Influence of Butanol Isomerization on Photothermal Hydrogen Production over Ti@TiO2 Core-Shell Nanoparticles
Abstract
:1. Introduction
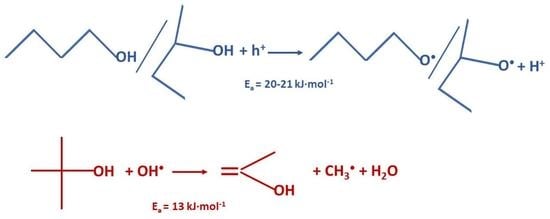
2. Results and Discussion
3. Materials and Methods
3.1. Chemical Reagents
3.2. Catalyst Preparation
3.3. Photocatalytic Experiments
3.4. Terephthalate Dosimetry
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abeab, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Oureshi, F.; Yusuf, M.; Kamyab, H.; Zaidi, S.; Khalil, M.J.; Khan, M.A.; Alam, M.A.; Masood, F.; Bazli, L.; Chelliapan, S.; et al. Current trends in hydrogen production, storage and applications in India: A review. Sustain. Energy Technol. Assess. 2022, 53, 102677. [Google Scholar]
- Voiry, D.; Shin, H.S.; Loh, K.P.; Chhowalla, M. Low-dimensional catalysts for hydrogen evolution and CO2 reduction. Nat. Rev. Chem. 2018, 2, 0105. [Google Scholar] [CrossRef]
- Qureshi, F.; Yusuf, M.; Kamyab, H.; Vo, D.-V.N.; Chelliapan, S.; Joo, S.-W.; Vasseghian, Y. Latest eco-friendly avenues on hydrogen production towards a circular bioeconomy: Current challenges, innovative insights, and future perspectives. Renew. Sustain. Energy Rev. 2022, 168, 112916. [Google Scholar] [CrossRef]
- Navarro, R.M.; Sanchez-Sanchez, M.C.; Alvarez-Galvan, M.C.; Valle, F.D.; Fierro, J.L.G. Hydrogen production from renewable sources: Biomass and photocatalytic opportunities. Energy Environ. Sci. 2009, 2, 35–54. [Google Scholar] [CrossRef]
- Qureshi, F.; Yusuf, M.; Pasha, A.A.; Khan, H.W.; Imteyaz, B.; Irshad, K. Sustainable and energy efficient hydrogen production via glycerol reforming technique: A review. Int. J. Hydrogen Energy 2022, 47, 41397–41420. [Google Scholar] [CrossRef]
- Kollmannsberger, S.L.; Walenta, C.A.; Courtois, C.; Tschurl, M.; Heiz, U. Thermal control of selectivity in photocatalytic, water-free alcohol photoreforming. ACS Catal. 2018, 8, 11076–11084. [Google Scholar] [CrossRef]
- Fang, S.; Hu, Y.H. Thermo-photo catalysis: A whole greater than the sum of its parts. Chem. Soc. Rev. 2022, 51, 3609–3647. [Google Scholar] [CrossRef]
- El Hakim, S.; Chave, T.; Nikitenko, S.I. Deciphering the reaction mechanisms of photothermal hydrogen production using H/D kinetic isotope effect. Cat. Sci. Technol. 2022, 12, 5252–5256. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.Q.; Yates, J.T. Photocatalysis on TiO2 surfaces—Principles, mechanisms, and selected results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Panayotov, D.A.; Morris, J.R. Surface chemistry of Au/TiO2: Thermally and photolytically activated reactions. Surf. Sci. Rep. 2016, 71, 77–271. [Google Scholar] [CrossRef]
- Osterloh, F.E. Photocatalysis versus photosynthesis: A sensitivity analysis of devices for solar energy conversion and chemical transformations. ACS Energy Lett. 2017, 2, 445–453. [Google Scholar] [CrossRef]
- Fabian, D.M.; Hu, S.; Singh, N.; Houle, F.A.; Hisatomi, T.; Domen, K.; Osterloh, F.E.; Ardo, S. Particle suspension reactors and materials for solar-driven water splitting. Energy Environ. Sci. 2015, 8, 2825–2850. [Google Scholar] [CrossRef] [Green Version]
- Walenta, C.A.; Kollmannsberger, S.L.; Courtois, C.; Tschurl, M.; Heiz, U. Photocatalytic selectivity switch to C-C scission: α-methyl ejection of tert-butanol on TiO2(110). Phys. Chem. Chem. Phys. 2018, 20, 7105–7111. [Google Scholar] [CrossRef]
- Courtois, C.; Walenta, C.A.; Tschurl, M.; Heiz, U.; Friend, C.M. Regulating photochemical selectivity with temperature: Isobutanol on TiO2(110). J. Am. Chem. Soc. 2020, 142, 13072–13080. [Google Scholar] [CrossRef]
- Nishimoto, S.; Ohtani, B.; Shirai, H.; Kagiya, T. Photocatalytic degradation and dimerization of t-butyl alcohol by aqueous suspension of platinized titanium dioxide. J. Chem. Soc. Perkin Trans. 1986, 2, 661–665. [Google Scholar] [CrossRef]
- Nikitenko, S.I.; Chave, T.; Cau, C.; Brau, H.-P.; Flaud, V. Photothermal hydrogen production using noble-metal-free Ti@TiO2 core-shell nanoparticles under visible-NIR light irradiation. ACS Catal. 2015, 5, 4790–4795. [Google Scholar] [CrossRef]
