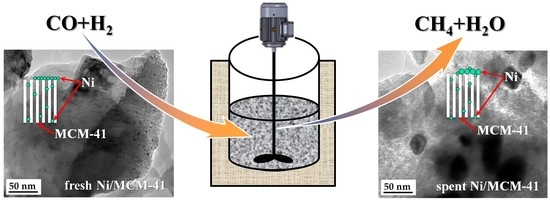
Catalytic Performance for CO Methanation over Ni/MCM-41 Catalyst in a Slurry-Bed Reactor
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalytic Performance for CO Methanation in a Slurry-Bed Reactor
2.2. Structure and Textural Properties Analysis
2.3. Catalyst Reducibility and Surface Properties Analysis
2.4. Relationship between Catalytic Activity and Surface Properties
2.5. Characterization of Spent Catalysts
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalyst Performance Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zeng, Y.; Ma, H.; Zhang, H.; Ying, W.; Fang, D. Ni-Ce-Al composite oxide catalysts synthesized by solution combustion method: Enhanced catalytic activity for CO methanation. Fuel 2015, 162, 16–22. [Google Scholar] [CrossRef]
- Li, J.; Li, P.; Li, J.; Tian, Z.; Yu, F. Highly-dispersed Ni-NiO nanoparticles anchored on an SiO2 support for an enhanced CO methanation performance. Catalysts 2019, 9, 506. [Google Scholar] [CrossRef] [Green Version]
- Meng, F.; Li, X.; Lv, X.; Li, Z. CO hydrogenation combined with water-gas-shift reaction for synthetic natural gas production: A thermodynamic and experimental study. Int. J. Coal Sci. Technol. 2018, 5, 439–451. [Google Scholar] [CrossRef] [Green Version]
- Hatta, A.H.; Jalil, A.A.; Hassan, N.S.; Hamid, M.Y.S.; Rahman, A.F.A.; Teh, L.P.; Prasetyoko, D. A review on recent bimetallic catalyst development for synthetic natural gas production via CO methanation. Int. J. Hydrog. Energy 2022, 47, 30981–31002. [Google Scholar] [CrossRef]
- Xu, B.; Meng, X.; Xin, Z.; Gao, W.; Yang, D.; Jin, D.; Zhao, R.; Dai, W. A novel CO methanation catalyst system based on acid-etched natural halloysites as supports. Ind. Eng. Chem. Res. 2022, 61, 13328–13340. [Google Scholar] [CrossRef]
- Shinde, V.M.; Madras, G. CO methanation toward the production of synthetic natural gas over highly active Ni/TiO2 catalyst. AIChE J. 2014, 60, 1027–1035. [Google Scholar] [CrossRef]
- Liu, S.-S.; Jin, Y.-Y.; Han, Y.; Zhao, J.; Ren, J. Highly stable and coking resistant Ce promoted Ni/SiC catalyst towards high temperature CO methanation. Fuel Process. Technol. 2018, 177, 266–274. [Google Scholar] [CrossRef]
- Zhang, J.; Bai, Y.; Zhang, Q.; Wang, X.; Zhang, T.; Tan, Y.; Han, Y. Low-temperature methanation of syngas in slurry phase over Zr-doped Ni/γ-Al2O3 catalysts prepared using different methods. Fuel 2014, 132, 211–218. [Google Scholar] [CrossRef]
- Meng, F.; Li, Z.; Liu, J.; Cui, X.; Zheng, H. Effect of promoter Ce on the structure and catalytic performance of Ni/Al2O3 catalyst for CO methanation in slurry-bed reactor. J. Nat. Gas Sci. Eng. 2015, 23, 250–258. [Google Scholar] [CrossRef]
- Ji, K.; Meng, F.; Xun, J.; Liu, P.; Zhang, K.; Li, Z.; Gao, J. Carbon deposition behavior of Ni catalyst prepared by combustion method in slurry methanation reaction. Catalysts 2019, 9, 570. [Google Scholar] [CrossRef] [Green Version]
- Mills, G.A.; Steffgen, F.W. Catalytic methanation. Catal. Rev. 1974, 8, 159–210. [Google Scholar] [CrossRef]
