Electrocatalytic Oxidation of Nitrophenols via Ag Nanoparticles Supported on Citric-Acid-Modified Polyaniline
Abstract
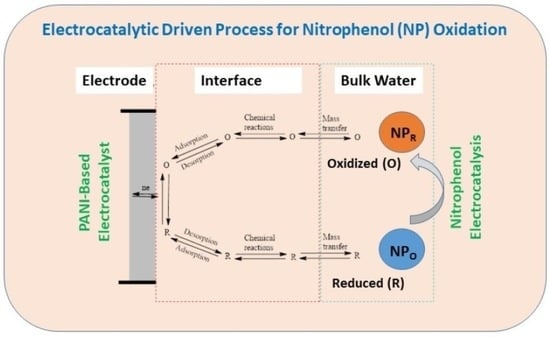
:1. Introduction
2. Results and Discussion
2.1. Thermogravimetric Analysis (TGA) and Atomic Absorption Spectroscopy (AAS)
2.2. FTIR Spectroscopy
2.3. X-ray Diffraction (XRD)
2.4. 13C Solid-State NMR Spectroscopy
2.5. Dye Adsorption Study
2.6. UV-Vis Spectroscopy
2.7. X-ray Photoelectron Spectroscopy (XPS)
2.8. Cyclic Voltammetry (CV)
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Synthesis of Ag@Polyaniline-Citric Acid Composite
3.2.2. Fabrication of Electrodes
3.2.3. Preparation of Phosphate-Buffered Saline (PBS-7) Solution
3.2.4. Preparation of Nitrophenol Solutions for Cyclic Voltammetry Study
3.2.5. Thermogravimetric Analysis (TGA)
3.2.6. Atomic Absorption Analysis (AAS)
3.2.7. Fourier Transform Infrared (FTIR) Spectroscopy
3.2.8. X-ray Diffraction (XRD)
3.2.9. 13C Solid-State NMR Spectroscopy
3.2.10. Solid-State UV-Vis Spectroscopy
3.2.11. X-ray Photoelectron Spectroscopy
3.2.12. Cyclic Voltammetry Analysis
3.2.13. Dye Adsorption Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sapurina, I.; Kazantseva, N.E.; Ryvkina, N.G.; Prokeš, J.; Sáha, P.; Stejskal, J. Electromagnetic Radiation Shielding by Composites of Conducting Polymers and Wood. J. Appl. Polym. Sci. 2005, 95, 807–814. [Google Scholar] [CrossRef]
- Turky, G.; Moussa, M.A.; Hasanin, M.; El-Sayed, N.S.; Kamel, S. Carboxymethyl Cellulose-Based Hydrogel: Dielectric Study, Antimicrobial Activity and Biocompatibility. Arab. J. Sci. Eng. 2021, 46, 17–30. [Google Scholar] [CrossRef]
- Abou Hammad, A.B.; Abd El-Aziz, M.E.; Hasanin, M.S.; Kamel, S. A Novel Electromagnetic Biodegradable Nanocomposite Based on Cellulose, Polyaniline, and Cobalt Ferrite Nanoparticles. Carbohydr. Polym. 2019, 216, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Mengoli, G.; Musiani, M.M.; Pelli, B.; Vecchi, E. Anodic Synthesis of Sulfur-bridged Polyaniline Coatings onto Fe Sheets. J. Appl. Polym. Sci. 1983, 28, 1125–1136. [Google Scholar] [CrossRef]
- Huang, S.C.; Ball, I.J.; Kaner, R.B. Polyaniline Membranes for Pervaporation of Carboxylic Acids and Water. Macromolecules 1998, 31, 5456–5464. [Google Scholar] [CrossRef]
- Clark, N.B.; Maher, L.J. Non-Contact, Radio Frequency Detection of Ammonia with a Printed Polyaniline Sensor. React. Funct. Polym. 2009, 69, 594–600. [Google Scholar] [CrossRef]
- Do Nascimento, G.M. Spectroscopy of Polyaniline Nanofibers. In Nanofibers; InTech: London, UK, 2010; ISBN 978-953-7619-86-2. [Google Scholar]
- Chen, Z.; Lu, C. Humidity Sensors: A Review of Materials and Mechanisms. Sens. Lett. 2005, 3, 274–295. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Qi, Q.; Zhang, T.; Wang, C. A Humidity Sensor Based on KCl-Doped SnO2 Nanofibers. Sens. Actuators B Chem. 2009, 138, 368–373. [Google Scholar] [CrossRef]
