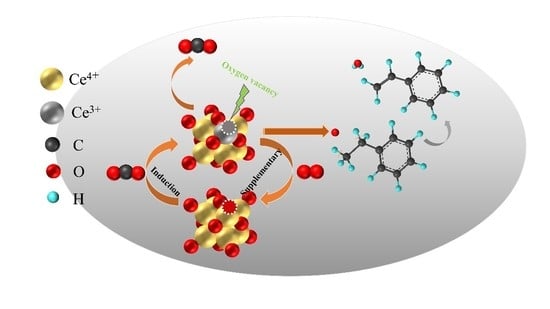
- A styrene yield of 91.4% was found for 10% k/CeO2 at 500 °C and CO2-O2 mixed atmosphere. The excellent catalytic performance of 10% k/CeO2 is attributed to the alkali metal oxide modified cerium oxide and carbon dioxide induced oxygen vacancies to promote the dehydrogenation of ethylbenzene.
- The proposed ODH strategy by using oxygen vacancies enriched catalysts offers an important insight into the efficient dehydrogenation of ethylbenzene at mild conditions.
Abstract
1. Introduction
2. Results and Discussion
2.1. Theoretical Analysis of Thermoneutral Oxidative Dehydrogenation
2.2. Synthesis of K/CeO2 Catalyst
2.2.1. Effect of Alkali Metals Modification on CeO2
2.2.2. Effect of K Loading Amounts on CeO2
2.3. Ethylbenzene ODH Performance
2.3.1. Optimization of Reaction Conditions in ODH
2.3.2. Reaction Stability
2.4. Surface Reaction Mechanism
3. Materials and Methods
3.1. Materials
3.2. Catalyst Synthesis
3.3. Catalyst Characterization
3.4. Catalytic Performance Testing under Oxygen-Free Conditions
3.5. Performance Testing of Oxidative Dehydrogenation of Ethylbenzene
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhu, X.; Gao, Y.; Wang, X.; Haribal, V.; Liu, J.; Neal, L.M.; Bao, Z.; Wu, Z.; Wang, H.; Li, F. A tailored multi-functional catalyst for ultra-efficient styrene production under a cyclic redox scheme. Nat. Commun. 2021, 12, 1329. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Li, W.-C.; Lu, W.-D.; Yan, B.; Qiu, B.; Gao, X.-Q.; Zhang, R.-P.; Zhou, S.-Z.; Lu, A.-H. Preparation of oxygen reactivity-tuned FeOx/BN catalyst for selectively oxidative dehydrogenation of ethylbenzene to styrene. Appl. Catal. B Environ. 2022, 305, 121070. [Google Scholar] [CrossRef]
- Mamedova, M.T. Oxidative Dehydrogenation of Ethylbenzene to Styrene on an Exhausted Aluminum Chromium Catalyst. Russ. J. Appl. Chem. 2020, 93, 488–493. [Google Scholar] [CrossRef]
- Xu, J.; Xue, B.; Liu, Y.-M.; Li, Y.-X.; Cao, Y.; Fan, K.-N. Mesostructured Ni-doped ceria as an efficient catalyst for styrene synthesis by oxidative dehydrogenation of ethylbenzene. Appl. Catal. A Gen. 2011, 405, 142–148. [Google Scholar] [CrossRef]
- Balasamy, R.J.; Tope, B.B.; Khurshid, A.; Al-Ali, A.A.S.; Atanda, L.A.; Sagata, K.; Asamoto, M.; Yahiro, H.; Nomura, K.; Sano, T.; et al. Ethylbenzene dehydrogenation over FeOx/(Mg,Zn)(Al)O catalysts derived from hydrotalcites: Role of MgO as basic sites. Appl. Catal. A Gen. 2011, 398, 113–122. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, X.; Li, Y.; Sui, Z.; Xu, X. A study on the catalytic role of Ce-Co species and coke deposited on ordered mesoporous Al2O3 in N2O-assisted oxidative dehydrogenation of ethylbenzene. J. Environ. Chem. Eng. 2022, 10, 108609. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; Gao, Q.; Sui, Z.; Xu, X. Promotional role of Ceria in N2O assisted selective oxidative dehydrogenation of ethylbenzene over Ce–Co2AlO4 spinel catalysts. J. Environ. Chem. Eng. 2021, 9, 105512. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; Sun, X.; Sui, Z.; Xu, X. Superior performance of K/Co2AlO4 catalysts for the oxidative dehydrogenation of ethylbenzene to styrene with N2O as an oxidant. J. Ind. Eng. Chem. 2022, 112, 67–75. [Google Scholar] [CrossRef]
