Efficient Pyrolysis of Low-Density Polyethylene for Regulatable Oil and Gas Products by ZSM-5, HY and MCM-41 Catalysts
Abstract
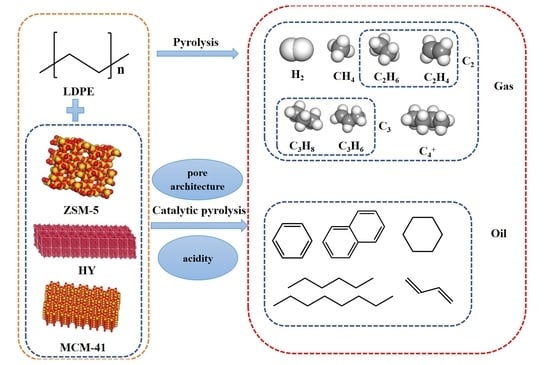
:1. Introduction
2. Results and Discussion
2.1. BET Results
2.2. Acid Properties of Zeolites
2.3. SEM and TEM Results
2.4. Effects of Temperature on Gas–Liquid–Solid Three-Phase Yield of LDPE Pyrolysis
2.5. Effects of Catalyst on the Composition and Quality of Gaseous Products
2.6. Effects of Catalyst on Oil Distribution and Quantity
2.7. Effect of Catalysts on Some Reaction Pathways
3. Materials and Methods
3.1. Materials
3.2. Experimental Setup
3.3. Characterization
3.4. Product Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aboul-Enein, A.A.; Awadallah, A.E. Production of nanostructured carbon materials using Fe–Mo/MgO catalysts via mild catalytic pyrolysis of polyethylene waste. Chem. Eng. J. 2018, 354, 802–816. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, J.; Williams, P.T. Fe–Ni–MCM-41 Catalysts for Hydrogen-Rich Syngas Production from Waste Plastics by Pyrolysis–Catalytic Steam Reforming. Energy Fuels 2017, 31, 8497–8504. [Google Scholar] [CrossRef]
- Maqsood, T.; Dai, J.; Zhang, Y.; Guang, M.; Li, B. Pyrolysis of plastic species: A review of resources and products. J. Anal. Appl. Pyrolysis 2021, 159, 105295. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, Y.; Ke, L.; Dai, L.; Wu, Q.; Cobb, K.; Zeng, Y.; Zou, R.; Liu, Y.; Ruan, R. A review on catalytic pyrolysis of plastic wastes to high-value products. Energy Convers. Manag. 2022, 254, 115243. [Google Scholar] [CrossRef]
- Tao, L.; Ma, X.; Ye, L.; Jia, J.; Wang, L.; Ma, P.; Liu, J. Interactions of lignin and LDPE during catalytic co-pyrolysis: Thermal behavior and kinetics study by TG-FTIR. J. Anal. Appl. Pyrolysis 2021, 158, 105267. [Google Scholar] [CrossRef]
- Sogancioglu, M.; Yel, E.; Ahmetli, G. Pyrolysis of waste high density polyethylene (HDPE) and low density polyethylene (LDPE) plastics and production of epoxy composites with their pyrolysis chars. J. Clean. Prod. 2017, 165, 369–381. [Google Scholar] [CrossRef]
- Miandad, R.; Rehan, M.; Barakat, M.A.; Aburiazaiza, A.S.; Khan, H.; Ismail, I.M.I.; Dhavamani, J.; Gardy, J.; Hassanpour, A.; Nizami, A.-S. Catalytic Pyrolysis of Plastic Waste: Moving Toward Pyrolysis Based Biorefineries. Front. Energy Res. 2019, 7, 27. [Google Scholar] [CrossRef]
- Miandad, R.; Barakat, M.A.; Aburiazaiza, A.S.; Rehan, M.; Nizami, A.S. Catalytic pyrolysis of plastic waste: A review. Process Saf. Environ. Prot. 2016, 102, 822–838. [Google Scholar] [CrossRef]
- Li, D.; Lei, S.; Wang, P.; Zhong, L.; Ma, W.; Chen, G. Study on the pyrolysis behaviors of mixed waste plastics. Renew. Energy 2021, 173, 662–674. [Google Scholar] [CrossRef]
- De Souza, M.J.B.; Silva, T.H.A.; Ribeiro, T.R.S.; Da Silva, A.O.S.; Pedrosa, A.M.G. Thermal and catalytic pyrolysis of polyvinyl chloride using micro/mesoporous ZSM-35/MCM-41 catalysts. J. Therm. Anal. Calorim. 2019, 140, 167–175. [Google Scholar] [CrossRef]
