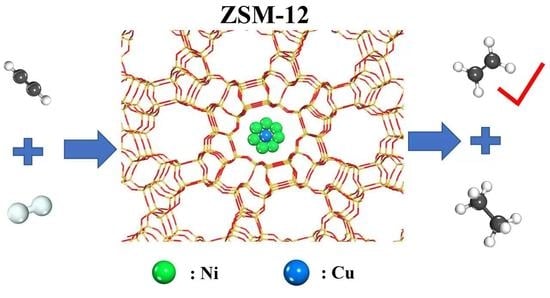
Semi-Hydrogenation of Acetylene to Ethylene Catalyzed by Bimetallic CuNi/ZSM-12 Catalysts
Abstract
:1. Introduction
2. Results and Discussions
2.1. Catalyst Characterizations
2.2. Catalytic Performance of 2 wt% Cu/ZSM-12 and 2 wt% Ni/ZSM-12
2.3. Catalytic Performance of CuNix/ZSM-12 Catalysts
2.4. Catalytic Stability Test of CuNi7/ZSM-12 Catalysts
3. Materials and Methods
3.1. Materials
3.2. Carrier Preparation
3.3. Catalyst Preparation
3.4. Catalyst Evaluation
3.5. Catalyst Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, T.-L.; Wang, H.; Li, B.; Krishna, R.; Wu, H.; Zhou, W.; Zhao, Y.; Han, Y.; Wang, X.; Zhu, W. Microporous metal–organic framework with dual functionalities for highly efficient removal of acetylene from ethylene/acetylene mixtures. Nat. Commun. 2015, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Neal, L.; Ding, D.; Wu, W.; Baroi, C.; Gaffney, A.M.; Li, F. Recent advances in intensified ethylene production—a review. ACS Catal. 2019, 9, 8592–8621. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Skoptsov, G.; Hu, J. Selective hydrogenation of acetylene to ethylene over bimetallic catalysts. Ind. Eng. Chem. Res. 2019, 58, 20620–20629. [Google Scholar] [CrossRef]
- Gui, X.; Liu, J.; Cao, Y.; Miao, Z.; Li, S.; Xing, Y.; Wang, D. Coal preparation technology: Status and development in China. Energy Environ. 2015, 26, 997–1013. [Google Scholar] [CrossRef]
- Zhang, X.; Song, X.; Wang, J.; Su, W.; Zhou, B.; Bai, Y.; Yu, G. Physico-chemical structure evolution characteristics of coal char during gasification in the presence of iron-based waste catalyst. Int. J. Coal Geol. 2020, 7, 456–463. [Google Scholar] [CrossRef]
- Schobert, H. Production of acetylene and acetylene-based chemicals from coal. Chem. Rev. 2014, 114, 1743–1760. [Google Scholar] [CrossRef]
- Ball, M.R.; Rivera-Dones, K.R.; Gilcher, E.B.; Ausman, S.F.; Hullfish, C.W.; Lebrón, E.A.; Dumesic, J.A. AgPd and CuPd catalysts for selective hydrogenation of acetylene. ACS Catal. 2020, 10, 8567–8581. [Google Scholar] [CrossRef]
- Thiruvenkataswamy, P.; Eljack, F.T.; Roy, N.; Mannan, M.S.; El-Halwagi, M.M. Safety and techno-economic analysis of ethylene technologies. J. Loss Prev. Process Ind. 2016, 39, 74–84. [Google Scholar] [CrossRef]
- Wang, S.; Uwakwe, K.; Yu, L.; Ye, J.; Zhu, Y.; Hu, J.; Chen, R.; Zhang, Z.; Zhou, Z.; Li, J. Highly efficient ethylene production via electrocatalytic hydrogenation of acetylene under mild conditions. Nat. Commun. 2021, 12, 1–9. [Google Scholar] [CrossRef]
- Tian, P.; Wei, Y.; Ye, M.; Liu, Z. Methanol to olefins (MTO): From fundamentals to commercialization. ACS Catal. 2015, 5, 1922–1938. [Google Scholar] [CrossRef]
- Pei, G.X.; Liu, X.Y.; Wang, A.; Lee, A.F.; Isaacs, M.A.; Li, L.; Pan, X.; Yang, X.; Wang, X.; Tai, Z. Ag alloyed Pd single-atom catalysts for efficient selective hydrogenation of acetylene to ethylene in excess ethylene. ACS Catal. 2015, 5, 3717–3725. [Google Scholar] [CrossRef]