- Somorjai, G.A. The catalytic hydrogenation of carbon monoxide. The formation of C1 hydrocarbons. Catal. Rev. 1981, 23, 189–202. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.; Wu, Y.; Qin, H.; Zhang, J. CO methanation over ZrO2/Al2O3 supported Ni catalysts: A comprehensive study. Fuel Process. Technol. 2014, 124, 61–69. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, X.; Liu, C.-J. Structural effect of Ni/TiO2 on CO methanation: Improved activity and enhanced stability. RSC Adv. 2022, 12, 721–727. [Google Scholar] [CrossRef]
- Meng, F.; Li, X.; Li, M.; Cui, X.; Li, Z. Catalytic performance of CO methanation over La-promoted Ni/Al2O3 catalyst in a slurry-bed reactor. Chem. Eng. J. 2017, 313, 1548–1555. [Google Scholar] [CrossRef]
- Shen, S.; Chen, J.; Koodali, R.T.; Hu, Y.; Xiao, Q.; Zhou, J.; Wang, X.; Guo, L. Activation of MCM-41 mesoporous silica by transition-metal incorporation for photocatalytic hydrogen production. Appl. Catal. B 2014, 150–151, 138–146. [Google Scholar] [CrossRef]
- Yang, M.; Lingjun, Z.; Xiaonan, Z.; Prasert, R.; Shurong, W. CO2 methanation over nickel-based catalysts supported on MCM-41 with in situ doping of zirconium. J. CO2 Util. 2020, 42, 101304. [Google Scholar] [CrossRef]
- Lensveld, D.J.; Gerbrand Mesu, J.; Jos van Dillen, A.; de Jong, K.P. Synthesis and characterisation of MCM-41 supported nickel oxide catalysts. Microporous Mesoporous Mater. 2001, 44–45, 401–407. [Google Scholar] [CrossRef]
- Qiu, S.; Zhang, X.; Liu, Q.; Wang, T.; Zhang, Q.; Ma, L. A simple method to prepare highly active and dispersed Ni/MCM-41 catalysts by co-impregnation. Catal. Commun. 2013, 42, 73–78. [Google Scholar] [CrossRef]
- Zhang, J.; Xin, Z.; Meng, X.; Tao, M. Synthesis, characterization and properties of anti-sintering nickel incorporated MCM-41 methanation catalysts. Fuel 2013, 109, 693–701. [Google Scholar] [CrossRef]
- Zyryanova, M.M.; Snytnikov, P.V.; Gulyaeva, R.V.; Amosov, Y.I.; Boronin, A.I.; Sobyanin, V.A. Performance of Ni/CeO2 catalysts for selective CO methanation in hydrogen-rich gas. Chem. Eng. J. 2014, 238, 189–197. [Google Scholar] [CrossRef]
- Munnik, P.; Velthoen, M.E.Z.; de Jongh, P.E.; de Jong, K.P.; Gommes, C.J. Nanoparticle growth in supported nickel catalysts during methanation reaction—Larger is better. Angew. Chem. Int. Ed. 2014, 53, 9493–9497. [Google Scholar] [CrossRef] [PubMed]
- Carraro, P.; Elías, V.; García Blanco, A.; Sapag, K.; Moreno, S.; Oliva, M.; Eimer, G. Synthesis and multi-technique characterization of nickel loaded MCM-41 as potential hydrogen-storage materials. Microporous Mesoporous Mater. 2014, 191, 103–111. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, S.Y. A study on hydrogen-storage behaviors of nickel-loaded mesoporous MCM-41. J. Colloid. Interf. Sci. 2010, 346, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Carraro, P.; Elías, V.; Blanco, A.A.G.; Sapag, K.; Eimer, G.; Oliva, M. Study of hydrogen adsorption properties on MCM-41 mesoporous materials modified with nickel. Int. J. Hydrog. Energy 2014, 39, 8749–8753. [Google Scholar] [CrossRef]
- Lehmann, T.; Wolff, T.; Hamel, C.; Veit, P.; Garke, B.; Seidel-Morgenstern, A. Physico-chemical characterization of Ni/MCM-41 synthesized by a template ion exchange approach. Microporous Mesoporous Mater. 2012, 151, 113–125. [Google Scholar] [CrossRef]
