Biotransformation of the Proteogenic Amino Acids Phenylalanine, Tyrosine and Tryptophan by Yarrowia Species: An Application to the Preparative Synthesis of Natural Phenylacetic Acid
Abstract
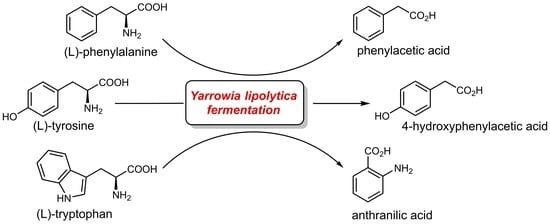
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials and General Methods
3.2. Microorganisms and Growth Media
3.3. Biotransformation Experiments and Preparative Synthesis of Phenylacetic Acid 6a and 4-Hydroxy-Phenylacetic Acid 6b
3.3.1. General Procedure for Biotransformation Experiments Using Aerobic Flasks
3.3.2. Bioreactor Based Preparative Synthesis of Phenylacetic Acid 6a and 4-Hydroxy-Phenylacetic Acid 6b
3.4. Analytical Methods and Characterisation of the Products Deriving from the Biotransformation Experiments
3.4.1. Extraction/Analysis Procedure (without Internal Standard)
3.4.2. Quantitative Analysis Procedure (with Internal Standard)
4. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hazelwood, L.A.; Daran, J.-M.; Maris, A.J.A.v.; Pronk, J.T.; Dickinson, J.R. The Ehrlich pathway for fusel alcohol production: A century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 2008, 74, 2259–2266. [Google Scholar] [CrossRef] [Green Version]
- Qian, X.; Yan, W.; Zhang, W.; Dong, W.; Ma, J.; Ochsenreither, K.; Jiang, M.; Xin, F. Current status and perspectives of 2-phenylethanol production through biological processes. Crit. Rev. Biotechnol. 2019, 39, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Farbood, M.I.; Blocker, R.W.; McLean, L.B.; Scharpf, L.G. Fermentation Process for Preparing Phenylacetic Acid Using Phenylalanine as A Starting Material. U.S. Patent US5420022, 30 May 1995. [Google Scholar]
- Matsumoto, M.; Narita, M.; Yamauchi, S.; Nakajima, T. Production of Phenylacetic Acid. Japanese Patent JPS59232095(A), 26 December 1984. [Google Scholar]
- Vidová, M.; Slezáková, I.; Rebroš, M.; Krištofíková, Ľ.; Rosenberg, M. Gluconobacter oxydans used to production of natural aroma—2-Phenylacetic acid in immobilized system (lentikats form). New Biotechnol. 2014, 31, S91–S92. [Google Scholar] [CrossRef]
- Mihaľ, M.; Červeňanský, I.; Markoš, J. Production of phenylacetic acid from L-phenylalanine in dual reactor membrane hybrid system. Chem. Eng. Process 2016, 110, 114–122. [Google Scholar] [CrossRef]
- Mihaľ, M.; Červeňanský, I.; Markoš, J. Application of immersed silicone rubber membrane module for biocatalytic production of 2-phenylethanol and phenylacetic acid. Chem. Eng. Process 2021, 166, 108474. [Google Scholar] [CrossRef]
- Cook, S.D. An historical review of phenylacetic acid. Plant Cell Physiol. 2019, 60, 243–254. [Google Scholar] [CrossRef]
- Woodward, A.W.; Bartel, B. Auxin: Regulation, action, and interaction. Ann. Bot. 2005, 95, 707–735. [Google Scholar] [CrossRef] [Green Version]
- Mu, W.; Yu, S.; Zhu, L.; Zhang, T.; Jiang, B. Recent research on 3-phenyllactic acid, a broad-spectrum antimicrobial compound. Appl. Microbiol. Biotechnol. 2012, 95, 1155–1163. [Google Scholar] [CrossRef]