- Olinga, T.E.; Fraysse, J.; Travers, J.P.; Dufresne, A.; Pron, A. Highly Conducting and Solution-Processable Polyaniline Obtained via Protonation with a New Sulfonic Acid Containing Plasticizing Functional Groups. Macromolecules 2000, 33, 2107–2113. [Google Scholar] [CrossRef]
- Liao, G.; Li, Q.; Xu, Z. The Chemical Modification of Polyaniline with Enhanced Properties: A Review. Prog. Org. Coat. 2019, 126, 35–43. [Google Scholar] [CrossRef]
- Zhang, L.; Long, Y.; Chen, Z.; Wan, M. The Effect of Hydrogen Bonding on Self-Assembled Polyaniline Nanostructures. Adv. Funct. Mater. 2004, 14, 693–698. [Google Scholar] [CrossRef]
- Ciriminna, R.; Meneguzzo, F.; Delisi, R.; Pagliaro, M. Citric Acid: Emerging Applications of Key Biotechnology Industrial Product. Chem. Cent. J. 2017, 11, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beygisangchin, M.; Abdul Rashid, S.; Shafie, S.; Sadrolhosseini, A.R.; Lim, H.N. Preparations, Properties, and Applications of Polyaniline and Polyaniline Thin Films—A Review. Polymers 2021, 13, 2003. [Google Scholar] [CrossRef] [PubMed]
- Murph, S.E.H.; Murphy, C.J.; Leach, A.; Gall, K. A Possible Oriented Attachment Growth Mechanism for Silver Nanowire Formation. Cryst. Growth Des. 2015, 15, 1968–1974. [Google Scholar] [CrossRef]
- Mudassir, M.A.; Hussain, S.Z.; Rehman, A.; Zaheer, W.; Asma, S.T.; Jilani, A.; Aslam, M.; Zhang, H.; Ansari, T.M.; Hussain, I. Development of Silver-Nanoparticle-Decorated Emulsion-Templated Hierarchically Porous Poly(1-Vinylimidazole) Beads for Water Treatment. ACS Appl. Mater. Interfaces 2017, 9, 24190–24197. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, Q.; Yang, X.; Ye, X.; Duan, H.; Zhang, J.; Zhao, B.; Huang, Y. Knitting Aryl Network Polymers-Incorporated Ag Nanoparticles: A Mild and Efficient Catalyst for the Fixation of CO2 as Carboxylic Acid. ACS Sustain. Chem. Eng. 2017, 5, 9634–9639. [Google Scholar] [CrossRef]
- Liao, G.; Gong, Y.; Zhong, L.; Fang, J.; Zhang, L.; Xu, Z.; Gao, H.; Fang, B. Unlocking the Door to Highly Efficient Ag-Based Nanoparticles Catalysts for NaBH4-Assisted Nitrophenol Reduction. Nano Res. 2019, 12, 2407–2436. [Google Scholar] [CrossRef]
- Bowers, G.N.; McComb, R.B.; Christensen, R.G.; Schaffer, R. High-Purity 4-Nitrophenol: Purification, Characterization, and Specifications for Use as a Spectrophotometric Reference Material. Clin. Chem. 1980, 26, 724–729. [Google Scholar] [CrossRef]
- Liu, Z.; Du, J.; Qiu, C.; Huang, L.; Ma, H.; Shen, D.; Ding, Y. Electrochemical Sensor for Detection of P-Nitrophenol Based on Nanoporous Gold. Electrochem. Commun. 2009, 11, 1365–1368. [Google Scholar] [CrossRef]
- Guo, X.; Zhou, H.; Fan, T.; Zhang, D. Electrochemical Detection of P-Nitrophenol on Surface Imprinted Gold with Lamellar-Ridge Architecture. Sens. Actuators B Chem. 2015, 220, 33–39. [Google Scholar] [CrossRef]
- Zhang, W.; Chang, J.; Chen, J.; Xu, F.; Wang, F.; Jiang, K.; Gao, Z. Graphene-Au Composite Sensor for Electrochemical Detection of Para-Nitrophenol. Res. Chem. Intermed. 2012, 38, 2443–2455. [Google Scholar] [CrossRef]
