Ethylene Dimerization Performance of NiBTCs Synthesized Using Different Solvents
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization
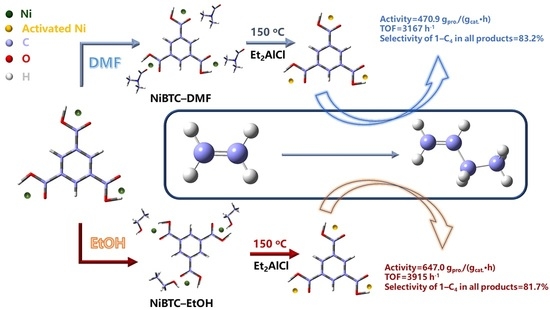
2.2. Ethylene Dimerization
2.3. The Stability of Catalyst
2.4. Postulated Mechanism for Ethylene Dimerization
2.5. Comparison with Other Catalysts
3. Materials and Methods
3.1. Reagents and Instruments
3.2. Catalysts Preparation
3.3. Catalytic Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, C. Designing catalysts for olefin polymerization and copolymerization: Beyond electronic and steric tuning. Nat. Rev. Chem. 2018, 2, 6–14. [Google Scholar] [CrossRef]
- Finiels, A.; Fajula, F.; Hulea, V. Nickel-based solid catalysts for ethylene oligomerization-a review. Catal. Sci. Technol. 2014, 4, 2412–2426. [Google Scholar] [CrossRef]
- Shin, D.Y.; Yoon, J.H.; Baik, H.; Lee, S.J. A way to avoid polymeric side products during the liquid-phase ethylene oligomerization with SBA-15 supported (bpy)Ni(II)Cl2 heterogeneous catalyst. Appl. Catal. A-G 2020, 590, 117363–117369. [Google Scholar] [CrossRef]
- Wang, J.; Alam, F.; Chang, Q.; Chen, Y.; Jiang, T. Catalytic behavior tuning via structural modifications of silylated-diphosphine Ni(II) complexes for ethylene selective dimerization. Appl. Organomet. Chem. 2020, 34, e5722. [Google Scholar] [CrossRef]
- Nicholas, C.P.; Laipert, L.; Prabhakar, S. Oligomerization of Light Olefins to Gasoline: An Advanced NMR Characterization of Liquid Products. Ind. Eng. Chem. Res 2016, 55, 9140–9146. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Q.; Solan, G.A.; Sun, W.-H. Recent advances in Ni-mediated ethylene chain growth: Nimine-donor ligand effects on catalytic activity, thermal stability and oligo-/polymer structure. Coordin. Chem. Rev. 2017, 350, 68–83. [Google Scholar] [CrossRef]
- Chen, L.; Huo, H.; Wang, L.; Kuang, Q.; Shi, W.; Zhang, N.; Li, Z.; Wang, J. Ethylene oligomerization studies utilizing nickel complexes bearing pyridine-imine ligands. Inorg. Chim. Acta 2019, 491, 67–75. [Google Scholar] [CrossRef]
- Feng, C.; Zhou, S.; Wang, D.; Zhao, Y.; Liu, S.; Li, Z.; Braunstein, P. Cooperativity in Highly Active Ethylene Dimerization by Dinuclear Nickel Complexes Bearing a Bifunctional PN Ligand. Organometallics 2020, 40, 184–193. [Google Scholar] [CrossRef]
- Olivier-Bourbigou, H.; Breuil, P.A.R.; Magna, L.; Michel, T.; Espada Pastor, M.F.; Delcroix, D. Nickel Catalyzed Olefin Oligomerization and Dimerization. Chem. Rev. 2020, 120, 7919–7983. [Google Scholar] [CrossRef]
- Sung Cho, H.; Deng, H.; Miyasaka, K.; Dong, Z.; Cho, M.; Neimark, A.V.; Ku Kang, J.; Yaghi, O.M.; Terasaki, O. Extra adsorption and adsorbate superlattice formation in metal-organic frameworks. Nature 2015, 527, 503–507. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444–1230456. [Google Scholar] [CrossRef] [Green Version]
- Hanikel, N.; Prevot, M.S.; Yaghi, O.M. MOF water harvesters. Nat. Nanotechnol. 2020, 15, 348–355. [Google Scholar] [CrossRef]
- Ji, Z.; Li, T.; Yaghi, O.M. Sequencing of metals in multivariate metal-organic frameworks. Science 2020, 369, 674–780. [Google Scholar] [CrossRef]