- Anuar Sharuddin, S.D.; Abnisa, F.; Wan Daud, W.M.A.; Aroua, M.K. A review on pyrolysis of plastic wastes. Energy Convers. Manag. 2016, 115, 308–326. [Google Scholar] [CrossRef]
- Nishu; Li, Y.; Liu, R. Catalytic pyrolysis of lignin over ZSM-5, alkali, and metal modified ZSM-5 at different temperatures to produce hydrocarbons. J. Energy Inst. 2022, 101, 111–121. [Google Scholar] [CrossRef]
- Ding, Y.L.; Wang, H.Q.; Xiang, M.; Yu, P.; Li, R.Q.; Ke, Q.P. The Effect of Ni-ZSM-5 Catalysts on Catalytic Pyrolysis and Hydro-Pyrolysis of Biomass. Front. Chem. 2020, 8, 790. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Gamliel, D.P.; Valla, J.A.; Bollas, G.M. The effect of ZSM-5 catalyst support in catalytic pyrolysis of biomass and compounds abundant in pyrolysis bio-oils. J. Anal. Appl. Pyrolysis 2016, 122, 7–12. [Google Scholar] [CrossRef]
- Wei, B.; Jin, L.; Wang, D.; Hu, H. Catalytic upgrading of lignite pyrolysis volatiles over modified HY zeolites. Fuel 2020, 259, 116234. [Google Scholar] [CrossRef]
- Ding, K.; Liu, S.; Huang, Y.; Liu, S.; Zhou, N.; Peng, P.; Wang, Y.; Chen, P.; Ruan, R. Catalytic microwave-assisted pyrolysis of plastic waste over NiO and HY for gasoline-range hydrocarbons production. Energy Convers. Manag. 2019, 196, 1316–1325. [Google Scholar] [CrossRef]
- Chi, Y.; Xue, J.; Zhuo, J.; Zhang, D.; Liu, M.; Yao, Q. Catalytic co-pyrolysis of cellulose and polypropylene over all-silica mesoporous catalyst MCM-41 and Al-MCM-41. Sci. Total Environ. 2018, 633, 1105–1113. [Google Scholar] [CrossRef]
- Yu, L.; Farinmade, A.; Ajumobi, O.; Su, Y.; John, V.T.; Valla, J.A. MCM-41/ZSM-5 composite particles for the catalytic fast pyrolysis of biomass. Appl. Catal. A Gen. 2020, 602, 117727. [Google Scholar] [CrossRef]
- Li, X.; Dong, L.; Zhang, J.; Hu, C.; Zhang, X.; Cai, Y.; Shao, S. In-situ catalytic upgrading of biomass-derived vapors using HZSM-5 and MCM-41: Effects of mixing ratios on bio-oil preparation. J. Energy Inst. 2019, 92, 136–143. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Han, T.U.; Kim, S.; Jae, J.; Jeon, J.-K.; Jung, S.-C.; Park, Y.-K. Catalytic co-pyrolysis of epoxy-printed circuit board and plastics over HZSM-5 and HY. J. Clean. Prod. 2017, 168, 366–374. [Google Scholar] [CrossRef]
- Li, K.; Lei, J.; Yuan, G.; Weerachanchai, P.; Wang, J.-Y.; Zhao, J.; Yang, Y. Fe-, Ti-, Zr- and Al-pillared clays for efficient catalytic pyrolysis of mixed plastics. Chem. Eng. J. 2017, 317, 800–809. [Google Scholar] [CrossRef]
- Kelkar, S.; Saffron, C.M.; Andreassi, K.; Li, Z.; Murkute, A.; Miller, D.J.; Pinnavaia, T.J.; Kriegel, R.M. A survey of catalysts for aromatics from fast pyrolysis of biomass. Appl. Catal. B Environ. 2015, 174–175, 85–95. [Google Scholar] [CrossRef]
- Abdalla, A.; Arudra, P.; Al-Khattaf, S.S. Catalytic cracking of 1-butene to propylene using modified H-ZSM-5 catalyst: A comparative study of surface modification and core-shell synthesis. Appl. Catal. A Gen. 2017, 533, 109–120. [Google Scholar] [CrossRef]