- Ma, H.-Y.; Wang, G.-C. Selective Hydrogenation of Acetylene on Pt n/TiO2 (n = 1, 2, 4, 8) Surfaces: Structure Sensitivity Analysis. ACS Catal. 2020, 10, 4922–4928. [Google Scholar] [CrossRef]
- Chesnokov, V.; Svintsitskii, D.; Chichkan, A.; Parmon, V. Effect of the structure of carbon support on the selectivity of Pt/C catalysts for the hydrogenation of acetylene to ethylene. Nanotechnol. Russ. 2018, 13, 246–255. [Google Scholar] [CrossRef]
- Hu, N.; Yang, C.; He, L.; Guan, Q.; Miao, R. Ni–Cu/Al2O3 catalysts for the selective hydrogenation of acetylene: A study on catalytic performance and reaction mechanism. New J. Chem. 2019, 43, 18120–18125. [Google Scholar] [CrossRef]
- Zhou, S.; Kang, L.; Zhou, X.; Xu, Z.; Zhu, M. Pure acetylene semihydrogenation over Ni–Cu bimetallic catalysts: Effect of the Cu/Ni ratio on catalytic performance. Nanomaterials 2020, 10, 509. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, J.; Feng, J.; He, Y.; Du, Y.; Li, D. Layered double hydroxide-derived Ni-Cu nanoalloy catalysts for semi-hydrogenation of alkynes: Improvement of selectivity and anti-coking ability via alloying of Ni and Cu. J. Catal. 2018, 359, 251–260. [Google Scholar] [CrossRef]
- Yang, B.; Burch, R.; Hardacre, C.; Headdock, G.; Hu, P. Origin of the Increase of Activity and Selectivity of Nickel Doped by Au, Ag, and Cu for Acetylene Hydrogenation. ACS Catal. 2012, 2, 1027–1032. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Lee, J.W.; Hong, C.-Y.; Mou, C.-Y.; Jang, B.W. Selective hydrogenation of acetylene in excess ethylene over SiO2 supported Au–Ag bimetallic catalyst. Appl. Catal. A Gen. 2012, 439, 8–14. [Google Scholar] [CrossRef]
- Gluhoi, A.C.; Bakker, J.W.; Nieuwenhuys, B.E. Gold, still a surprising catalyst: Selective hydrogenation of acetylene to ethylene over Au nanoparticles. Catal. Today 2010, 154, 13–20. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, L.; Liu, B.-J. Selective hydrogenation of acetylene on CuNi/Al2O3 catalyst. Chem. Eng. J. 2012, 32, 50–52. [Google Scholar]
- McCue, A.J.; McRitchie, C.J.; Shepherd, A.M.; Anderson, J.A. Cu/Al2O3 catalysts modified with Pd for selective acetylene hydrogenation. J. Catal. 2014, 319, 127–135. [Google Scholar] [CrossRef]
- Ravanchi, M.T.; Sahebdelfar, S.; Komeili, S. Acetylene selective hydrogenation: A technical review on catalytic aspects. Rev. Chem. Eng. 2018, 34, 215–237. [Google Scholar] [CrossRef]
- Ye, R.-P.; Liao, L.; Reina, T.R.; Liu, J.; Chevella, D.; Jin, Y.; Fan, M.; Liu, J. Engineering Ni/SiO2 catalysts for enhanced CO2 methanation. Fuel 2021, 285, 119151. [Google Scholar] [CrossRef]