- Wang, C.; Li, G.; Guo, H. Heterogeneous dimerization of ethylene by coordinatively unsaturated metal sites in two forms of Ni-MIL-77. Mol. Catal. 2022, 524, 112340–112348. [Google Scholar] [CrossRef]
- Ma, L.; Falkowski, J.M.; Abney, C.; Lin, W. A series of isoreticular chiral metal-organic frameworks as a tunable platform for asymmetric catalysis. Nat. Chem. 2010, 2, 838–846. [Google Scholar] [CrossRef]
- Li, Z.; Schweitzer, N.M.; League, A.B.; Bernales, V.; Peters, A.W.; Getsoian, A.B.; Wang, T.C.; Miller, J.T.; Vjunov, A.; Fulton, J.L.; et al. Sintering-Resistant Single-Site Nickel Catalyst Supported by Metal-Organic Framework. J. Am. Chem. Soc. 2016, 138, 1977–1982. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Y.; Han, Y.; Sheng, D.; Shan, D.; Liu, X.; Cheng, A. Ultrathin Nickel-Based Metal–Organic Framework Nanosheets as Reusable Heterogeneous Catalyst for Ethylene Dimerization. ACS Appl. Nano Mater. 2018, 2, 136–142. [Google Scholar] [CrossRef]
- Mlinar, A.N.; Keitz, B.K.; Gygi, D.; Bloch, E.D.; Long, J.R.; Bell, A.T. Selective Propene Oligomerization with Nickel(II)-Based Metal–Organic Frameworks. ACS Catal. 2014, 4, 717–721. [Google Scholar] [CrossRef] [Green Version]
- Canivet, J.; Aguado, S.; Schuurman, Y.; Farrusseng, D. MOF-supported selective ethylene dimerization single-site catalysts through one-pot postsynthetic modification. J. Am. Chem. Soc. 2013, 135, 4195–4198. [Google Scholar] [CrossRef]
- Metzger, E.D.; Brozek, C.K.; Comito, R.J.; Dinca, M. Selective Dimerization of Ethylene to 1-Butene with a Porous Catalyst. ACS Cent. Sci. 2016, 2, 148–153. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Alalouni, M.R.; Dong, X.; Cao, Z.; Cheng, Q.; Zheng, L.; Meng, L.; Guan, C.; Liu, L.; Abou-Hamad, E.; et al. Highly Active Heterogeneous Catalyst for Ethylene Dimerization Prepared by Selectively Doping Ni on the Surface of a Zeolitic Imidazolate Framework. J. Am. Chem. Soc. 2021, 143, 7144–7153. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, O.M.; Li, H.; Groy, T.L. Construction of Porous Solids from Hydrogen-Bonded Metal Complexes of 1,3,5-Benzenetricarboxylic Acid. J. Am. Chem. Soc. 1996, 118, 9096–9101. [Google Scholar] [CrossRef]
- Naik Shreyanka, S.; Theerthagiri, J.; Lee, S.J.; Yu, Y.; Choi, M.Y. Multiscale design of 3D metal–organic frameworks (M−BTC, M: Cu, Co, Ni) via PLAL enabling bifunctional electrocatalysts for robust overall water splitting. Chem. Eng. J. 2022, 446, 137045. [Google Scholar] [CrossRef]
- Du, P.; Dong, Y.; Liu, C.; Wei, W.; Liu, D.; Liu, P. Fabrication of hierarchical porous nickel based metal-organic framework (Ni-MOF) constructed with nanosheets as novel pseudo-capacitive material for asymmetric supercapacitor. J. Colloid Interface Sci. 2018, 518, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Song, X.; Li, S.; Zhang, J.; Yang, X.; Shen, P.; Gao, L.; Wei, R.; Zhang, J.; Xiao, G. 3D-monoclinic M–BTC MOF (M = Mn, Co, Ni) as highly efficient catalysts for chemical fixation of CO2 into cyclic carbonates. J. Ind. Eng. Chem. 2018, 58, 296–303. [Google Scholar] [CrossRef]