- Gurdeep Singh, H.K.; Yusup, S.; Quitain, A.T.; Abdullah, B.; Ameen, M.; Sasaki, M.; Kida, T.; Cheah, K.W. Biogasoline production from linoleic acid via catalytic cracking over nickel and copper-doped ZSM-5 catalysts. Environ. Res. 2020, 186, 109616. [Google Scholar] [CrossRef]
- Li, W.; Zheng, J.; Luo, Y.; Tu, C.; Zhang, Y.; Da, Z. Hierarchical Zeolite Y with Full Crystallinity: Formation Mechanism and Catalytic Cracking Performance. Energy Fuels 2017, 31, 3804–3811. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, G.; Qin, L.; Li, H.; Chen, Y.; Liu, B. Synthesis and catalytic cracking performance of mesoporous zeolite Y. Catal. Commun. 2016, 73, 98–102. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, M.; Shao, J.; Yang, H.; Zeng, K.; Chen, Y.; Luo, J.; Agblevor, F.A.; Chen, H. The conversion of biomass to light olefins on Fe-modified ZSM-5 catalyst: Effect of pyrolysis parameters. Sci. Total Environ. 2018, 628, 350–357. [Google Scholar] [CrossRef]
- Wong, S.L.; Ngadi, N.; Abdullah, T.A.T.; Inuwa, I.M. Conversion of low density polyethylene (LDPE) over ZSM-5 zeolite to liquid fuel. Fuel 2017, 192, 71–82. [Google Scholar] [CrossRef]
- Roozbehani, B.; Sakaki, S.A.; Shishesaz, M.; Abdollahkhani, N.; Hamedifar, S. Taguchi method approach on catalytic degradation of polyethylene and polypropylene into gasoline. Clean Technol. Environ. Policy 2015, 17, 1873–1882. [Google Scholar] [CrossRef]
- Mo, Y.; Zhao, L.; Wang, Z.; Chen, C.L.; Tan, G.Y.; Wang, J.Y. Enhanced styrene recovery from waste polystyrene pyrolysis using response surface methodology coupled with Box-Behnken design. Waste Manag. 2014, 34, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-P.; Cao, J.-P.; Wei, F.; Zhao, X.-Y.; Feng, X.-B.; Huang, X.; Zhao, M.; Wei, X.-Y. Sulfation-acidified HZSM-5 catalyst for in-situ catalytic conversion of lignite pyrolysis volatiles to light aromatics. Fuel 2019, 255, 115784. [Google Scholar] [CrossRef]
- Tang, Z.; Chen, W.; Hu, J.; Li, S.; Chen, Y.; Yang, H.; Chen, H. Co-pyrolysis of microalgae with low-density polyethylene (LDPE) for deoxygenation and denitrification. Bioresour. Technol. 2020, 311, 123502. [Google Scholar] [CrossRef]
- Song, J.; Sima, J.; Pan, Y.; Lou, F.; Du, X.; Zhu, C.; Huang, Q. Dielectric Barrier Discharge Plasma Synergistic Catalytic Pyrolysis of Waste Polyethylene into Aromatics-Enriched Oil. ACS Sustain. Chem. Eng. 2021, 9, 11448–11457. [Google Scholar] [CrossRef]
- Namchot, W.; Jitkarnka, S. Catalytic pyrolysis of waste tire using HY/MCM-41 core-shell composite. J. Anal. Appl. Pyrolysis 2016, 121, 297–306. [Google Scholar] [CrossRef]
- Wei, B.; Yang, H.; Hu, H.; Wang, D.; Jin, L. Enhanced production of light tar from integrated process of in-situ catalytic upgrading lignite tar and methane dry reforming over Ni/mesoporous Y. Fuel 2020, 279, 118533. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, L.; Bai, Y.; Li, F. Catalytic upgrading of volatile from coal pyrolysis over faujasite zeolites. J. Anal. Appl. Pyrolysis 2018, 132, 184–189. [Google Scholar] [CrossRef]