- Liu, H.; Chai, M.; Pei, G.; Liu, X.; Li, L.; Kang, L.; Wang, A.; Zhang, T. Effect of IB-metal on Ni/SiO2 catalyst for selective hydrogenation of acetylene. Chin. J. Catal. 2020, 41, 1099–1108. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, J.; Zhao, B.; He, L.; Wang, A.; Wang, B. Insight into the effects of Cu component and the promoter on the selectivity and activity for efficient removal of acetylene from ethylene on Cu-based catalyst. J. Phys. Chem. C 2017, 121, 27936–27949. [Google Scholar] [CrossRef]
- Wang, L.; Li, F.; Chen, Y.; Chen, J. Selective hydrogenation of acetylene on SiO2-supported Ni-Ga alloy and intermetallic compound. J. Energy Chem. 2019, 29, 40–49. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, J. Selective hydrogenation of acetylene on SiO2 supported Ni-In bimetallic catalysts: Promotional effect of In. Appl. Surf. Sci. 2016, 387, 16–27. [Google Scholar] [CrossRef]
- Zhao, J.; Hua, Z.; Liu, Z.; Li, Y.; Guo, L.; Bu, W.; Cui, X.; Ruan, M.; Chen, H.; Shi, J. Direct fabrication of mesoporous zeolite with a hollow capsular structure. ChemComm 2009, 48, 7578–7580. [Google Scholar] [CrossRef]
- Komatsu, T.; Kishi, T.; Gorai, T. Preparation and catalytic properties of uniform particles of Ni3Ge intermetallic compound formed inside the mesopores of MCM-41. J. Catal. 2008, 259, 174–182. [Google Scholar] [CrossRef]
- Huang, W.; Pyrz, W.; Lobo, R.F.; Chen, J.G. Selective hydrogenation of acetylene in the presence of ethylene on K+-β-zeolite supported Pd and PdAg catalysts. Appl. Catal. A Gen. 2007, 333, 254–263. [Google Scholar] [CrossRef]
- Zhou, S.; Kang, L.; Xu, Z.; Zhu, M. Catalytic performance and deactivation of Ni/MCM-41 catalyst in the hydrogenation of pure acetylene to ethylene. RSC Adv. 2020, 10, 1937–1945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, F.; Chen, X.; Tu, Z.; Lu, Z.-H.; Feng, G.; Zhang, R. Graphene aerogel supported Ni for CO2 hydrogenation to methane. Ind. Eng. Chem. Res. 2021, 60, 12235–12243. [Google Scholar] [CrossRef]
- Li, A.; Yao, D.; Yang, Y.; Yang, W.; Li, Z.; Lv, J.; Huang, S.; Wang, Y.; Ma, X. Active Cu0–Cuσ+ Sites for the Hydrogenation of Carbon–Oxygen Bonds over Cu/CeO2 Catalysts. ACS Catal. 2022, 12, 1315–1325. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, X.; Li, L.; Liu, X.; Huang, Y.; Pan, X.; Wang, A.; Li, J.; Zhang, T. PdZn intermetallic nanostructure with Pd-Zn-Pd ensembles for highly active and chemoselective semi-hydrogenation of acetylene. ACS Catal. 2016, 6, 1054–1061. [Google Scholar] [CrossRef]
- Kang, J.H.; Shin, E.W.; Kim, W.J.; Park, J.D.; Moon, S.H. Selective Hydrogenation of Acetylene on TiO2-Added Pd Catalysts. J. Catal. 2002, 208, 310–320. [Google Scholar] [CrossRef]
- Kang, L.; Cheng, B.; Zhu, M. Pd/MCM-41 catalyst for acetylene hydrogenation to ethylene. R. Soc. Open Sci. 2019, 6, 191155. [Google Scholar] [CrossRef]
- Zhou, S.; Shang, L.; Zhao, Y.; Shi, R.; Waterhouse, G.I.N.; Huang, Y.C.; Zheng, L.; Zhang, T. Pd Single-Atom Catalysts on Nitrogen-Doped Graphene for the Highly Selective Photothermal Hydrogenation of Acetylene to Ethylene. Adv. Mater. 2019, 31, e1900509. [Google Scholar] [CrossRef]