- Gan, Q.; He, H.; Zhao, K.; He, Z.; Liu, S. Morphology-dependent electrochemical performance of Ni-1,3,5-benzenetricarboxylate metal-organic frameworks as an anode material for Li-ion batteries. J. Colloid Interface Sci. 2018, 530, 127–136. [Google Scholar] [CrossRef]
- Maiti, S.; Pramanik, A.; Manju, U.; Mahanty, S. Reversible Lithium Storage in Manganese 1,3,5-Benzenetricarboxylate Metal-Organic Framework with High Capacity and Rate Performance. ACS Appl. Mater. Interfaces 2015, 7, 16357–16363. [Google Scholar] [CrossRef]
- Singh, M.P.; Dhumal, N.R.; Kim, H.J.; Kiefer, J.; Anderson, J.A. Influence of Water on the Chemistry and Structure of the Metal−Organic Framework Cu3(btc)2. J. Phys. Chem. C 2016, 120, 17323–17333. [Google Scholar] [CrossRef]
- Sánchez-Andújar, M.; Gómez-Aguirre, L.C.; Pato Doldán, B.; Yáñez-Vilar, S.; Artiaga, R.; Llamas-Saiz, A.L.; Manna, R.S.; Schnelle, F.; Lang, M.; Ritter, F.; et al. First-order structural transition in the multiferroic perovskite-like formate [(CH3)2NH2][Mn(HCOO)3]. CrystEngComm 2014, 16, 3558–3566. [Google Scholar] [CrossRef] [Green Version]
- Sun, K.; Li, L.; Yu, X.; Liu, L.; Meng, Q.; Wang, F.; Zhang, R. Functionalization of mixed ligand metal-organic frameworks as the transport vehicles for drugs. J. Colloid Interface Sci. 2017, 486, 128–135. [Google Scholar] [CrossRef]
- Zeng, G.J.; Chen, Y.; Chen, L.; Xiong, P.X.; Wei, M.D. Hierarchical cerium oxide derived from metal-organic frameworks for high performance supercapacitor electrodes. Electrochim. Acta 2016, 222, 773–780. [Google Scholar] [CrossRef]
- He, J.H.; Zhang, Y.T.; Pan, Q.H.; Yu, J.H.; Ding, H.; Xu, R.R. Three metal-organic frameworks prepared from mixed solvents of DMF and HAc. Micropor. Mesopor. Mat 2006, 90, 145–152. [Google Scholar] [CrossRef]
- Israr, F.; Kim, D.K.; Kim, Y.; Oh, S.J.; Ng, K.C.; Chun, W. Synthesis of porous Cu-BTC with ultrasonic treatment: Effects of ultrasonic power and solvent condition. Ultrason. Sonochem. 2016, 29, 186–193. [Google Scholar] [CrossRef]
- Xu, J.; Yang, C.; Xue, Y.; Wang, C.; Cao, J.; Chen, Z. Facile synthesis of novel metal-organic nickel hydroxide nanorods for high performance supercapacitor. Electrochim. Acta 2016, 211, 595–602. [Google Scholar] [CrossRef]
- Yi, F.Y.; Chen, D.; Wu, M.K.; Han, L.; Jiang, H.L. Chemical Sensors Based on Metal-Organic Frameworks. ChemPlusChem 2016, 81, 675–690. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, G.; Xu, Y.; Zhang, H.; Guo, X.; Liu, Y.; Pang, H. A new strategy for the controllable growth of MOF@PBA architectures. J. Mater. Chem. A 2019, 7, 17266–17271. [Google Scholar] [CrossRef]
- Wang, X.; Jian, H.; Xiao, Q.; Huang, S. Ammonium nickel phosphate on nickel foam with a Ni3+-rich surface for ultrasensitive nonenzymatic glucose sensors. Appl. Surf. Sci. 2018, 459, 40–47. [Google Scholar] [CrossRef]
- Raihana, M.K.; Padmanathan, N.; Eswaramoorthi, V.; McNulty, D.; Sahadevan, J.; Mohanapriya, P.; Muthu, S.E. Reduced graphene oxide/VSB-5 composite micro/nanorod electrode for high energy density supercapattery. Electrochim. Acta 2021, 391, 138903–138914. [Google Scholar] [CrossRef]