- Singh, R.K.; Ruj, B. Time and temperature depended fuel gas generation from pyrolysis of real world municipal plastic waste. Fuel 2016, 174, 164–171. [Google Scholar] [CrossRef]
- Lok, C.M.; Van Doorn, J.; Aranda Almansa, G. Promoted ZSM-5 catalysts for the production of bio-aromatics, a review. Renew. Sustain. Energy Rev. 2019, 113, 109248. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, X.; Chen, L.; Zhao, B.; Yang, S.; Xie, X. Comparision of catalytic fast pyrolysis of biomass to aromatic hydrocarbons over ZSM-5 and Fe/ZSM-5 catalysts. J. Anal. Appl. Pyrolysis 2016, 121, 342–346. [Google Scholar] [CrossRef]
- Xu, D.; Yang, S.; Su, Y.; Shi, L.; Zhang, S.; Xiong, Y. Simultaneous production of aromatics-rich bio-oil and carbon nanomaterials from catalytic co-pyrolysis of biomass/plastic wastes and in-line catalytic upgrading of pyrolysis gas. Waste Manag. 2021, 121, 95–104. [Google Scholar] [CrossRef]
- Liu, W.-W.; Hu, C.-W.; Yang, Y.; Tong, D.-M.; Zhu, L.-F.; Zhang, R.-N.; Zhao, B.-H. Study on the effect of metal types in (Me)-Al-MCM-41 on the mesoporous structure and catalytic behavior during the vapor-catalyzed co-pyrolysis of pubescens and LDPE. Appl. Catal. B Environ. 2013, 129, 202–213. [Google Scholar] [CrossRef]
- Sun, K.; Themelis, N.J.; Bourtsalas, A.C.; Huang, Q. Selective production of aromatics from waste plastic pyrolysis by using sewage sludge derived char catalyst. J. Clean. Prod. 2020, 268, 122038. [Google Scholar] [CrossRef]
- Casoni, A.I.; Nievas, M.L.; Moyano, E.L.; Álvarez, M.; Diez, A.; Dennehy, M.; Volpe, M.A. Catalytic pyrolysis of cellulose using MCM-41 type catalysts. Appl. Catal. A Gen. 2016, 514, 235–240. [Google Scholar] [CrossRef]








| Catalysts | BET Surface Area (m2/g) | Total Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| ZSM-5 | 361 | 0.206 | 0.411 |
| HY | 701 | 0.392 | 0.811 |
| MCM-41 | 962 | 0.718 | 3.653 |
| Catalysts | Acid Content (μmol/g) | |||
|---|---|---|---|---|
| Weak Acidity | Medium Acidity | Strong Acidity | Total Acidity | |
| ZSM-5 | 368 | 838 | 549 | 1755 |
| HY | 366 | 748 | 652 | 1766 |
| MCM-41 | 138 | 221 | - | 359 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Li, Y.; Zhou, Y.; Deng, S.; Zhang, H. Efficient Pyrolysis of Low-Density Polyethylene for Regulatable Oil and Gas Products by ZSM-5, HY and MCM-41 Catalysts. Catalysts 2023, 13, 382. https://doi.org/10.3390/catal13020382
Liu T, Li Y, Zhou Y, Deng S, Zhang H. Efficient Pyrolysis of Low-Density Polyethylene for Regulatable Oil and Gas Products by ZSM-5, HY and MCM-41 Catalysts. Catalysts. 2023; 13(2):382. https://doi.org/10.3390/catal13020382
Chicago/Turabian StyleLiu, Ting, Yincui Li, Yifan Zhou, Shengnan Deng, and Huawei Zhang. 2023. "Efficient Pyrolysis of Low-Density Polyethylene for Regulatable Oil and Gas Products by ZSM-5, HY and MCM-41 Catalysts" Catalysts 13, no. 2: 382. https://doi.org/10.3390/catal13020382