- Zhao, H.; Jiang, Z.; Chang, X.; Xue, H.; Yahefu, W.; Zhang, X. 4-hydroxyphenylacetic acid prevents acute APAP-induced liver injury by increasing phase II and antioxidant enzymes in mice. Front. Pharmacol. 2018, 9, 653. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Xi, R.; Zhang, Z.; Li, W.; Liu, Y.; Jin, F.; Wang, X. 4-hydroxyphenylacetic acid attenuated inflammation and edema via suppressing HIF-1α in seawater aspiration-induced lung injury in rats. Int. J. Mol. Sci. 2014, 15, 12861–12884. [Google Scholar] [CrossRef]
- Surburg, H.; Panten, J. Common Fragrance and Flavor Materials: Preparation, Properties and Uses, 6th ed.; Wiley: Hoboken, NJ, USA, 2016; ISBN 9783527331604. [Google Scholar]
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients, 6th ed.; CRC Press: Boca Raton, FL, USA, 2010; ISBN 978-1-4200-9077-2. [Google Scholar]
- Adams, T.B.; Cohen, S.M.; Doull, J.; Feron, V.J.; Goodman, J.I.; Marnett, L.J.; Munro, I.C.; Portoghese, P.S.; Smith, R.L.; Waddell, W.J.; et al. The FEMA GRAS assessment of phenethyl alcohol, aldehyde, acid, and related acetals and esters used as flavor ingredients. Food Chem. Toxicol. 2005, 43, 1179–1206. [Google Scholar] [CrossRef]
- Li, Z.; Wu, S.; Zhou, Y.; Lukito, B.R. Bioproduction of phenethyl alcohol, aldehyde, acid, amine, and related compounds. International Patent WO 2018/217168 A1, 29 November 2018. [Google Scholar]
- Nielsen, D.; Machas, M.; McKenna, R. Microbial production of 2-phenylethanol from renewable substrates. U.S. Patent US2020/0231992 A1, 23 July 2020. [Google Scholar]
- Zhang, L.H.; Liu, Q.; Pan, H.; Li, X.; Guo, D.Y. Metabolic engineering of Escherichia coli to high efficient synthesis phenylacetic acid from phenylalanine. AMB Express 2017, 7, 7. [Google Scholar] [CrossRef] [Green Version]
- Mao, Z.; Liu, L.; Zhang, Y.; Yuan, J. Efficient synthesis of phenylacetate and 2-phenylethanol by modular cascade biocatalysis. Chem. Bio. Chem. 2020, 21, 2676–2679. [Google Scholar] [CrossRef]
- Zhan, Y.; Zhou, M.; Wang, H.; Chen, L.; Li, Z.; Cai, D.; Wen, Z.; Ma, X.; Chen, S. Efficient synthesis of 2-phenylethanol from l-phenylalanine by engineered Bacillus licheniformis using molasses as carbon source. Appl. Microbiol. Biotechnol. 2020, 104, 7507–7520. [Google Scholar] [CrossRef]
- Gu, Y.; Ma, J.; Zhu, Y.; Xu, P. Refactoring Ehrlich pathway for high-yield 2-phenylethanol production in Yarrowia lipolytica. ACS Synth. Biol. 2020, 9, 623–633. [Google Scholar] [CrossRef] [Green Version]
- Sekar, B.S.; Li, X.; Li, Z. Bioproduction of natural phenethyl acetate, phenylacetic acid, ethyl phenylacetate, and phenethyl phenylacetate from renewable feedstock. ChemSusChem 2022, 15, e202102645. [Google Scholar] [CrossRef]
- Regulation (EC) No.1334/2008 of the European Parliament and of The Council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods and amending Council Regulation (EEC) No.1601/91, Regulations (EC) No.2232/96 and (EC) No.110/2008 and Directive 2000/13/EC. Off. J. Eur. Union 2008, 354, 34.
- U.S. Food & Drugs Administration. Code of Federal Regulations-Title 21-Food and Drugs; U.S. Government Publishing Office: Washington, DC, USA, 2018.
