Transfer Hydrogenation of Biomass-Like Phenolic Compounds and 2-PrOH over Ni-Based Catalysts Prepared Using Supercritical Antisolvent Coprecipitation
Abstract
:1. Introduction
2. Results
2.1. Catalyst Properties
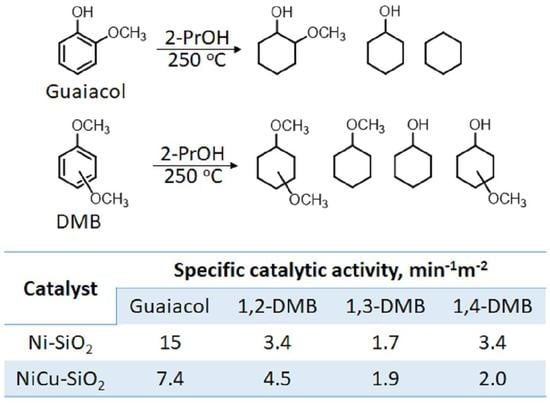
2.2. Transfer Hydrogenation of Guaiacol
2.3. Transfer Hydrogenation of Dimethoxybenzenes
2.4. Kinetic Studies
3. Materials and Methods
3.1. Materials
3.1.1. Catalyst Preparation and Characterization
3.1.2. Batch Experiments
3.1.3. Kinetic Calculations
3.1.4. Product Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gilkey, M.J.; Xu, B. Heterogeneous catalytic transfer hydrogenation as an effective pathway in biomass upgrading. ACS Catal. 2016, 6, 1420–1436. [Google Scholar] [CrossRef]
- Thanigaivel, S.; Priya, A.K.; Dutta, K.; Rajendran, S.; Sekar, K.; Jalil, A.A.; Soto-Moscoso, M. Role of nanotechnology for the conversion of lignocellulosic biomass into biopotent energy: A biorefinery approach for waste to value-added products. Fuel 2022, 322, 124236. [Google Scholar] [CrossRef]
- Ke, L.; Wu, Q.; Zhou, N.; Xiong, J.; Yang, Q.; Zhang, L.; Wang, Y.; Dai, L.; Zou, R.; Liu, Y.; et al. Lignocellulosic biomass pyrolysis for aromatic hydrocarbons production: Pre and in-process enhancement methods. Renew. Sustain. Energy Rev. 2022, 165, 112607. [Google Scholar] [CrossRef]
- Gnanasekaran, L.; Priya, A.K.; Thanigaivel, S.; Hoang, T.K.A.; Soto-Moscoso, M. The conversion of biomass to fuels via cutting-edge technologies: Explorations from natural utilization systems. Fuel 2023, 331, 125668. [Google Scholar] [CrossRef]
- Scholze, B.; Meier, D. Characterization of the water-insoluble fraction from pyrolysis oil (pyrolytic lignin). Part I. PY-GC/MS, FTIR, and functional groups. J. Anal. Appl. Pyrolysis 2001, 60, 41–54. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, P.; El Azab, I.H.; Bin Xu, B.; Guo, Z.; Elnaggar, A.Y.; Mersal, G.A.M.; Liu, X.; Zhi, Y.; Lin, Z.; et al. An efficient bifunctional Ni-Nb2O5 Nanocatalysts for the hydrodeoxygenation of anisole. Chin. J. Chem. Eng. 2022, 49, 187–197. [Google Scholar] [CrossRef]
- Shafaghat, H.; Rezaei, P.S.; Ashri Wan Daud, W.M. Effective parameters on selective catalytic hydrodeoxygenation of phenolic compounds of pyrolysis bio-oil to high-value hydrocarbons. RSC Adv. 2015, 5, 103999–104042. [Google Scholar] [CrossRef]
- Shuai, L.; Luterbacher, J. Organic Solvent effects in biomass conversion reactions. ChemSusChem 2016, 9, 133–155. [Google Scholar] [CrossRef] [Green Version]
- Shafaghat, H.; Tsang, Y.F.; Jeon, J.K.; Kim, J.M.; Kim, Y.; Kim, S.; Park, Y.K. In-situ hydrogenation of bio-oil/bio-oil phenolic compounds with secondary alcohols over a synthesized mesoporous Ni/CeO2 catalyst. Chem. Eng. J. 2020, 382, 122912. [Google Scholar] [CrossRef]
- Philippov, A.A.; Nesterov, N.N.; Pakharukova, V.P.; Martyanov, O.N. High-loaded ni-based catalysts obtained via supercritical antisolvent coprecipitation in transfer hydrogenation of anisole: Influence of the support. Appl. Catal. A Gen. 2022, 643, 118792. [Google Scholar] [CrossRef]
- Park, Y.K.; Ha, J.M.; Oh, S.; Lee, J. Bio-oil upgrading through hydrogen transfer reactions in supercritical solvents. Chem. Eng. J. 2021, 404, 126527. [Google Scholar] [CrossRef]