- Nikitenko, S.I.; Chave, T.; Le Goff, X. Insights into the photothermal hydrogen production from glycerol aqueous solutions over noble metal-free Ti@TiO2 core-shell nanoparticles. Part. Part. Syst. Charact. 2018, 35, 1800265. [Google Scholar] [CrossRef]
- El Hakim, S.; Chave, T.; Nada, A.A.; Roualdes, S.; Nikitenko, S.I. Tailoring noble metal-free Ti@TiO2 photocatalyst for boosting photothermal hydrogen production. Front. Catal. 2021, 1, 669260. [Google Scholar] [CrossRef]
- López-Tendallo, F.J.; Hidalgo-Carrillo, J.; Montes-Jiménez, V.; Sánchez-López, E.; Urbano, F.J.; Marinas, A. Photocatalytic production of hydrogen from binary mixtures of C-3 alcohols on Pt/TiO2: Influence of alcohol structure. Catal. Today 2019, 328, 2–7. [Google Scholar] [CrossRef]
- López, C.R.; Pulido Melián, E.; Ortega Méndez, J.A.; Santiago, D.E.; Doña Rodríguez, J.M.; González Díaz, O. Comparative study of alcohols as sacrificial agents in H2 production by heterogenous photocatalysis using Pt/TiO2 catalysts. J. Photochem. Photobiol. A Chem. 2015, 312, 45–54. [Google Scholar] [CrossRef]
- Yasuda, M.; Matsumoto, T.; Yamashita, T. Sacrificial hydrogen production over TiO2-based photocatalysis: Polyols, carboxylic acids, and saccharides. Renew. Sustain. Energy Rev. 2018, 81, 1627–1635. [Google Scholar] [CrossRef]
- Kennedy, J.; Bahruji, H.; Bowker, M.; Davies, P.R.; Bouleghlimat, E.; Issarapanacheewin, S. Hydrogen generation by photocatalytic reforming of potential biofuels: Polyols, cyclic alcohols, and saccharides. J. Photochem. Photobiol. A Chem. 2018, 356, 451–456. [Google Scholar] [CrossRef]
- Bahruji, H.; Bowker, M.; Davies, P.R.; Pedrono, F. New insights into the mechanism of photocatalytic reforming on Pd/TiO2. Appl. Catal. B Environ. 2011, 107, 205–209. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A. Understanding hydroxyl radical (•OH) generation process in photocatalysis. ACS Energy Lett. 2016, 1, 356–359. [Google Scholar] [CrossRef] [Green Version]
- Mark, G.; Tauber, A.; Laupert, R.; Schuchmann, H.-P.; Schulz, D.; Mues, A.; von Sonntag, C. OH-radical formation by ultrasound in aqueous solution. Part II: Terephtalate and Fricke dosimetry and the influence of various conditions on the sonolytic yield. Ultrason. Sonochem. 1998, 5, 41–52. [Google Scholar] [CrossRef]
- Bahnemann, D.W.; Hilgendorff, M.; Memming, R. Charge carrier dynamics at TiO2 particles: Reactivity of free and trapped holes. J. Phys. Chem. B 1997, 101, 4265–4275. [Google Scholar] [CrossRef]
- Kim, G.; Choi, H.J.; Kim, H.; Kim, J.; Monllor-Satoca, D.; Kim, M.; Park, H. Temperature-boosted photocatalytic H2 production and charge transfer kinetics on TiO2 under UV and visible light. Photochem. Photobiol. Sci. 2016, 15, 1247–1253. [Google Scholar] [CrossRef]
- Eyring, H. The activated complex in chemical reactions. J. Chem. Phys. 1935, 3, 107–115. [Google Scholar] [CrossRef]
- Jain, P.; Yu, S. Isotope effects in plasmonic photosynthesis. Angew. Chem. Int. Ed. 2020, 59, 22480–22483. [Google Scholar]





| Alcohol | Ea, kJ·mol−1 |
|---|---|
| 1-BuOH | 21 ± 2 |
| 2-BuOH | 20 ± 2 |
| t-BuOH | 13 ± 2 |
| Glycerol | 25 ± 5 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Hakim, S.; Bathias, M.; Chave, T.; Nikitenko, S.I. Influence of Butanol Isomerization on Photothermal Hydrogen Production over Ti@TiO2 Core-Shell Nanoparticles. Catalysts 2022, 12, 1662. https://doi.org/10.3390/catal12121662
El Hakim S, Bathias M, Chave T, Nikitenko SI. Influence of Butanol Isomerization on Photothermal Hydrogen Production over Ti@TiO2 Core-Shell Nanoparticles. Catalysts. 2022; 12(12):1662. https://doi.org/10.3390/catal12121662
Chicago/Turabian StyleEl Hakim, Sara, Mathéo Bathias, Tony Chave, and Sergey I. Nikitenko. 2022. "Influence of Butanol Isomerization on Photothermal Hydrogen Production over Ti@TiO2 Core-Shell Nanoparticles" Catalysts 12, no. 12: 1662. https://doi.org/10.3390/catal12121662