- Pan, D.; Ru, Y.; Liu, T.; Wang, Y.; Yu, F.; Chen, S.; Yan, X.; Fan, B.; Li, R. Highly efficient and stable ordered mesoporous Ti-Al composite oxide catalyst for oxidative dehydrogenation of ethylbenzene to styrene with CO2. Chem. Eng. Sci. 2022, 250, 117388. [Google Scholar] [CrossRef]
- Song, K.; Wang, S.; Sun, Q.; Xu, D. Study of oxidative dehydrogenation of ethylbenzene with CO2 on supported CeO2-Fe2O3 binary oxides. Arab. J. Chem. 2020, 13, 7357–7369. [Google Scholar] [CrossRef]
- Wang, Q.; Li, X.; Li, W.; Feng, J. Promoting effect of Fe in oxidative dehydrogenation of ethylbenzene to styrene with CO2 (I) preparation and performance of Ce1−xFexO2 catalyst. Catal. Commun. 2014, 50, 21–24. [Google Scholar] [CrossRef]
- Wang, T.; Guan, X.; Lu, H.; Liu, Z.; Ji, M. Nanoflake-assembled Al2O3-supported CeO2-ZrO2 as an efficient catalyst for oxidative dehydrogenation of ethylbenzene with CO2. Appl. Surf. Sci. 2017, 398, 1–8. [Google Scholar] [CrossRef]
- Li, X.; Feng, J.; Fan, H.; Wang, Q.; Li, W. The dehydrogenation of ethylbenzene with CO2 over CexZr1−xO2 solid solutions. Catal. Commun. 2015, 59, 104–107. [Google Scholar] [CrossRef]
- Ji, M.; Zhang, X.; Wang, J.; Park, S.-E. Ethylbenzene dehydrogenation with CO2 over Fe-doped MgAl2O4 spinel catalysts: Synergy effect between Fe2+ and Fe3+. J. Mol. Catal. A Chem. 2013, 371, 36–41. [Google Scholar] [CrossRef]
- Ch Prathap, V.; Ramana Kumar, V.; Venkata Rao, M.; Nagaiah, P.; Rama Rao, K.S.; David Raju, B. Promotional role of Ceria in CeO2/MgAl2O4 spinel catalysts in CO2 assisted selective oxidative dehydrogenation of ethylbenzene to styrene. J. Ind. Eng. Chem. 2019, 79, 97–105. [Google Scholar]
- Reddy Benjaram, M.; Lee, S.-C.; Han, D.-S.; Park, S.-E. Utilization of carbon dioxide as soft oxidant for oxydehydrogenation of ethylbenzene to styrene over V2O5–CeO2/TiO2–ZrO2 catalyst. Appl. Catal. B Environ. 2009, 87, 230–238. [Google Scholar] [CrossRef]
- Jiang, N.; Burri, A.; Park, S.-E. Ethylbenzene to styrene over ZrO2-based mixed metal oxide catalysts with CO2 as soft oxidant. Chin. J. Catal. 2016, 37, 3–15. [Google Scholar] [CrossRef]
- Rao, M.V.; Venkateshwarlu, V.; Thirupathaiah, K.; Raju, M.A.; Nagaiah, P.; Mura, K.L.; Raju, B.D.; Rao, K.S.R. Oxidative dehydrogenation of ethylbenzene over γ-Al2O3 supported ceria-lanthanum oxide catalysts: Influence of Ce/La composition. Arab. J. Chem. 2020, 13, 772–782. [Google Scholar]
- Wang, T.; Qi, L.; Lu, H.; Ji, M. Flower-like Al2O3-supported iron oxides as an efficient catalyst for oxidative dehydrogenation of ethlybenzene with CO2. J. CO2 Util. 2017, 17, 162–169. [Google Scholar] [CrossRef]
- Castro Antonio, J.R.; Marques Samuel, P.D.; Soares João, M.; Filho Josue, M.; Saraiva Gilberto, D.; Oliveira Alcineia, C. Nanosized aluminum derived oxides catalysts prepared with different methods for styrene production. Chem. Eng. J. 2012, 209, 345–355. [Google Scholar] [CrossRef]
- Balasamy, R.J.; Khurshid, A.; Al-Ali, A.A.S.; Atanda, L.A.; Sagata, K.; Asamoto, M.; Yahiro, H.; Nomura, K.; Sano, T.; Takehira, K. Ethylbenzene dehydrogenation over binary FeOx–MeOy/Mg(Al)O catalysts derived from hydrotalcites. Appl. Catal. A Gen. 2010, 390, 225–234. [Google Scholar] [CrossRef]
- Rao, K.N.; Reddy, B.M.; Abhishek, B.; Seo, Y.-H.; Jiang, N.; Park, S.-E. Effect of ceria on the structure and catalytic activity of V2O5/TiO2–ZrO2 for oxidehydrogenation of ethylbenzene to styrene utilizing CO2 as soft oxidant. Appl. Catal. B Environ. 2009, 91, 649–656. [Google Scholar] [CrossRef]