- Yfanti, V.L.; Lemonidou, A.A. Effect of hydrogen donor on glycerol hydrodeoxygenation to 1,2-propanediol. Catal. Today 2020, 355, 727–736. [Google Scholar] [CrossRef]
- Chen, H.; Xu, Q.; Zhang, D.; Liu, W.; Liu, X.; Yin, D. Highly efficient synthesis of γ-valerolactone by catalytic conversion of biomass-derived levulinate esters over support-free mesoporous Ni. Renew. Energy 2021, 163, 1023–1032. [Google Scholar] [CrossRef]
- Reddy Kannapu, H.P.; Mullen, C.A.; Elkasabi, Y.; Boateng, A.A. Catalytic transfer hydrogenation for stabilization of bio-oil oxygenates: Reduction of p-cresol and furfural over bimetallic Ni-Cu catalysts using isopropanol. Fuel Process. Technol. 2015, 137, 220–228. [Google Scholar] [CrossRef]
- Philippov, A.A.; Chibiryaev, A.M.; Martyanov, O.N. Base-free transfer hydrogenation of menthone by sub- and supercritical alcohols. J. Supercrit. Fluids 2019, 145, 162–168. [Google Scholar] [CrossRef]
- Shafaghat, H.; Kim, J.M.; Lee, I.G.; Jae, J.; Jung, S.C.; Park, Y.K. Catalytic hydrodeoxygenation of crude bio-oil in supercritical methanol using supported nickel catalysts. Renew. Energy 2019, 144, 159–166. [Google Scholar] [CrossRef]
- Alekseev, E.S.; Alentiev, A.Y.; Belova, A.S.; Bogdan, V.I.; Bogdan, T.V.; Bystrova, A.V.; Gafarova, E.R.; Golubeva, E.N.; Grebenik, E.A.; Gromov, O.I.; et al. Supercritical fluids in chemistry. Russ. Chem. Rev. 2020, 89, 1337–1427. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, J.; Kim, U.J.; Choi, J.W. Conversion of lignin to phenol-rich oil fraction under supercritical alcohols in the presence of metal catalysts. Energy Fuels 2015, 29, 5154–5163. [Google Scholar] [CrossRef]
- Goto, M. Chemical recycling of plastics using sub- and supercritical fluids. J. Supercrit. Fluids 2009, 47, 500–507. [Google Scholar] [CrossRef]
- Tang, Z.-R.; Edwards, J.K.; Bartley, J.K.; Taylor, S.H.; Carley, A.F.; Herzing, A.A.; Kiely, C.J.; Hutchings, G.J. Nanocrystalline cerium oxide produced by supercritical antisolvent precipitation as a support for high-activity gold catalysts. J. Catal. 2007, 249, 208–219. [Google Scholar] [CrossRef]
- Nesterov, N.S.; Pakharukova, V.P.; Martyanov, O.N. Water as a cosolvent—Effective tool to avoid phase separation in bimetallic Ni-Cu catalysts obtained via supercritical antisolvent approach. J. Supercrit. Fluids 2017, 130, 133–139. [Google Scholar] [CrossRef]
- Reverchon, E. Supercritical antisolvent precipitation of micro- and nano-particles. J. Supercrit. Fluids 1999, 15, 1–21. [Google Scholar] [CrossRef]
- Nesterov, N.S.; Paharukova, V.P.; Yakovlev, V.A.; Martyanov, O.N. The facile synthesis of Ni-Cu catalysts stabilized in SiO2 Framework via a supercritical antisolvent approach. J. Supercrit. Fluids 2016, 112, 119–127. [Google Scholar] [CrossRef]
- Nesterov, N.S.; Smirnov, A.A.; Pakharukova, V.P.; Yakovlev, V.A.; Martyanov, O.N. Advanced green approaches for the synthesis of nicu-containing catalysts for the hydrodeoxygenation of anisole. Catal. Today 2021, 379, 262–271. [Google Scholar] [CrossRef]
- Philippov, A.; Nesterov, N.; Pakharukova, V.; Kozhevnikov, I.; Martyanov, O. Advanced high-loaded Ni—Cu catalysts in transfer hydrogenation of anisole: Unexpected effect of Cu addition. Catalysts 2022, 12, 1307. [Google Scholar] [CrossRef]