- Tao, M.; Meng, X.; Xin, Z.; Bian, Z.; Lv, Y.; Gu, J. Synthesis and characterization of well dispersed nickel-incorporated SBA-15 and its high activity in syngas methanation reaction. Appl. Catal. A 2016, 516, 127–134. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, T.; Li, B.; Jiang, T.; Ma, L.; Zhang, X.; Liu, Q. Aqueous phase reforming of sorbitol to bio-gasoline over Ni/HZSM-5 catalysts. Appl. Energy 2012, 97, 509–513. [Google Scholar] [CrossRef]
- van de Loosdrecht, J.; van der Kraan, A.M.; van Dillen, A.J.; Geus, J.W. Metal-support interaction: Titania-supported and silica-supported nickel catalysts. J. Catal. 1997, 170, 217–226. [Google Scholar] [CrossRef]
- Meng, F.; Li, X.; Shaw, G.M.; Smith, P.J.; Morgan, D.J.; Perdjon, M.; Li, Z. Sacrificial carbon strategy toward enhancement of slurry methanation activity and stability over Ni-Zr/SiO2 catalyst. Ind. Eng. Chem. Res. 2018, 57, 4798–4806. [Google Scholar] [CrossRef]
- Meng, F.; Wang, L.; Li, X.; Perdjon, M.; Li, Z. Mesoporous nano Ni-Al2O3 catalyst for CO2 methanation in a continuously stirred tank reactor. Catal. Commun. 2022, 164, 106437. [Google Scholar] [CrossRef]
- Jia, C.; Gao, J.; Li, J.; Gu, F.; Xu, G.; Zhong, Z.; Su, F. Nickel catalysts supported on calcium Titanate for enhanced CO methanation. Catal. Sci. Technol. 2013, 3, 490–499. [Google Scholar] [CrossRef]
- Wu, C.; Wang, L.; Williams, P.T.; Shi, J.; Huang, J. Hydrogen production from biomass gasification with Ni/MCM-41 catalysts: Influence of Ni content. Appl. Catal. B 2011, 108–109, 6–13. [Google Scholar] [CrossRef]









| Catalyst | Specific Surface Area (m2/g) a | Pore Volume (cm3/g) b | Average Pore Size (nm) c | Crystallite Size of NiO/Ni (nm) d |
|---|---|---|---|---|
| MCM-41 | 1188 | 1.41 | 3.1 | - |
| 4Ni/MCM-41 | 979 | 1.40 | 3.3 | 10.7/9.0 |
| 12Ni/MCM-41 | 921 | 1.39 | 4.9 | 15.8/15.6 |
| 20Ni/MCM-41 | 838 | 1.38 | 5.2 | 17.4/17.2 |
| 28Ni/MCM-41 | 804 | 1.35 | 5.1 | 20.9/19.2 |
| 20Ni/SiO2 | 153 | 1.38 | 24.1 | 17.2/16.8 |
| Catalyst | DNi(%) a | Scat(m2·g−1cat) b | NNi(×1019·g−1) c | dH(nm) d |
|---|---|---|---|---|
| 4Ni/MCM-41 | 3.34 | 0.86 | 1.32 | 30.1 |
| 12Ni/MCM-41 | 3.18 | 2.27 | 3.49 | 31.8 |
| 20Ni/MCM-41 | 2.67 | 2.97 | 4.57 | 37.8 |
| 28Ni/MCM-41 | 1.80 | 2.62 | 4.04 | 56.3 |
| 20Ni/SiO2 | 1.66 | 1.85 | 2.84 | 60.7 |
| Catalyst | Specific Surface Area (m2/g) a | Pore Volume (cm3/g) b | Average Pore Size (nm) c | Ni Crystallite Size (nm) d |
|---|---|---|---|---|
| 4Ni/MCM-41 | 783 | 0.78 | 3.25 | 9.0/17.7 |
| 12Ni/MCM-41 | 584 | 0.53 | 5.00 | 15.6/21.1 |
| 20Ni/MCM-41 | 488 | 0.53 | 4.07 | 17.2/23.0 |
| 28Ni/MCM-41 | 322 | 0.50 | 4.84 | 19.2/24.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Qin, J.; Zhou, Y.; Zheng, H.; Meng, F. Catalytic Performance for CO Methanation over Ni/MCM-41 Catalyst in a Slurry-Bed Reactor. Catalysts 2023, 13, 598. https://doi.org/10.3390/catal13030598
Zhang G, Qin J, Zhou Y, Zheng H, Meng F. Catalytic Performance for CO Methanation over Ni/MCM-41 Catalyst in a Slurry-Bed Reactor. Catalysts. 2023; 13(3):598. https://doi.org/10.3390/catal13030598
Chicago/Turabian StyleZhang, Guoqiang, Jinyu Qin, Yuan Zhou, Huayan Zheng, and Fanhui Meng. 2023. "Catalytic Performance for CO Methanation over Ni/MCM-41 Catalyst in a Slurry-Bed Reactor" Catalysts 13, no. 3: 598. https://doi.org/10.3390/catal13030598