Well-Defined Ultrasmall V-NiP2 Nanoparticles Anchored g-C3N4 Nanosheets as Highly Efficient Visible-Light-Driven Photocatalysts for H2 Evolution
Abstract
:1. Introduction
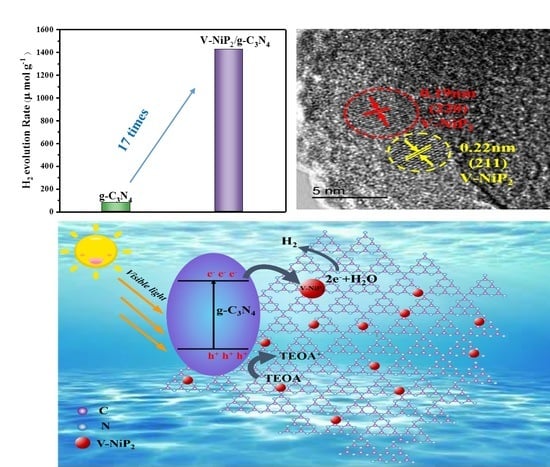
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Synthesis of the V-NiP2/g-C3N4 Composite
3.2.1. Synthesis of Bulk g-C3N4
3.2.2. Synthesis of the NiV-LDH/g-C3N4 Precursor
3.2.3. Synthesis of V-NiP2/g-C3N4 Composite
3.3. Characterizations
3.4. Photocatalytic Hydrogen Evolution Measurements
3.5. Photoelectrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.; Gong, Y.; Zhang, L.; Gao, H.; Yang, G.; Fang, B. Semiconductor polymeric graphitic carbon nitride photocatalysts: The “holy grail” for the photocatalytic hydrogen evolution reaction under visible light. Energy Environ. Sci. 2019, 12, 2080–2147. [Google Scholar] [CrossRef]
- Wang, W.; Bai, X.; Ci, Q.; Du, L.; Ren, X.; Phillips, D. Near-Field Drives Long-Lived Shallow Trapping of Polymeric C3N4 for Efficient Photocatalytic Hydrogen Evolution. Adv. Funct. Mater. 2021, 31, 795–802. [Google Scholar] [CrossRef]
- Shao, Z.; Meng, X.; Lai, H.; Zhang, D.; Pu, X.; Su, C.; Li, H.; Ren, X.; Geng, Y. Coralline-like Ni2P decorated novel tetrapod-bundle Cd0.9Zn0.1S ZB/WZ homojunctions for highly efficient visible-light photocatalytic hydrogen evolution. Chin. J. Catal. 2021, 42, 439–449. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, X.; Cao, Z. Unraveling the Catalytic Performance of the Nonprecious Metal Single-Atom-Embedded Graphitic s-Triazine-Based C3N4 for CO2 Hydrogenation. ACS Appl. Mater. Inter. 2022, 14, 35844–35853. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Qiu, B.; Du, M.; Ji, J.; Muhammad Nasir, M. Xing, J. Zhang. Dopant-Induced Edge and Basal Plane Catalytic Sites on Ultrathin C3N4 Nanosheets for Photocatalytic Water Reduction. ACS Sustain. Chem. Eng. 2020, 8, 7497–7502. [Google Scholar] [CrossRef]
- Liu, E.; Chen, J.; Ma, Y.; Feng, J.; Jia, J.; Fan, J.; Hu, X. Fabrication of 2D SnS2/g-C3N4 heterojunction with enhanced H2 evolution during photocatalytic water splitting. J. Colliod Interf. Sci. 2018, 524, 313–324. [Google Scholar] [CrossRef]
- Wang, X.; Wang, F.; Sang, Y.; Liu, H. Full-Spectrum Solar-Light-Activated Photocatalysts for Light Chemical Energy Conversion. Adv. Energy Mater. 2017, 7, 1700473–1700488. [Google Scholar] [CrossRef]
- Zou, Q.; Feng, K.; Zhong, J.; Mai, Y.; Zhou, Y. Single-Metal-Atom Polymeric Unimolecular Micelles for Switchable Photocatalytic H2 Evolution. CCS Chem. 2020, 2, 1963–1971. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, H.; Cui, G.; Dong, Y.; Wang, G.; Jiang, P.; Wu, X.; Zhao, N. A photochemical synthesis route to typical transition metal sulfides as highly efficient cocatalyst for hydrogen evolution: From the case of NiS/g-C3N4. Appl. Catal. B-Environ. 2018, 225, 284–290. [Google Scholar] [CrossRef]
- Chen, M.; Guo, C.; Hou, S.; Lv, J.; Zhang, Y.; Zhang, H.; Xu, J. A novel Z-scheme AgBr/P-g-C3N4 heterojunction photocatalyst: Excellent photocatalytic performance and photocatalytic mechanism for ephedrine degradation. Appl. Catal. B-Environ. 2020, 266, 118614. [Google Scholar] [CrossRef]
- Ran, J.; Ma, T.; Guo, G.; Du, X.; Qiao, S. Porous P-doped graphitic carbon nitride nanosheets for synergistically enhanced visible-light photocatalytic H2 production. Energy Environ. Sci. 2015, 8, 3708–3717. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Yan, J.; Zhang, X.; Zhao, M.; Jiang, Q. Ag2O modified g-C3N4 for highly efficient photocatalytic hydrogen generation under visible light irradiation. J. Mater. Chem. A 2015, 3, 15710–15714. [Google Scholar] [CrossRef]