- De Castro, I.B.D.; Graça, I.; Rodríguez-García, L.; Kennema, M.; Rinaldi, R.; Meemken, F. Elucidating the reactivity of methoxyphenol positional isomers towards hydrogen-transfer reactions by ATR-IR spectroscopy of the liquid-solid interface of RANEY® Ni. Catal. Sci. Technol. 2018, 8, 3107–3114. [Google Scholar] [CrossRef] [Green Version]
- Viar, N.; Requies, J.M.; Agirre, I.; Iriondo, A.; Gil-Calvo, M.; Arias, P.L. Ni-Cu bimetallic catalytic system for producing 5-hydroxymethylfurfural-derived value-added biofuels. ACS Sustain. Chem. Eng. 2020, 8, 11183–11193. [Google Scholar] [CrossRef]
- Ganesan, M.; Liu, C.C.; Pandiyarajan, S.; Lee, C.T.; Chuang, H.C. Post-supercritical CO2 electrodeposition approach for Ni-Cu alloy fabrication: An innovative eco-friendly strategy for high-performance corrosion resistance with durability. Appl. Surf. Sci. 2022, 577, 151955. [Google Scholar] [CrossRef]
- Sequeira, C.A.C.; Cardoso, D.S.P.; Amaral, L.; Šljukić, B.; Santos, D.M.F. On the performance of commercially available corrosion-resistant nickel alloys: A review. Corros. Rev. 2016, 34, 187–200. [Google Scholar] [CrossRef]
- Guo, Q.; Wu, M.; Wang, K.; Zhang, L.; Xu, X. Catalytic hydrodeoxygenation of algae bio-oil over bimetallic Ni-Cu/ZrO2 catalysts. Ind. Eng. Chem. Res. 2015, 54, 890–899. [Google Scholar] [CrossRef]
- Gao, B.; Zhang, J.; Yang, J.-H. Bimetallic Cu-Ni/MCM-41 catalyst for efficiently selective transfer hydrogenation of furfural into furfural alcohol. Mol. Catal. 2022, 517, 112065. [Google Scholar] [CrossRef]
- Szczyglewska, P.; Feliczak-Guzik, A.; Nowak, I. Ordered mesoporous silica-supported metal catalysts for hydrodeoxygenation of anisole derivatives. Microporous Mesoporous Mater. 2021, 312, 110691. [Google Scholar] [CrossRef]
- Ardiyanti, A.R.; Khromova, S.A.; Venderbosch, R.H.; Yakovlev, V.A.; Heeres, H.J. Catalytic hydrotreatment of fast-pyrolysis oil using non-sulfided bimetallic Ni-Cu catalysts on a δ-Al2O3 support. Appl. Catal. B Environ. 2012, 117–118, 105–117. [Google Scholar] [CrossRef]




| Catalyst | k × 10−2, min−1 | |||
|---|---|---|---|---|
| Guaiacol | 1,2-DMB | 1,3-DMB | 1,4-DMB | |
| Ni-SiO2 | 5.9 | 1.3 | 0.65 | 1.3 |
| NiCu-SiO2 | 2.6 | 1.6 | 0.67 | 0.71 |
| Catalyst | k/SCO × 10−3, min−1×m−2 | ||||
|---|---|---|---|---|---|
| SCO, m2/g | Guaiacol | 1,2-DMB | 1,3-DMB | 1,4-DMB | |
| Ni-SiO2 | 35 | 15 | 3.4 | 1.7 | 3.4 |
| NiCu-SiO2 | 32 | 7.4 | 4.5 | 1.9 | 2.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Philippov, A.; Nesterov, N.; Martyanov, O. Transfer Hydrogenation of Biomass-Like Phenolic Compounds and 2-PrOH over Ni-Based Catalysts Prepared Using Supercritical Antisolvent Coprecipitation. Catalysts 2022, 12, 1655. https://doi.org/10.3390/catal12121655
Philippov A, Nesterov N, Martyanov O. Transfer Hydrogenation of Biomass-Like Phenolic Compounds and 2-PrOH over Ni-Based Catalysts Prepared Using Supercritical Antisolvent Coprecipitation. Catalysts. 2022; 12(12):1655. https://doi.org/10.3390/catal12121655
Chicago/Turabian StylePhilippov, Alexey, Nikolay Nesterov, and Oleg Martyanov. 2022. "Transfer Hydrogenation of Biomass-Like Phenolic Compounds and 2-PrOH over Ni-Based Catalysts Prepared Using Supercritical Antisolvent Coprecipitation" Catalysts 12, no. 12: 1655. https://doi.org/10.3390/catal12121655