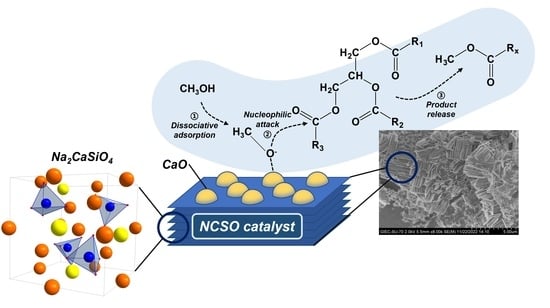
Sodium Silicates Modified Calcium Oxide as a High-Performance Solid Base Catalyst for Biodiesel Production
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
2.2. Influence of Reaction Conditions on Biodiesel Production
2.2.1. Influence of the Reaction Time
2.2.2. Influence of Reaction Temperature
2.2.3. Influence of Catalyst Loading
2.2.4. Influence of the Methanol/Oil Molar Ratio
2.3. Reusability of NCSO
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. Catalyst Characterization
3.4. Transesterification of Soybean Oil
3.5. Product Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zul, N.A.; Ganesan, S.; Hamidon, T.S.; Oh, W.-D.; Hussin, M.H. A review on the utilization of calcium oxide as a base catalyst in biodiesel production. J. Environ. Chem. Eng. 2021, 9, 105741. [Google Scholar] [CrossRef]
- Munyentwali, A.; Li, H.; Yang, Q. Review of advances in bifunctional solid acid/base catalysts for sustainable biodiesel production. Appl. Catal. A Gen. 2022, 633, 118525. [Google Scholar] [CrossRef]
- Orege, J.I.; Oderinde, O.; Kifle, G.A.; Ibikunle, A.A.; Raheem, S.A.; Ejeromedoghene, O.; Okeke, E.S.; Olukowi, O.M.; Orege, O.B.; Fagbohun, E.O.; et al. Recent advances in heterogeneous catalysis for green biodiesel production by transesterification. Energy Convers. Manag. 2022, 258, 115406. [Google Scholar] [CrossRef]
- Jamil, U.; Husain Khoja, A.; Liaquat, R.; Raza Naqvi, S.; Nor Nadyaini Wan Omar, W.; Aishah Saidina Amin, N. Copper and calcium-based metal organic framework (MOF) catalyst for biodiesel production from waste cooking oil: A process optimization study. Energy Convers. Manag. 2020, 215, 112934. [Google Scholar] [CrossRef]
- Demirbas, A. Biodiesel from waste cooking oil via base-catalytic and supercritical methanol transesterification. Energy Convers. Manag. 2009, 50, 923–927. [Google Scholar] [CrossRef]
- Yildiz, I.; Acikkalp, E.; Caliskan, H.; Mori, K. Environmental pollution cost analyses of biodiesel and diesel fuels for a diesel engine. J. Environ. Manag. 2019, 243, 218–226. [Google Scholar] [CrossRef]
- Qiu, F.; Li, Y.; Yang, D.; Li, X.; Sun, P. Biodiesel production from mixed soybean oil and rapeseed oil. Appl. Energy 2011, 88, 2050–2055. [Google Scholar] [CrossRef]
- Liu, X.; Xing, S.; Yang, L.; Fu, J.; Lv, P.; Zhang, X.; Li, M.; Wang, Z. Highly active and durable Ca-based solid base catalyst for biodiesel production. Fuel 2021, 302, 121094. [Google Scholar] [CrossRef]
- Lee, J.C.; Lee, B.; Ok, Y.S.; Lim, H. Preliminary techno-economic analysis of biodiesel production over solid-biochar. Bioresour. Technol. 2020, 306, 123086. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Q.; Sun, X.; Wu, C.; Gao, Z. Kinetics studies of biodiesel production from waste cooking oil using FeCl3-modified resin as heterogeneous catalyst. Renew. Energy 2017, 107, 522–530. [Google Scholar] [CrossRef]
- Rabie, A.M.; Shaban, M.; Abukhadra, M.R.; Hosny, R.; Ahmed, S.A.; Negm, N.A. Diatomite supported by CaO/MgO nanocomposite as heterogeneous catalyst for biodiesel production from waste cooking oil. J. Mol. Liq. 2019, 279, 224–231. [Google Scholar] [CrossRef]
- Chen, G.-Y.; Shan, R.; Yan, B.-B.; Shi, J.-F.; Li, S.-Y.; Liu, C.-Y. Remarkably enhancing the biodiesel yield from palm oil upon abalone shell-derived CaO catalysts treated by ethanol. Fuel Process. Technol. 2016, 143, 110–117. [Google Scholar] [CrossRef]
- Kouzu, M.; Hidaka, J.-s. Transesterification of vegetable oil into biodiesel catalyzed by CaO: A review. Fuel 2012, 93, 1–12. [Google Scholar] [CrossRef]
- Mazaheri, H.; Ong, H.C.; Amini, Z.; Masjuki, H.H.; Mofijur, M.; Su, C.H.; Anjum Badruddin, I.; Khan, T.M.Y. An Overview of Biodiesel Production via Calcium Oxide Based Catalysts: Current State and Perspective. Energies 2021, 14, 3950. [Google Scholar] [CrossRef]