- Xue, W.; Hu, X.; Liu, E.; Fan, J. Novel reduced graphene oxidesupported Cd0.5Zn0.5S/g-C3N4 Z-scheme heterojunction photocatalyst for enhanced hydrogen evolution. Appl. Surf. Sci. 2018, 447, 783–794. [Google Scholar] [CrossRef]
- Zhou, W.; Jia, T.; Zhang, D.; Zheng, Z.; Hong, W.; Chen, X. The enhanced cocatalyst free photocatalytic hydrogen evolution and stability based on indenofluorene-containing donor-acceptor conjugated polymer dots/g-C3N4 nanosheets heterojunction. Appl. Catal. B-Environ. 2019, 259, 118067. [Google Scholar] [CrossRef]
- Ye, S.; Wang, R.; Wu, M. A review on g-C3N4 for photocatalytic water splitting and CO2 reduction. Appl. Surf. Sci. 2015, 358, 15–27. [Google Scholar] [CrossRef]
- Zheng, Y.; Lin, L.; Wang, B. Graphitic carbon nitride polymers toward sustainable photoredox catalysis. Angew. Chem. Inter. Ed. 2015, 54, 12868–12884. [Google Scholar] [CrossRef]
- Kong, L.; Dong, Y.; Jiang, P.; Wang, G.; Zhang, H. Light-assisted rapid preparation of a Ni/g-C3N4 magnetic composite for robust photocatalytic H2 evolution from water. J. Mater. Chem. A 2016, 4, 9998–10007. [Google Scholar] [CrossRef]
- Zhao, H.; Dong, Y.; Jiang, P. In situ light-assis-ted preparation of MoS2 on graphitic C3N4 nanosheets for enhanced photocatalytic H2 production from water. J. Mater. Chem. A 2015, 14, 7375–7381. [Google Scholar] [CrossRef]
- Dong, Y.; Kong, L.; Wang, G.; Jiang, P.; Zhao, N.; Zhang, H. Photochemical synthe-sis of CoxP as cocatalyst for boosting photocatalytic H2 production via spatial charge separation. Appl. Catal. B Environ. 2017, 211, 245–251. [Google Scholar] [CrossRef]
- Tian, L.; Min, S.; Wang, F.; Zhang, Z. Metallic vanadium nitride as a noble-metal-free cocatalyst efficiently catalyze photocatalytic hydrogen production with CdS nanoparticles under visible light irradiation. J. Phys. Chem. C 2019, 123, 28640–28650. [Google Scholar] [CrossRef]
- Shen, R.; Xie, J.; Zhang, H.; Zhang, A.; Chen, X.; Li, X. Enhanced solar fuel H2 generation over g-C3N4 nanosheet photocatalysts by the synergetic effect of noble metal-free Co2P cocatalyst and the environmental phosphorylation strategy. ACS Sustain. Chem. Eng. 2017, 6, 816–826. [Google Scholar] [CrossRef]
- Dong, H.; Hong, S.; Zou, Y.; Zhang, X.; Lu, Z.; Han, J.; Wang, L.; Ni, L.; Li, C.; Wang, Y. Fabrication of 2D/0D Heterojunction Based on the Dual Controls of Micro/Nano-Morphology and Structure Towards High-Efficiency Photocatalytic H2 Production. ChemCatChem 2019, 11, 5651–5660. [Google Scholar] [CrossRef]
- Pi, M.; Zhang, D.; Wang, S.; Chen, S. Enhancing electrocatalytic hydrogen evolution of WP2 three-dimensional nanowire arrays via mo doping—Sciencedirect. Mater. Lett. 2018, 213, 315–318. [Google Scholar] [CrossRef]
- Zeng, D.; Zhou, T.; Ong, W.; Mingda, W.; Duan, X.; Xu, W.; Chen, Y.; Zhu, Y. Sub-5 nm ultra-fine FeP nanodots as efficient co-catalysts modified porous g-C3N4 for precious-metal-free photocatalytic hydrogen evolution under visible light. ACS Appl. Mater. Interfaces 2019, 11, 5651–5660. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Shen, J.; Liu, Q.Q.; Yang, X.F.; Tang, H. Porous MoP network structure as co-catalyst for H2 evolution over g-C3N4 nanosheets. Appl. Surf. Sci. 2018, 462, 822–830. [Google Scholar] [CrossRef]