- Dilks, A. The identification of peroxy-features at polymer surfaces by ESCA. J. Polym. Sci. Pol. Chem. Edit. 1981, 19, 1319–1327. [Google Scholar] [CrossRef]
- Gedrich, K.; Senkovska, I.; Klein, N.; Stoeck, U.; Henschel, A.; Lohe, M.R.; Baburin, I.A.; Mueller, U.; Kaskel, S. A highly porous metal-organic framework with open nickel sites. Angew. Chem. Int. Ed. 2010, 49, 8489–8492. [Google Scholar] [CrossRef]
- Maniam, P.; Stock, N. Investigation of porous Ni-based metal-organic frameworks containing paddle-wheel type inorganic building units via high-throughput methods. Inorg. Chem 2011, 50, 5085–5097. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Ru, Q.; Qiao, C.; Sun, X.; Jia, C.; Wang, Y.; Zhang, Y. Adsorption desulfurization of model gasoline by metal–organic framework Ni3(BTC)2. J. Energy Chem. 2019, 32, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Israr, F.; Kim, D.K.; Kim, Y.; Chun, W. Scope of various solvents and their effects on solvothermal synthesis of Ni-BTC. Quim. Nova 2016, 39, 669–675. [Google Scholar] [CrossRef]
- Murad, A.; Liew, J.Y.C.; Yaacob, M.H.; Noor, I.M.; Osman, N.H.; Kamarudin, M.A.; Tan, S.T.; Lee, H.K.; Talib, Z.A.; Alresheedi, M.T.; et al. Effect of nickel ion concentration on structural, optical and electrical properties towards Ni–H3BTC-MOF formation for nonlinear saturable absorption phenomenon. J. Phys. Chem. Sol. 2022, 167, 110743–110751. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Guo, C.; Chen, T.; Guo, C.; Lu, Y.; Wang, J. CdS(ZB)/CdS(WZ)/Ni-BTC photocatalytic selective oxidation of benzyl alcohol to benzaldehyde coupled with hydrogen evolution. Appl. Surf. Sci. 2022, 571, 151284–151296. [Google Scholar] [CrossRef]
- Taherinia, D.; Hatami, H.; Mirzaee Valadi, F. Trimetallic Co-Ni-Mn metal-organic framework as an efficient electrocatalyst for alkaline oxygen evolution reaction. J. Electroanal. Chem. 2022, 922, 116720–116729. [Google Scholar] [CrossRef]
- Israr, F.; Chun, D.; Kim, Y.; Kim, D.K. High yield synthesis of Ni-BTC metal-organic framework with ultrasonic irradiation: Role of polar aprotic DMF solvent. Ultrason. Sonochem. 2016, 31, 93–101. [Google Scholar] [CrossRef]
- Nyamato, G.S.; Alam, M.G.; Ojwach, S.O.; Akerman, M.P. (Pyrazolyl)-(phosphinoyl)pyridine iron(II), cobalt(II) and nickel(II) complexes: Synthesis, characterization and ethylene oligomerization studies. J. Organomet. Chem. 2015, 783, 64–72. [Google Scholar] [CrossRef]
- Rhinehart, J.L.; Brown, L.A.; Long, B.K. A robust Ni(II) alpha-diimine catalyst for high temperature ethylene polymerization. J. Am. Chem. Soc. 2013, 135, 16316–16319. [Google Scholar] [CrossRef]
- Andrei, R.D.; Popa, M.I.; Fajula, F.; Hulea, V. Heterogeneous oligomerization of ethylene over highly active and stable Ni-AlSBA-15 mesoporous catalysts. J. Catal. 2015, 323, 76–84. [Google Scholar] [CrossRef]
- Chen, L.; Jiang, Y.; Huo, H.; Liu, J.; Li, Y.; Li, C.; Zhang, N.; Wang, J. Metal-organic framework-based composite Ni@MOF as Heterogenous catalyst for ethylene trimerization. Appl. Catal. A-G 2020, 594, 117457–117465. [Google Scholar] [CrossRef]
- Brogaard, R.Y.; Olsbye, U. Ethene Oligomerization in Ni-Containing Zeolites: Theoretical Discrimination of Reaction Mechanisms. ACS Catal. 2016, 6, 1205–1214. [Google Scholar] [CrossRef]