- Teo, S.H.; Rashid, U.; Choong, S.Y.T.; Taufiq-Yap, Y.H. Heterogeneous calcium-based bimetallic oxide catalyzed transesterification of Elaeis guineensis derived triglycerides for biodiesel production. Energy Convers. Manag. 2017, 141, 20–27. [Google Scholar] [CrossRef]
- Sudsakorn, K.; Saiwuttikul, S.; Palitsakun, S.; Seubsai, A.; Limtrakul, J. Biodiesel production from Jatropha Curcas oil using strontium-doped CaO/MgO catalyst. J. Environ. Chem. Eng. 2017, 5, 2845–2852. [Google Scholar] [CrossRef]
- Dias, A.P.S.; Ramos, M. On the storage stability of CaO biodiesel catalyst. Hydration and carbonation poisoning. J. Environ. Chem. Eng. 2021, 9, 104917. [Google Scholar] [CrossRef]
- Malins, K. The potential of K3PO4, K2CO3, Na3PO4 and Na2CO3 as reusable alkaline catalysts for practical application in biodiesel production. Fuel Process. Technol. 2018, 179, 302–312. [Google Scholar] [CrossRef]
- de Lima, A.L.; Ronconi, C.M.; Mota, C.J.A. Heterogeneous basic catalysts for biodiesel production. Catal. Sci. Technol. 2016, 6, 2877–2891. [Google Scholar] [CrossRef]
- Rahmani Vahid, B.; Haghighi, M. Urea-nitrate combustion synthesis of MgO/MgAl2O4 nanocatalyst used in biodiesel production from sunflower oil: Influence of fuel ratio on catalytic properties and performance. Energy Convers. Manag. 2016, 126, 362–372. [Google Scholar] [CrossRef]
- Rodríguez-Ramírez, R.; Romero-Ibarra, I.; Vazquez-Arenas, J. Synthesis of sodium zincsilicate (Na2ZnSiO4) and heterogeneous catalysis towards biodiesel production via Box-Behnken design. Fuel 2020, 280, 118668. [Google Scholar] [CrossRef]
- Ibrahim, M.L.; Nik Abdul Khalil, N.N.A.; Islam, A.; Rashid, U.; Ibrahim, S.F.; Sinar Mashuri, S.I.; Taufiq-Yap, Y.H. Preparation of Na2O supported CNTs nanocatalyst for efficient biodiesel production from waste-oil. Energy Convers. Manag. 2020, 205, 112445. [Google Scholar] [CrossRef]
- Sánchez Faba, E.M.; Ferrero, G.O.; Dias, J.M.; Eimer, G.A. Na-Ce-modified-SBA-15 as an effective and reusable bimetallic mesoporous catalyst for the sustainable production of biodiesel. Appl. Catal. A Gen. 2020, 604, 117769. [Google Scholar] [CrossRef]
- Rijo, B.; Fernando, E.; Ramos, M.; Dias, A.P.S. Biodiesel production over sodium carbonate and bicarbonate catalysts. Fuel 2022, 323, 124383. [Google Scholar] [CrossRef]
- Wan, L.; Liu, H.; Skala, D. Biodiesel production from soybean oil in subcritical methanol using MnCO3/ZnO as catalyst. Appl. Catal. B Environ. 2014, 152–153, 352–359. [Google Scholar] [CrossRef]
- Wang, J.; Yang, L.; Luo, W.; Yang, G.; Miao, C.; Fu, J.; Xing, S.; Fan, P.; Lv, P.; Wang, Z. Sustainable biodiesel production via transesterification by using recyclable Ca2MgSi2O7 catalyst. Fuel 2017, 196, 306–313. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Yang, L.; Yang, G.; Miao, C.; Lv, P. Natural albite as a novel solid basic catalyst for the effective synthesis of biodiesel: Characteristics and performance. Energy 2017, 141, 1650–1660. [Google Scholar] [CrossRef]
- Pavlović, S.M.; Marinković, D.M.; Kostić, M.D.; Janković-Častvan, I.M.; Mojović, L.V.; Stanković, M.V.; Veljković, V.B. A CaO/zeolite-based catalyst obtained from waste chicken eggshell and coal fly ash for biodiesel production. Fuel 2020, 267, 117171. [Google Scholar] [CrossRef]
- Singh, V.; Hameed, B.H.; Sharma, Y.C. Economically viable production of biodiesel from a rural feedstock from eastern India, P. pinnata oil using a recyclable laboratory synthesized heterogeneous catalyst. Energy Convers. Manag. 2016, 122, 52–62. [Google Scholar] [CrossRef]
- Samart, C.; Sreetongkittikul, P.; Sookman, C. Heterogeneous catalysis of transesterification of soybean oil using KI/mesoporous silica. Fuel Process. Technol. 2009, 90, 922–925. [Google Scholar] [CrossRef]