- Jia, J.F.; Haraki, K.; Kondo, J.N.; Domen, K.; Tamaru, K. Selective hydrogenation of acetylene over Au/Al2O3 catalyst. J. Phys. Chem. B 2000, 104, 11153–11156. [Google Scholar] [CrossRef]
- Sun, X.; Li, F.; Shi, J.; Zheng, Y.; Su, H.; Sun, L.; Peng, S.; Qi, C. Gold nanoparticles supported on MgOx-Al2O3 composite oxide: An efficient catalyst for selective hydrogenation of acetylene. Appl. Surf. Sci. 2019, 487, 625–633. [Google Scholar] [CrossRef]
- Pei, G.X.; Liu, X.Y.; Wang, A.; Su, Y.; Li, L.; Zhang, T. Selective hydrogenation of acetylene in an ethylene-rich stream over silica supported Ag-Ni bimetallic catalysts. Appl. Catal. A-Gen. 2017, 545, 90–96. [Google Scholar] [CrossRef]
- Simanullang, W.F.; Ma, J.; Shimizu, K.-I.; Furukawa, S. Silica-decorated Ni–Zn alloy as a highly active and selective catalyst for acetylene semihydrogenation. Catal. Sci. Technol. 2021, 11, 4016–4020. [Google Scholar] [CrossRef]
- Esmaeili, E.; Mortazavi, Y.; Khodadadi, A.A.; Rashidi, A.M.; Rashidzadeh, M. The role of tin-promoted Pd/MWNTs via the management of carbonaceous species in selective hydrogenation of high concentration acetylene. Appl. Surf. Sci. 2012, 263, 513–522. [Google Scholar] [CrossRef]
- Zhao, L.; Wei, Z.; Zhu, M.; Dai, B. Catalytic performance of a Ti added Pd/SiO2 catalyst for acetylene hydrogenation. J. Ind. Eng. Chem. 2012, 18, 45–48. [Google Scholar] [CrossRef]










| Samples | SBET m2/g | Vp cm3/g | Dp nm | Ni (wt%) | Cu (wt%) | Cu/Ni |
|---|---|---|---|---|---|---|
| CuNi5/ZSM-12 | 361.7 | 0.142 | 6.987 | 1.56 | 0.34 | 5.04 |
| CuNi7/ZSM-12 | 361.9 | 0.139 | 7.236 | 1.73 | 0.27 | 6.91 |
| CuNi9/ZSM-12 | 365.0 | 0.140 | 6.858 | 1.80 | 0.21 | 9.46 |
| CuNi11/ZSM-12 | 374.2 | 0.144 | 6.952 | 1.82 | 0.17 | 11.65 |
| H-ZSM-12 | 385.8 | 0.149 | 7.093 | - | - | - |
| Samples | Relative Acidity Amount | ||
|---|---|---|---|
| Ⅰ (a) | Ⅱ (a) | Total Acid | |
| Ni/ZSM-12 | 1 | 0.41 | 1.41 |
| CuNi7/ZSM-12 | 0.94 | 0.38 | 1.32 |
| Cu/ZSM-12 | 0.91 | 0.27 | 1.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, S.; Zhang, C.; Wu, M.; Ye, R.; Shi, D.; Li, M.; Zhao, P.; Zhang, R.; Feng, G. Semi-Hydrogenation of Acetylene to Ethylene Catalyzed by Bimetallic CuNi/ZSM-12 Catalysts. Catalysts 2022, 12, 1072. https://doi.org/10.3390/catal12091072
Hu S, Zhang C, Wu M, Ye R, Shi D, Li M, Zhao P, Zhang R, Feng G. Semi-Hydrogenation of Acetylene to Ethylene Catalyzed by Bimetallic CuNi/ZSM-12 Catalysts. Catalysts. 2022; 12(9):1072. https://doi.org/10.3390/catal12091072
Chicago/Turabian StyleHu, Song, Chong Zhang, Mingyu Wu, Runping Ye, Depan Shi, Mujin Li, Peng Zhao, Rongbin Zhang, and Gang Feng. 2022. "Semi-Hydrogenation of Acetylene to Ethylene Catalyzed by Bimetallic CuNi/ZSM-12 Catalysts" Catalysts 12, no. 9: 1072. https://doi.org/10.3390/catal12091072