- Sharma, P.; Dwivedi, R.; Dixit, R.; Batra, M.; Prasad, R. Mechanism evolution for the oxidative dehydrogenation of ethyl benzene to styrene over V2O5/TiO2 catalyst: Computational and kinetic approach. RSC Adv. 2015, 5, 39635–39642. [Google Scholar] [CrossRef]
- Zhou, W.; Lu, W.; Sun, Z.; Qian, J.; He, M.; Chen, Q.; Sun, S. Fe assisted Co-containing hydrotalcites catalyst for the efficient aerobic oxidation of ethylbenzene to acetophenone. Appl. Catal. A Gen. 2021, 624, 118322. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, Z.; Nelson, N.C.; Sadow, A.D.; Slowing, I.I.; Overbury, S.H. Role Of CO2 As a Soft Oxidant For Dehydrogenation of Ethylbenzene to Styrene over a High-Surface-Area Ceria Catalyst. ACS Catal. 2015, 5, 6426–6435. [Google Scholar] [CrossRef]
- Wang, H.; Cao, F.-X.; Song, Y.-H.; Yang, G.-Q.; Ge, H.-Q.; Liu, Z.-T.; Qu, Y.-Q.; Liu, Z.-W. Two-step hydrothermally synthesized Ce1-xZrxO2 for oxidative dehydrogenation of ethylbenzene with carbon dioxide. J. CO2 Util. 2019, 34, 99–107. [Google Scholar] [CrossRef]
- Burri, D.R.; Choi, K.M.; Han, D.-S.; Koo, J.-B.; Park, S.-E. CO2 utilization as an oxidant in the dehydrogenation of ethylbenzene to styrene over MnO2-ZrO2 catalysts. Catal. Today 2006, 115, 242–247. [Google Scholar] [CrossRef]
- Burri, A.; Jiang, N.; Yahyaoui, K.; Park, S.-E. Ethylbenzene to styrene over alkali doped TiO2-ZrO2 with CO2 as soft oxidant. Appl. Catal. A Gen. 2015, 495, 192–199. [Google Scholar] [CrossRef]
- Ansari, M.B.; Park, S.-E. Carbon dioxide utilization as a soft oxidant and promoter in catalysis. Energy Environ. Sci. 2012, 5, 9419–9437. [Google Scholar] [CrossRef]
- Centi, G.; Quadrelli, E.A.; Perathoner, S. Catalysis for CO2 conversion: A key technology for rapid introduction of renewable energy in the value chain of chemical industries. Energy Environ. Sci. 2013, 6, 1711–1731. [Google Scholar] [CrossRef]
- Mukherjee, D.; Park, S.-E.; Reddy, B.M. CO2 as a soft oxidant for oxidative dehydrogenation reaction: An eco benign process for industry. J. CO2 Util. 2016, 16, 301–312. [Google Scholar] [CrossRef]
- Fan, H.-X.; Feng, J.; Li, W.-Y. Promotional effect of oxygen storage capacity on oxy-dehydrogenation of ethylbenzene with CO2 over κ-Ce2Zr2O8(111). Appl. Surf. Sci. 2019, 486, 411–419. [Google Scholar] [CrossRef]
- Xu, J.; Wang, L.-C.; Liu, Y.-M.; Cao, Y.; He, H.-Y.; Fan, K.-N. Mesostructured CeO2 as an Effective Catalyst for Styrene Synthesis by Oxidative Dehydrogenation of Ethylbenzene. Catal. Lett. 2009, 133, 307–313. [Google Scholar] [CrossRef]
- Gao, Y.; Haeri, F.; He, F.; Li, F. Alkali Metal-Promoted LaxSr2–xFeO4−δ Redox Catalysts for Chemical Looping Oxidative Dehydrogenation of Ethane. ACS Catal. 2018, 8, 1757–1766. [Google Scholar] [CrossRef]
- Li, Y.; Cui, Y.; Zhang, X.; Lu, Y.; Fang, W.; Yang, Y. Optimum ratio of K2O to CeO2 in a wet-chemical method prepared catalysts for ethylbenzene dehydrogenation. Catal. Commun. 2016, 73, 12–15. [Google Scholar] [CrossRef]
- da Costa Borges Soares, M.; Barbosa, F.F.; Torres, M.A.M.; Pergher, S.B.C.; Essayem, N.; Braga, T.P. Preferential adsorption of CO2 on cobalt ferrite sites and its role in oxidative dehydrogenation of ethylbenzene. Braz. J. Chem. Eng. 2021, 38, 495–510. [Google Scholar] [CrossRef]