- Alam, M.K.; Rahman, M.M.; Abbas, M.; Torati, S.R.; Asiri, A.M.; Kim, D.; Kim, C.G. Ultra-Sensitive 2-Nitrophenol Detection Based on Reduced Graphene Oxide/ZnO Nanocomposites. J. Electroanal. Chem. 2017, 788, 66–73. [Google Scholar] [CrossRef]
- Hasan, Z.; Jhung, S.H. Removal of Hazardous Organics from Water Using Metal-Organic Frameworks (MOFs): Plausible Mechanisms for Selective Adsorptions. J. Hazard. Mater. 2015, 283, 329–339. [Google Scholar] [CrossRef]
- Hussain, S.; Muhammad Junaid, H.; Tahir Waseem, M.; Rauf, W.; Jabbar Shaikh, A.; Anjum Shahzad, S. Aggregation-Induced Emission of Quinoline Based Fluorescent and Colorimetric Sensors for Rapid Detection of Fe3+ and 4-Nitrophenol in Aqueous Medium. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 272, 121021. [Google Scholar] [CrossRef]
- Wang, C.; Xu, J.; Zhang, R.; Zhao, W. Facile and Low-Energy-Consumption Synthesis of Dual-Functional Carbon Dots from Cornus Walteri Leaves for Detection of p-Nitrophenol and Photocatalytic Degradation of Dyes. Colloids Surf. A Physicochem. Eng. Asp. 2022, 640, 128351. [Google Scholar] [CrossRef]
- Ma, F.; Jin, S.; Li, Y.; Feng, Y.; Tong, Y.; Ye, B.-C. Pyrolysis-Derived Materials of Mn-Doped ZIF-67 for the Electrochemical Detection of o-Nitrophenol. J. Electroanal. Chem. 2022, 904, 115932. [Google Scholar] [CrossRef]
- Narouie, S.; Hossein Rounaghi, G.; Saravani, H.; Shahbakhsh, M. Poly (Biphenol/Biphenoquinone - Vanadium (IV)) Modified Electrode as Selective Sensor for Detection of 4-Nitrophenol. Microchem. J. 2022, 172, 106945. [Google Scholar] [CrossRef]
- Dan, X.; Ruiyi, L.; Qinsheng, W.; Yongqiang, Y.; Guangli, W.; Zaijun, L. Thermal-Switchable Sensor Based on Palladium-Graphene Composite and Poly(N-Isopropylacrylamide) for Electrochemical Detection of 4-Nitrophenol. Microchem. J. 2022, 172, 106970. [Google Scholar] [CrossRef]
- Lei, L.; Li, C.; Huang, W.; Wu, K. Simultaneous Detection of 4-Chlorophenol and 4-Nitrophenol Using a Ti 3 C 2 T x MXene Based Electrochemical Sensor. Analyst 2021, 146, 7593–7600. [Google Scholar] [CrossRef]
- Xu, H.; Wang, H.; Lu, Y.; Zeng, Y.; Yang, Y.; Zhang, Z.; Wang, H.; Wang, X.; Li, L. CeO2 Quantum Dots for Highly Selective and Ultrasensitive Fluorescence Detection of 4-Nitrophenol via the Fluorescence Resonance Energy Transfer Mechanism. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 262, 120115. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.-L.; Xia, X.; Xu, Q.-Q.; Wang, J.; Sun, J.-C.; Zhang, Y.; Li, S.-S. Superior Conductivity FeSe2 for Highly Sensitive Electrochemical Detection of P-Nitrophenol and o-Nitrophenol Based on Synergistic Effect of Adsorption and Catalysis. Sens. Actuators B Chem. 2021, 348, 130692. [Google Scholar] [CrossRef]