- Yue, X.Z.; Yi, S.S.; Wang, R.W.; Zhang, Z.T.; Qiu, S.L. A novel and highly efficient earth-abundant Cu3P with TiO2 “P-N” heterojunction nanophotocatalyst for hydrogen evolution from water. Nanoscale 2016, 8, 17516–17523. [Google Scholar] [CrossRef]
- Alshorifi, T.; Alswat, A.; Mannaa, A.; Alotaibi, T.; El-Bahy, M.; Salama, S. Facile and Green Synthesis of Silver Quantum Dots Immobilized onto a Polymeric CTS–PEO Blend for the Photocatalytic Degradation ofFacile and Green Synthesis of Silver Quantum Dots Immobilized onto a Polymeric CTS–PEO Blend for the Photocatalytic Degradation of p-Nitrophenol. ACS Omega 2022, 6, 30432–30441. [Google Scholar] [CrossRef]
- Mannaa, A.; Qasim, F.; Alshorifi, T.; I-Bahy, M.E.; Salama, S. Role of NiO Nanoparticles in Enhancing Structure Properties of TiO2 and Its Applications in Photodegradation and Hydrogen Evolution. ACS Omega 2022, 6, 30386–30400. [Google Scholar] [CrossRef]
- El-Hakam, A.; Shorifi, T.A.L.; Salama, S.; Gamal, S.; El-Yazeed, W.S.A.; Ibrahim, A.A.; Ahmed, I. Application of nanostructured mesoporous silica/bismuth vanadate composite catalysts for the degradation of methylene blue and brilliant green. J. Mater. Res. Technol. 2022, 18, 1963–1976. [Google Scholar] [CrossRef]
- Yan, X.; Jin, Z. Interface Engineering: NiAl-LDH in-situ derived NiP2 quantum dots and Cu3P nanoparticles ingeniously constructed p-n heterojunction for photocatalytic hydrogen evolution. Chem. Eng. J. 2020, 420, 2. [Google Scholar] [CrossRef]
- Yan, X.; An, H.; Chen, Z.; Yang, G. Significantly enhanced charge transfer efficiency and surface reaction on NiP2/g-C3N4 heterojunction for photocatalytic hydrogen evolution. Chin. J. Chem. Eng. 2022, 43, 31–39. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Q.; Qiao, X.; Hou, D.; Li, D. One-pot Hydrothermal Synthesis of Willow Branch-shaped MoS2/CdS Heterojunctions for Photocatalytic H2 Production Under Visible Light Irradiation. Chin. J. Catal. 2019, 40, 371–379. [Google Scholar] [CrossRef]
- Zhang, K.; Feng, S.; Wang, J.; Azcatl, A.; Lu, N.; Addou, R.; Wan, N.; Zhou, C.; Lerach, J.; Bojan, V.; et al. Manganese doping of monolayer MoS2: The substrate is critical. Nano Lett. 2015, 15, 6586–6591. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Ma, M.; Ren, X.; Liu, Z.; Du, G.; Asiri, A.M.; Sun, X. A Mn-doped Ni2P nanosheet array: An efficient and durable hydrogen evolution reaction electrocatalyst in alkaline media. Chem. Commun. 2017, 53, 11048–11051. [Google Scholar] [CrossRef] [PubMed]
- Bolara, S.; Shita, S.; Kumara, J.S.; Murmu, N.C.; Ganeshc, R.S.; Inokawa, H.; Kuila, T. Optimization of active surface area of flower like MoS2 using V-doping towards enhanced hydrogen evolution reaction in acidic and basic medium. Appl. Catal. B-Environ. 2019, 254, 432–442. [Google Scholar] [CrossRef]
- Bi, L.; Xu, D.; Zhang, L.; Lin, Y.; Wang, D.; Xie, T. Metal Ni-loaded g-C3N4 for enhanced photocatalytic H2 evolution activity: the change in surface band bending. Phys. Chem. Chem. Phys. 2015, 17, 29899–29905. [Google Scholar] [CrossRef]
- Lu, Y.; Chu, D.; Zhu, M.; Du, Y.; Yang, P. Exfoliated carbon nitride nanosheets decorated with NiS as an efficient noble-metal-free visible-light-driven photocatalyst for hydrogen evolution. Phys. Chem. Chem. Phys. 2015, 17, 17355–17361. [Google Scholar] [CrossRef]