- Bahador, F.; Foroutan, R.; Nourafkan, E.; Peighambardoust, S.J.; Esmaeili, H. Enhancement of Biodiesel Production from Chicken Fat Using MgO and MgO@Na2O Nanocatalysts. Chem. Eng. Technol. 2020, 44, 77–84. [Google Scholar] [CrossRef]
- Koppala, S.; Swamiappan, S. Glowing Combustion Synthesis, Characterization, and Toxicity Studies of Na2CaSiO4 Powders. Mater. Manuf. Process. 2015, 30, 1476–1481. [Google Scholar] [CrossRef]
- Huang, J.; Zou, Y.; Yaseen, M.; Qu, H.; He, R.; Tong, Z. Fabrication of hollow cage-like CaO catalyst for the enhanced biodiesel production via transesterification of soybean oil and methanol. Fuel 2021, 290, 119799. [Google Scholar] [CrossRef]
- Roschat, W.; Phewphong, S.; Thangthong, A.; Moonsin, P.; Yoosuk, B.; Kaewpuang, T.; Promarak, V. Catalytic performance enhancement of CaO by hydration-dehydration process for biodiesel production at room temperature. Energy Convers. Manag. 2018, 165, 1–7. [Google Scholar] [CrossRef]
- Wang, J.-X.; Chen, K.-T.; Wu, J.-S.; Wang, P.-H.; Huang, S.-T.; Chen, C.-C. Production of biodiesel through transesterification of soybean oil using lithium orthosilicate solid catalyst. Fuel Process. Technol. 2012, 104, 167–173. [Google Scholar] [CrossRef]
- Yan, B.; Zhang, Y.; Chen, G.; Shan, R.; Ma, W.; Liu, C. The utilization of hydroxyapatite-supported CaO-CeO2 catalyst for biodiesel production. Energy Convers. Manag. 2016, 130, 156–164. [Google Scholar] [CrossRef]
- Chen, G.-Y.; Shan, R.; Shi, J.-F.; Yan, B.-B. Transesterification of palm oil to biodiesel using rice husk ash-based catalysts. Fuel Process. Technol. 2015, 133, 8–13. [Google Scholar] [CrossRef]
- Shan, R.; Zhao, C.; Yuan, H.; Wang, S.; Wang, Y. Transesterification of vegetable oil using stable natural diatomite-supported catalyst. Energy Convers. Manag. 2017, 138, 547–555. [Google Scholar] [CrossRef]
- Tasić, M.B.; Stamenković, O.S.; Veljković, V.B. Cost analysis of simulated base-catalyzed biodiesel production processes. Energy Convers. Manag. 2014, 84, 405–413. [Google Scholar] [CrossRef]
- Albuquerque, M.C.G.; Azevedo, D.C.S.; Cavalcante, C.L.; Santamaría-González, J.; Mérida-Robles, J.M.; Moreno-Tost, R.; Rodríguez-Castellón, E.; Jiménez-López, A.; Maireles-Torres, P. Transesterification of ethyl butyrate with methanol using MgO/CaO catalysts. J. Mol. Catal. A Chem. 2009, 300, 19–24. [Google Scholar] [CrossRef]
- Black, L.; Garbev, K.; Stemmermann, P.; Hallam, K.R.; Allen, G.C. Characterisation of crystalline C-S-H phases by X-ray photoelectron spectroscopy. Cem. Concr. Res. 2003, 33, 899–911. [Google Scholar] [CrossRef]
- Shan, R.; Chen, G.; Yan, B.; Shi, J.; Liu, C. Porous CaO-based catalyst derived from PSS-induced mineralization for biodiesel production enhancement. Energy Convers. Manag. 2015, 106, 405–413. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, H.; Ren, H.; Cheng, Q.; Cui, Y.; Zhang, J. Development KCl/CaO as a catalyst for biodiesel production by tri-component coupling transesterification. Environ. Prog. Sustain. Energy 2018, 38, 647–653. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Fu, J.; Xing, S.; Li, M.; Liu, X.; Yang, L.; Lv, P. Sodium Silicates Modified Calcium Oxide as a High-Performance Solid Base Catalyst for Biodiesel Production. Catalysts 2023, 13, 775. https://doi.org/10.3390/catal13040775
Zhang S, Fu J, Xing S, Li M, Liu X, Yang L, Lv P. Sodium Silicates Modified Calcium Oxide as a High-Performance Solid Base Catalyst for Biodiesel Production. Catalysts. 2023; 13(4):775. https://doi.org/10.3390/catal13040775
Chicago/Turabian StyleZhang, Shunpan, Junying Fu, Shiyou Xing, Ming Li, Xiaochun Liu, Lingmei Yang, and Pengmei Lv. 2023. "Sodium Silicates Modified Calcium Oxide as a High-Performance Solid Base Catalyst for Biodiesel Production" Catalysts 13, no. 4: 775. https://doi.org/10.3390/catal13040775