- Antohe, I.; Iordache, I.; Antohe, V.-A.A.; Socol, G. A Polyaniline/Platinum Coated Fiber Optic Surface Plasmon Resonance Sensor for Picomolar Detection of 4-Nitrophenol. Sci. Rep. 2021, 11, 10086. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yang, C.; Chen, Y.; Zhu, Z.; Zhou, L. Cuttlefish Ink-Based N and S Co-Doped Carbon Quantum Dots as a Fluorescent Sensor for Highly Sensitive and Selective Para -Nitrophenol Detection. Anal. Methods 2021, 13, 5351–5359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.-Y.; Yi, F.-Y.; Liu, L.-J.; Yan, G.-P.; Liu, H.; Guo, J.-F. An Europium(iii) Metal–Organic Framework as a Multi-Responsive Luminescent Sensor for Highly Sensitive and Selective Detection of 4-Nitrophenol and I − and Fe 3+ Ions in Water. Dalt. Trans. 2021, 50, 15593–15601. [Google Scholar] [CrossRef]
- Amiripour, F.; Ghasemi, S.; Azizi, S.N. Förster Resonance Energy Transfer-Based Molecularly Imprinted Polymer/Amine-Functionalized Metal-Organic Framework Nanocomposite for Trace Level Detection of 4-Nitrophenol. Anal. Chim. Acta 2022, 1202, 339638. [Google Scholar] [CrossRef]
- Hashemzaei, Z.; Saravani, H.; Sharifitabar, M.; Shahbakhsh, M. Copper Nanowires/Poly (Naphtoquinone Chromium (III)) for Simultaneous Voltammetric Detection of Para - Aminophenol, Phenol and Para - Nitrophenol. Microchem. J. 2022, 175, 107210. [Google Scholar] [CrossRef]
- Peng, X.; Wang, Y.; Luo, Z.; Zhang, B.; Mei, X.; Yang, X. Facile Synthesis of Fluorescent Sulfur Quantum Dots for Selective Detection of P-Nitrophenol in Water Samples. Microchem. J. 2021, 170, 106735. [Google Scholar] [CrossRef]
- Fu, J.; Zhou, S.; Zhao, P.; Wu, X.; Tang, S.; Chen, S.; Yang, Z.; Zhang, Z. A Dual-Response Ratiometric Fluorescence Imprinted Sensor Based on Metal-Organic Frameworks for Ultrasensitive Visual Detection of 4-Nitrophenol in Environments. Biosens. Bioelectron. 2022, 198, 113848. [Google Scholar] [CrossRef]
- Cao, K.; Si, C.; Zhang, H.; Hu, J.; Zheng, D. An Electrochemical Sensor Based on a Glassy Carbon Electrode Modified with Optimized Cu–Fe3O4 Nanocomposite for 4-Nitrophenol Detection. J. Mater. Sci. Mater. Electron. 2022, 33, 2386–2398. [Google Scholar] [CrossRef]
- Ganash, A.A.; Aljubairy, R.A. Efficient Electrochemical Detection of Hazardous Para-Nitrophenol Based on a Carbon Paste Electrode Modified with Green Synthesized Gold/Iron Oxide Nanocomposite. Chem. Pap. 2022, 76, 3169–3183. [Google Scholar] [CrossRef]
- Nie, X.; Deng, P.; Wang, H.; Tang, Y. An Electrochemical Sensor Based on a Nitrogen-Doped Carbon Material and PEI Composites for Sensitive Detection of 4-Nitrophenol. Nanomaterials 2021, 12, 86. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Huang, T.; Meng, M.; Yan, Y. Fluorescent Polydopamine Based Molecularly Imprinted Sensor for Ultrafast and Selective Detection of P-Nitrophenol in Drinking Water. Microchim. Acta 2022, 189, 25. [Google Scholar] [CrossRef] [PubMed]
- Coats, A.W.; Redfern, J.P. Thermogravimetric Analysis. A Review. Analyst 1963, 88, 906. [Google Scholar] [CrossRef]