- Moussa, S.; Concepción, P.; Arribas, M.A.; Martínez, A. The nature of active Ni sites and the role of Al species in the oligomerization of ethylene on mesoporous Ni-Al-MCM-41 catalysts. Appl. Catal. A-G 2020, 608, 117831–117841. [Google Scholar] [CrossRef]
- Lever, A.B.P.; Lewis, J.; Nyholm, R.S. Pyraxine Metal Complexes. Part III.* Derivatives of Nickel (II). J. Chem. Soc. 1963, 1, 5042–5048. [Google Scholar] [CrossRef]
- Wu, J.X.; Yuan, W.W.; Xu, M.; Gu, Z.Y. Ultrathin 2D nickel zeolitic imidazolate framework nanosheets for electrocatalytic reduction of CO2. Chem. Commun. 2019, 55, 11634–11637. [Google Scholar] [CrossRef]
- Jafari, N.; Zeinali, S.; Shadmehr, J. Room temperature resistive gas sensor based on ZIF-8/MWCNT/AgNPs nanocomposite for VOCs detection. J. Mate. Sci.-Mater. El 2019, 30, 12339–12350. [Google Scholar] [CrossRef]
- Borges, W.M.S.; Guerreiro, M.C.; Anconi, C.P.A.; Magalhães, K.T.; Castro, G.M.M.; Neto, J.L.; Rossi, M.A.L.S. Coordination of iron (III) to modified silica surface containing pyrazine acid groups and its application in advanced oxidative processes. Surf. Interfaces 2022, 29, 101770–101779. [Google Scholar] [CrossRef]
- Sun, X.; Man, J.; Liu, K.; Liu, W.; Sun, J.; Zhang, N.; Zhou, Y.; Geng, Z.; Li, S.; Sun, J. Uniform lithium deposition enabled by a carbon nanotubes framework modified with nanosized ZIF-8 particles for dendrite-free lithium metal anode. Appl. Surf. Sci. 2023, 616, 156474–156484. [Google Scholar] [CrossRef]
- Mohammadi, A.; Nakhaei Pour, A. Triethylenetetramine-impregnated ZIF-8 nanoparticles for CO2 adsorption. J. CO2 Util 2023, 69, 102424–102437. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Hu, C.; Zuo, C.; Wang, P.; Chen, W.; Ao, T. Efficient removal of tetracycline by a hierarchically porous ZIF-8 metal organic framework. Environ. Res. 2021, 198, 111254–111266. [Google Scholar] [CrossRef]
- Chen, C.; Alalouni, M.R.; Xiao, P.; Li, G.; Pan, T.; Shen, J.; Cheng, Q.; Dong, X. Ni-Loaded 2D Zeolitic Imidazolate Framework as a Heterogeneous Catalyst with Highly Activity for Ethylene Dimerization. Ind. Eng. Chem. Res. 2022, 61, 14374–14381. [Google Scholar] [CrossRef]
- Li, D.; Li, F.; Yu, H.; Guo, L.; Huang, J.; Li, J.; Li, C. Nickel-modified triphenylamine-based conjugated porous polymers as precatalyst for ethylene oligomerization. Inorg. Chim. Acta 2023, 544, 121228–121238. [Google Scholar] [CrossRef]
- Li, D.; Guo, L.; Li, F.; Huang, J.; Li, J.; Li, M.; Li, C. Synthesis and catalytic behavior of nickel heterogenized in covalent organic frameworks as precatalysts in ethylene oligomerization. Micropor. Mesopor. Mat. 2022, 338, 111979–111992. [Google Scholar] [CrossRef]
- Metzger, E.D.; Comito, R.J.; Wu, Z.; Zhang, G.; Dubey, R.C.; Xu, W.; Miller, J.T.; Dincă, M. Highly Selective Heterogeneous Ethylene Dimerization with a Scalable and Chemically Robust MOF Catalyst. ACS Sustain. Chem. Eng. 2019, 7, 6654–6661. [Google Scholar] [CrossRef]
- Liu, B.; Jie, S.; Bu, Z.; Li, B.-G. Postsynthetic modification of mixed-linker metal–organic frameworks for ethylene oligomerization. RSC Adv. 2014, 4, 62343–62346. [Google Scholar] [CrossRef]