- Bhunia, M.K.; Yamauchi, K.; Takanabe, K. Harvesting Solar Light with Crystalline Carbon Nitrides for Efficient Photocatalytic Hydrogen Evolution. Angew. Chem. Int. Ed. 2014, 41, 11001–11005. [Google Scholar] [CrossRef]
- Zhao, H.; Sun, S.N.; Jiang, P.P.; Xu, Z.J. Graphitic C3N4 modified by Ni2P cocatalyst: An efficient, robust and low cost photocatalyst for visible-light-driven H2 evolution from water. Chem. Eng. J. 2017, 35, 296–303. [Google Scholar] [CrossRef]
- Wang, J.; Chen, J.; Wang, P.; Hou, J.; Wang, C.; Ao, Y. Robust photocatalytic hydrogen evolution over amorphous ruthenium phosphide quantum dots modified g-C3N4 nanosheet. Appl. Catal. B-Environ. 2018, 239, 578–585. [Google Scholar] [CrossRef]
- Lin, Q.; Li, L.; Liang, S.; Liu, M.; Bi, J.; Wu, L. Efficient synthesis of monolayer carbon nitride 2D nanosheet with tunable concentration and enhanced visible-light photocatalytic activities. Appl. Catal. B Environ. 2015, 163, 135–142. [Google Scholar] [CrossRef]
- Chen, Z.; Song, Y.; Cai, J.; Zheng, X.; Han, D.; Wu, Y.; Zang, Y.; Niu, S.; Liu, Y.; Zhu, J.; et al. Tailoring the d-band centers enables Co4N nanosheets to be highly active for hydrogen evolution catalysis. Angew. Chem. Int. Ed. 2018, 57, 5076–5080. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhang, S.; Li, C.; Zhu, C.; Chen, Y.; Gao, P.; Qi, L.; Zhang, X. Hollow CoP nanopaticle/N-doped graphene hybrids as highly active and stable bifunctional catalysts for full water splitting. Nanoscale 2016, 8, 10902–10907. [Google Scholar] [CrossRef]
- Jiang, P.; Liu, Q.; Sun, X.P. NiP2 nanosheet arrays supported on carbon cloth: An efficient 3D hydrogen evolution cathode in both acidic and alkaline solutions. Nanoscale 2014, 22, 13440–13445. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Kong, L.; Jiang, P.; Wang, G.; Zhao, N.; Zhang, H.; Tang, B. A general strategy to fabricate NixP as highly efficient cocatalyst via photo-reduction deposition for hydrogen evolution. ACS Sustain. Chem. Eng. 2017, 5, 6845–6853. [Google Scholar] [CrossRef]
- Xiao, L.; Tong, S.; Zhuo, W.; Zhang, K.; Peng, X.; Han, Y. Enhanced photocatalytic hydrogen evolution by loading Cd0.5Zn0.5S QDs onto Ni2P porous nanosheets. Nanoscale Res. Lett. 2018, 13, 31–40. [Google Scholar] [CrossRef]
- Luo, Y.; Qin, J.; Yang, G.; Luo, S.; Zhao, Z.; Ma, M.C.J. N-Ni-S coordination sites of NiS/C3N4 formed by an electrochemical-pyrolysis strategy for boosting oxygen evolution reaction. Chem. Eng. J. 2021, 410, 128394. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, M.; Cao, L.; Liu, Q.; Li, X.; Chen, Q.; Liu, D.; Li, W.; Huang, J.; Feng, L. Well-Defined Ultrasmall V-NiP2 Nanoparticles Anchored g-C3N4 Nanosheets as Highly Efficient Visible-Light-Driven Photocatalysts for H2 Evolution. Catalysts 2022, 12, 998. https://doi.org/10.3390/catal12090998
Niu M, Cao L, Liu Q, Li X, Chen Q, Liu D, Li W, Huang J, Feng L. Well-Defined Ultrasmall V-NiP2 Nanoparticles Anchored g-C3N4 Nanosheets as Highly Efficient Visible-Light-Driven Photocatalysts for H2 Evolution. Catalysts. 2022; 12(9):998. https://doi.org/10.3390/catal12090998
Chicago/Turabian StyleNiu, Mengfan, Liyun Cao, Qianqian Liu, Xiaoyi Li, Qian Chen, Dinghan Liu, Wenbin Li, Jianfeng Huang, and Liangliang Feng. 2022. "Well-Defined Ultrasmall V-NiP2 Nanoparticles Anchored g-C3N4 Nanosheets as Highly Efficient Visible-Light-Driven Photocatalysts for H2 Evolution" Catalysts 12, no. 9: 998. https://doi.org/10.3390/catal12090998