- Mohamed, M.H.; Dolatkhah, A.; Aboumourad, T.; Dehabadi, L.; Wilson, L.D. Investigation of Templated and Supported Polyaniline Adsorbent Materials. RSC Adv. 2015, 5, 6976–6984. [Google Scholar] [CrossRef]
- Wyrzykowski, D.; Hebanowska, E.; Nowak-Wiczk, G.; Makowski, M.; Chmurzyński, L. Thermal Behaviour of Citric Acid and Isomeric Aconitic Acids. J. Therm. Anal. Calorim. 2011, 104, 731–735. [Google Scholar] [CrossRef] [Green Version]
- Heydari Gharahcheshmeh, M.; Gleason, K.K. Texture and Nanostructural Engineering of Conjugated Conducting and Semiconducting Polymers. Mater. Today Adv. 2020, 8, 100086. [Google Scholar] [CrossRef]
- Trchová, M.; Stejskal, J. Polyaniline: The Infrared Spectroscopy of Conducting Polymer Nanotubes (IUPAC Technical Report). Pure Appl. Chem. 2011, 83, 1803–1817. [Google Scholar] [CrossRef]
- Sima, F.; Ristoscu, C.; Duta, L.; Gallet, O.; Anselme, K.; Mihailescu, I.N. Laser Thin Films Deposition and Characterization for Biomedical Applications. In Laser Surface Modification of Biomaterials; Elsevier: Amsterdam, The Netherlands, 2016; pp. 77–125. ISBN 9780081009420. [Google Scholar]
- Kora, A.J.; Beedu, S.R.; Jayaraman, A. Size-Controlled Green Synthesis of Silver Nanoparticles Mediated by Gum Ghatti (Anogeissus Latifolia) and Its Biological Activity. Org. Med. Chem. Lett. 2012, 2, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zujovic, Z.; Kilmartin, P.A.; Travas-Sejdic, J. The Applications of Solid-State NMR to Conducting Polymers. The Special Case on Polyaniline. Molecules 2020, 25, 444. [Google Scholar] [CrossRef] [Green Version]
- Wishart, D.S.; Knox, C.; Guo, A.C.; Eisner, R.; Young, N.; Gautam, B.; Hau, D.D.; Psychogios, N.; Dong, E.; Bouatra, S.; et al. HMDB: A Knowledgebase for the Human Metabolome. Nucleic Acids Res. 2009, 37, D603–D610. [Google Scholar] [CrossRef]
- Stejskal, J. Conducting Polymer-Silver Composites. Chem. Pap. 2013, 67, 814–848. [Google Scholar] [CrossRef]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Appel, J. Freundlich’s Adsorption Isotherm. Surf. Sci. 1973, 39, 237–244. [Google Scholar] [CrossRef]
- Sips, R. On the Structure of a Catalyst Surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Waware, U.S.; Arukula, R.; Hamouda, A.M.S.; Kasak, P. Electrochemical and X-Ray Photoelectron Spectroscopic Investigations of Conductive Polymers. Ionics (Kiel) 2020, 26, 831–838. [Google Scholar] [CrossRef] [Green Version]
- Neoh, K.G.; Kang, E.T.; Tan, K.L. Spectroscopic Studies of Protonation, Oxidation and Light Irradiation of Polyaniline Solutions. Polymer (Guildf) 1992, 33, 2292–2298. [Google Scholar] [CrossRef]
- Wan, M. Absorption Spectra of Thin Film of Polyaniline. J. Polym. Sci. Part A Polym. Chem. 1992, 30, 543–549. [Google Scholar] [CrossRef]
- Cao, Y.; Smith, P.; Heeger, A.J. Spectroscopic Studies of Polyaniline in Solution and in Spin-Cast Films. Synth. Met. 1989, 32, 263–281. [Google Scholar] [CrossRef]