| Catalyst | Time (h) | Ethylene Pressure (bar) | Activity gpro./(gcat.·h) | TOF (h−1) | Selectivity (%) | |||
|---|---|---|---|---|---|---|---|---|
| 1-C4 | Other C4 | C6 | ≥C8 | |||||
| Ni(NO3)2 6H2O + H3BTC + Et2AlCl | 0.5 | 10 | 0 | 0 | 0 | 0 | 0 | 0 |
| H3BTC + Et2AlCl | 0.5 | 10 | 0 | 0 | 0 | 0 | 0 | 0 |
| NiBTC–DMF | 0.5 | 10 | 196.9 | 1325 | 86.5 | 9.40 | 1.30 | 2.80 |
| 1.0 | 10 | 157.2 | 1057 | 76.9 | 16.5 | 2.30 | 4.30 | |
| 0.5 | 20 | 470.9 | 3167 | 83.2 | 8.30 | 2.51 | 5.99 | |
| NiBTC–EtOH | 0.5 | 10 | 291.6 | 1764 | 78.0 | 16.9 | 1.55 | 3.55 |
| 1.0 | 10 | 279.4 | 1691 | 77.9 | 14.7 | 2.87 | 4.53 | |
| 0.5 | 20 | 647.0 | 3915 | 81.7 | 12.3 | 2.76 | 3.24 | |
| Entry | Amount of Product Obtained (g) | Amount of Product Obtained from the Filtrate (g) |
|---|---|---|
| NiBTC–DMF | 0.492 | 0.009 |
| NiBTC–EtOH | 0.729 | 0.035 |
| Entry | Ni Content % a | Al Content % a | Activity gpro./(gcat·h) | TOF (h−1) | Selectivity (%) | |||
|---|---|---|---|---|---|---|---|---|
| 1-C4 | Other C4 | C6 | ≥C8 | |||||
| NiBTC–DMF–R | 20.89 | 7.54 | 106.2 | 714.3 | 85.1 | 4.31 | 0.39 | 10.2 |
| NiBTC–EtOH–R | 24.77 | 6.54 | 121.0 | 732.1 | 74.9 | 11.3 | 1.30 | 12.5 |
| Entry | Activity gpro./(gcat.·h) | TOF (h−1) | Selectivity (%) | |||
|---|---|---|---|---|---|---|
| 1-C4 | Other C4 | C6 | ≥C8 | |||
| NiBTC–DMF | 196.9 | 1325 | 86.5 | 9.40 | 1.30 | 2.80 |
| NiBTC–EtOH | 291.6 | 1764 | 78.0 | 16.9 | 1.55 | 3.55 |
| Ni(pyz)2Cl2 | 149.7 | 1261 | 79.1 | 12.2 | 0.78 | 7.92 |
| α–Ni(im)2 | 184.7 | 1075 | 71.9 | 18.7 | 1.78 | 7.62 |
| Entry | Ethylene Pressure (bar) | Al/Ni Molar Ratio | Activity gpro./(gcat.·h) | Selectivity of 1-C4 (%) | Ref. |
|---|---|---|---|---|---|
| Ni–ZIF–8 | 50 | 4640 | 2130 | 85.1 | [21] |
| 45 Ni–ZIF–L | 30 | 7187 | 71.96 | 86.3 | [61] |
| Ni@TAPA–CPPs | 5 | 500 | 251.92 | 29.6 | [62] |
| Ni@MAPA–COF | 7 | 500 | 202.51 | 47.5 | [63] |
| Ni@MOF | 10 | 800 | 43.52 | 43.7 | [51] |
| 30Ni@(Fe)–MIL–101 | 30 | 70 | 205.0 | Not report | [19] |
| Ni(1%)–MFU–4l | 50 | 500 | 198.0 | 92.0 | [20] |
| Ni(7.5%)–CFA-1 | 50 | 2000 | 22.23 | 87.1 | [64] |
| IRMOF–3–Ni | 20 | 100 | 125.8 | 35.0 (C4 %) | [65] |
| MixMOF–Ni-b | 20 | 100 | 492.9 | 92.7 (C4 %) | [65] |
| NiBTC–DMF | 20 | 100 | 470.9 | 83.2 | This work |
| NiBTC–EtOH | 20 | 100 | 647.0 | 81.7 | This work |
| Entry | Yield % | Content of Ni wt.% a |
|---|---|---|
| NiBTC–DMF | 56.08 | 31.16 |
| NiBTC–EtOH | 65.63 | 34.64 |
| Ni(pyz)2Cl2 | 50.38 | 19.68 |
| α–Ni(im)2 | 41.22 | 28.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Li, G.; Guo, H. Ethylene Dimerization Performance of NiBTCs Synthesized Using Different Solvents. Catalysts 2023, 13, 640. https://doi.org/10.3390/catal13030640
Wang C, Li G, Guo H. Ethylene Dimerization Performance of NiBTCs Synthesized Using Different Solvents. Catalysts. 2023; 13(3):640. https://doi.org/10.3390/catal13030640
Chicago/Turabian StyleWang, Cong, Gang Li, and Hongchen Guo. 2023. "Ethylene Dimerization Performance of NiBTCs Synthesized Using Different Solvents" Catalysts 13, no. 3: 640. https://doi.org/10.3390/catal13030640