- Wang, Z.; Qu, Z.; Quan, X.; Wang, H. Selective catalytic oxidation of ammonia to nitrogen over ceria–zirconia mixed oxides. Appl. Catal. A Gen. 2012, 411, 131–138. [Google Scholar] [CrossRef]
- Xu, H.; Sun, M.; Liu, S.; Li, Y.; Wang, J.; Chen, Y. Effect of the calcination temperature of cerium–zirconium mixed oxides on the structure and catalytic performance of WO3/CeZrO2 monolithic catalyst for selective catalytic reduction of NOx with NH3. RSC Adv. 2017, 7, 24177–24187. [Google Scholar] [CrossRef]
- Hong, W.-J.; Iwamoto, S.; Inoue, M. Direct NO decomposition over a Ce–Mn mixed oxide modified with alkali and alkaline earth species and CO2-TPD behavior of the catalysts. Catal. Today 2011, 164, 489–494. [Google Scholar] [CrossRef]
- Miyakoshi, A.; Ueno, A.; Ichikawa, M. XPS and TPD characterization of manganese-substituted iron-potassium oxide catalysts which are selective for dehydrogenation of ethylbenzene into styrene. Appl. Catal. A-Gen. 2001, 219, 249–258. [Google Scholar] [CrossRef]
- Chen, S.; Zeng, L.; Mu, R.; Xiong, C.; Zhao, Z.J.; Zhao, C.; Pei, C.; Peng, L.; Luo, J.; Fan, L.S.; et al. Modulating Lattice Oxygen in Dual-Functional Mo-V-O Mixed Oxides for Chemical Looping Oxidative Dehydrogenation. J. Am. Chem. Soc. 2019, 141, 18653–18657. [Google Scholar] [CrossRef] [PubMed]
- Carrero, C.A.; Schloegl, R.; Wachs, I.E.; Schomaecker, R. Critical Literature Review of the Kinetics for the Oxidative Dehydrogenation of Propane over Well-Defined Supported Vanadium Oxide Catalysts. ACS Catal. 2014, 4, 3357–3380. [Google Scholar] [CrossRef]
- Song, H.; Wang, W.; Sun, J.; Wang, X.; Zhang, X.; Chen, S.; Pei, C.; Zhao, Z.-J. Chemical looping oxidative propane dehydrogenation controlled by oxygen bulk diffusion over FeVO4 oxygen carrier pellets. Chin. J. Chem. Eng. 2023, 53, 409–420. [Google Scholar] [CrossRef]
- Wang, H.; Yang, G.-Q.; Song, Y.-H.; Liu, Z.-T.; Liu, Z.-W. Defect-rich Ce1−xZrxO2 solid solutions for oxidative dehydrogenation of ethylbenzene with CO2. Catal. Today 2019, 324, 39–48. [Google Scholar] [CrossRef]
- Schumacher, L.; Hess, C. The active role of the support in propane ODH over VOx/CeO2 catalysts studied using multiple operando spectroscopies. J. Catal. 2021, 398, 29–43. [Google Scholar] [CrossRef]














| Temperature (°C) | XC8H10 (g) + CO2 (g) + YO2 (g) = XC8H8 (g) + CO (g) + XH2O (g) | |||
|---|---|---|---|---|
| EB (mol) | CO2 (mol) | O2 (mol) | ΔH (kJ) (Dynamic to 0) | |
| 300 | 2.3226 | 1 | 0.6613 | 0 |
| 350 | 2.3245 | 1 | 0.6622 | 0 |
| 400 | 2.3256 | 1 | 0.6628 | 0 |
| 450 | 2.3259 | 1 | 0.6629 | 0 |
| 500 | 2.3254 | 1 | 0.6627 | 0 |
| 550 | 2.3239 | 1 | 0.6619 | 0 |
| 600 | 2.3214 | 1 | 0.6607 | 0 |
| 650 | 2.3176 | 1 | 0.6588 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.; Zhang, J.; Li, K.; Wang, H.; Zhu, X. Efficient Oxidative Dehydrogenation of Ethylbenzene over K/CeO2 with Exceptional Styrene Yield. Catalysts 2023, 13, 781. https://doi.org/10.3390/catal13040781
Sun H, Zhang J, Li K, Wang H, Zhu X. Efficient Oxidative Dehydrogenation of Ethylbenzene over K/CeO2 with Exceptional Styrene Yield. Catalysts. 2023; 13(4):781. https://doi.org/10.3390/catal13040781
Chicago/Turabian StyleSun, He, Juping Zhang, Kongzhai Li, Hua Wang, and Xing Zhu. 2023. "Efficient Oxidative Dehydrogenation of Ethylbenzene over K/CeO2 with Exceptional Styrene Yield" Catalysts 13, no. 4: 781. https://doi.org/10.3390/catal13040781