- Masters, J.G.; Sun, Y.; MacDiarmid, A.G.; Epstein, A.J. Polyaniline: Allowed Oxidation States. Synth. Met. 1991, 41, 715–718. [Google Scholar] [CrossRef]
- Monkman, A.; Adams, P. Structural Characterisation of Polyaniline Free Standing Films. Synth. Met. 1991, 41, 891–896. [Google Scholar] [CrossRef]
- Stejskal, J.; Kratochvíl, P.; Radhakrishnan, N. Polyaniline Dispersions 2. UV—Vis Absorption Spectra. Synth. Met. 1993, 61, 225–231. [Google Scholar] [CrossRef]
- Stafström, S.; Brédas, J.L.; Epstein, A.J.; Woo, H.S.; Tanner, D.B.; Huang, W.S.; MacDiarmid, A.G. Polaron Lattice in Highly Conducting Polyaniline: Theoretical and Optical Studies. Phys. Rev. Lett. 1987, 59, 1464–1467. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.C.; Cushman, R.J.; Zhang, D. Experimentally Determined PH-Potential Phase Diagram for Polyaniline; Clues for Optically Distinguishable Sub-Phase in the Conductor Form. Synth. Met. 1989, 29, 401–408. [Google Scholar] [CrossRef]
- Foix, D.; Martinez, H.; Pradel, A.; Ribes, M.; Gonbeau, D. XPS Valence Band Spectra and Theoretical Calculations for Investigations on Thiogermanate and Thiosilicate Glasses. Chem. Phys. 2006, 323, 606–616. [Google Scholar] [CrossRef]
- Briggs, D. Surface Analysis of Polymers by XPS and Static SIMS; Cambridge University Press: Cambridge, UK, 1998; ISBN 9780521352222. [Google Scholar]
- Taguchi, M.; Takata, Y.; Chainani, A. Hard X-Ray Photoelectron Spectroscopy: A Few Recent Applications. J. Electron Spectros. Relat. Phenom. 2013, 190, 242–248. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications. Surf. Technol. 1983, 20, 91–92. [Google Scholar]
- Xu, R.; Wang, D.; Zhang, J.; Li, Y. Shape-Dependent Catalytic Activity of Silver Nanoparticles for the Oxidation of Styrene. Chem. Asian J. 2006, 1, 888–893. [Google Scholar] [CrossRef] [PubMed]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A Practical Beginner’s Guide to Cyclic Voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Gautam, V.; Singh, K.P.; Yadav, V.L. Multicomponent Template Effects - Preparation of Highly Porous Polyaniline Nanorods Using Crude Lemon Juice and Its Application for Selective Detection of Catechol. ACS Sustain. Chem. Eng. 2018, 6, 2256–2268. [Google Scholar] [CrossRef]
- Dobre, N.; Golgovici, F.; Anicap, L.; Buda, M. Cyclic Voltammetry of Silver Nanoparticles on Platinum, Gold and Glassy Carbon Electrodes. Rev. Chim. 2014, 65, 578–581. [Google Scholar]
- Jiang, W.; Chen, P.; Li, X.; Wu, G.; Zheng, H.; Li, C.; Fang, L.; Jiang, H. π-Conjugation Extension and Defects Introduction into g-C3N4 by Phenanthroline Molecular Doping to Form a Metal-Free Electrochemical Sensor towards Effective 4-Nitrophenol Detection. Diam. Relat. Mater. 2021, 119, 108557. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, Y.; Souri, M.; Luo, X.; Boehm, A.M.; Li, R.; Zhang, Y.; Wang, T.; Kim, D.Y.; Mei, J.; et al. Influence of Dopant Size and Electron Affinity on the Electrical Conductivity and Thermoelectric Properties of a Series of Conjugated Polymers. J. Mater. Chem. A 2018, 6, 16495–16505. [Google Scholar] [CrossRef]
- Sapurina, I.; Stejskal, J. The Mechanism of the Oxidative Polymerization of Aniline and the Formation of Supramolecular Polyaniline Structures. Polym. Int. 2008, 57, 1295–1325. [Google Scholar] [CrossRef]
- Marimuthu, S.; Antonisamy, A.J.; Malayandi, S.; Rajendran, K.; Tsai, P.-C.; Pugazhendhi, A.; Ponnusamy, V.K. Silver Nanoparticles in Dye Effluent Treatment: A Review on Synthesis, Treatment Methods, Mechanisms, Photocatalytic Degradation, Toxic Effects and Mitigation of Toxicity. J. Photochem. Photobiol. B Biol. 2020, 205, 111823. [Google Scholar] [CrossRef] [PubMed]
- Dolatkhah, A.; Jani, P.; Wilson, L.D. Redox-Responsive Polymer Template as an Advanced Multifunctional Catalyst Support for Silver Nanoparticles. Langmuir 2018, 34, 10560–10568. [Google Scholar] [CrossRef] [PubMed]











| Composite | Weight (%) of Various Components | Aniline: CHI Feed Ratio | PANI: Non- PANI Ratio | |||
|---|---|---|---|---|---|---|
| TGA Thermal Events | AAS Result | |||||
| First | Second | Third | Ag Content | |||
| Water | non-PANI | PANI | ||||
| Ag@PANI | 1.9 | 24.2 | 67.7 | 6.2 | ∞ | 2.8 |
| Ag@P-CA | 0.51 | 8.3 | 86.1 | 5.1 | ∞ | 10.4 |
| Composites | Sips Isotherm | |||
|---|---|---|---|---|
| KS | Qm (µmol·g−1) | ns | Adj R2 | |
| PANI | 0.00272 ± 0.00029 | 515 ± 23 | 1.56 ± 0.24 | 0.977 |
| Ag@P-CA | 0.00057 ± 0.00036 | 1520 ± 371 | 0.85 ± 0.15 | 0.981 |
| Ag@PANI | 0.00186 ± 0.00035 | 733 ± 56 | 1.25 ± 0.21 | 0.975 |
| Highlighted Region | Spectral Range (nm) | Energy Range (eV) | Spectral Band Assignment | Samples without Relative Absorption Bands |
|---|---|---|---|---|
| Red (I, IV) [64,65] | 500–600 | 2.1–2.5 | PG form of PANI | P-CA |
| Blue (V) [58,59,60,61] | 610–650 | 1.9–2.0 | Quinoid units | none |
| Yellow (II) [59,62] | 315–360 | 3.4–3.9 | Benzenoid units | none |
| Green (III) [63,64,65] | 400–430 | 2.9–3.1 | Polarons | none |
| Sample | Estimate of Half the Bandgap Energy (eV) | Estimate of Bandgap Energy (eV) |
|---|---|---|
| PANI | 2.3 | 4.6 |
| P-CA | 0.2 | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khani, M.; Sammynaiken, R.; Wilson, L.D. Electrocatalytic Oxidation of Nitrophenols via Ag Nanoparticles Supported on Citric-Acid-Modified Polyaniline. Catalysts 2023, 13, 465. https://doi.org/10.3390/catal13030465
Khani M, Sammynaiken R, Wilson LD. Electrocatalytic Oxidation of Nitrophenols via Ag Nanoparticles Supported on Citric-Acid-Modified Polyaniline. Catalysts. 2023; 13(3):465. https://doi.org/10.3390/catal13030465
Chicago/Turabian StyleKhani, Milad, Ramaswami Sammynaiken, and Lee D. Wilson. 2023. "Electrocatalytic Oxidation of Nitrophenols via Ag Nanoparticles Supported on Citric-Acid-Modified Polyaniline" Catalysts 13, no. 3: 465. https://doi.org/10.3390/catal13030465