- Serra, S.; Fuganti, C.; Brenna, E. Biocatalytic preparation of natural flavours and fragrances. Trends Biotechnol. 2005, 23, 193–198. [Google Scholar] [CrossRef]
- Fronza, G.; Fuganti, C.; Pedrocchi-Fantoni, G.; Serra, S.; Zucchi, G.; Fauhl, C.; Guillou, C.; Reniero, F. Stable isotope characterization of raspberry ketone extracted from Taxus baccata and obtained by oxidation of the accompanying alcohol (betuligenol). J. Agric. Food Chem. 1999, 47, 1150–1155. [Google Scholar] [CrossRef]
- Fronza, G.; Fuganti, C.; Serra, S.; Burke, A.; Guillou, C.; Reniero, F. The positional δ(18O) values of extracted and synthetic vanillin. Helv. Chim. Acta 2001, 84, 351–359. [Google Scholar] [CrossRef]
- Fronza, G.; Fuganti, C.; Serra, S.; Cisero, M.; Koziet, J. Stable isotope labeling pattern of resveratrol and related natural stilbenes. J. Agric. Food Chem. 2002, 50, 2748–2754. [Google Scholar] [CrossRef] [PubMed]
- Aleu, J.; Fronza, G.; Fuganti, C.; Serra, S.; Fauhl, C.; Guillou, C.; Reniero, F. Differentiation of natural and synthetic phenylacetic acids by 2H NMR of the derived benzoic acids. Eur. Food Res. Technol. 2002, 214, 63–66. [Google Scholar] [CrossRef]
- Brenna, E.; Fronza, G.; Fuganti, C.; Gatti, F.G.; Grande, V.; Serra, S.; Guillou, C.; Reniero, F.; Serra, F. Stable isotope characterization of the ortho-oxygenated phenylpropanoids: Coumarin and melilotol. J. Agric. Food Chem. 2005, 53, 9383–9388. [Google Scholar] [CrossRef] [PubMed]
- Serra, S. Recent advances in the synthesis of carotenoid-derived flavours and fragrances. Molecules 2015, 20, 12817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serra, S.; De Simeis, D. Fungi-mediated biotransformation of the isomeric forms of the apocarotenoids ionone, damascone and theaspirane. Molecules 2018, 24, 19. [Google Scholar] [CrossRef] [Green Version]
- Serra, S.; Castagna, A.; Valentino, M. Biocatalytic synthesis of natural dihydrocoumarin by microbial reduction of coumarin. Catalysts 2019, 9, 665. [Google Scholar] [CrossRef] [Green Version]
- Serra, S.; Marzorati, S.; Valentino, M. Two biotechnological approaches to the preparative synthesis of natural dihydrocoumarin. Catalysts 2022, 12, 28. [Google Scholar] [CrossRef]
- Castagna, A.; Serra, S.; Valentino, M. Process for Preparing Phenylacetic Acid. International Patent WO 2021/205299 A1, 14 October 2021. [Google Scholar]
- Bourdichon, F.; Casaregola, S.; Farrokh, C.; Frisvad, J.C.; Gerds, M.L.; Hammes, W.P.; Harnett, J.; Huys, G.; Laulund, S.; Ouwehand, A.; et al. Food fermentations: Microorganisms with technological beneficial use. Int. J. Food Microbiol. 2012, 154, 87–97. [Google Scholar] [CrossRef]
- Czerucka, D.; Piche, T.; Rampal, P. Review article: Yeast as probiotics—Saccharomyces boulardii. Aliment. Pharmacol. Ther. 2007, 26, 767–778. [Google Scholar] [CrossRef]
- Silva, V.C.; Peres, M.F.S.; Gattas, E.A.L. Application of methylotrophic yeast Pichia pastoris in the field of food industry—A review. J. Food Agric. Environ. 2009, 7, 268–273. [Google Scholar]
- Groenewald, M.; Boekhout, T.; Neuvéglise, C.; Gaillardin, C.; van Dijck, P.W.M.; Wyss, M. Yarrowia lipolytica: Safety assessment of an oleaginous yeast with a great industrial potential. Crit. Rev. Microbiol. 2014, 40, 187–206. [Google Scholar] [CrossRef]
- Abeln, F.; Chuck, C.J. The history, state of the art and future prospects for oleaginous yeast research. Microb. Cell Fact. 2021, 20, 221. [Google Scholar] [CrossRef]
- Braga, A.; Freitas, B.; Cordeiro, A.; Belo, I. Valorization of crude glycerol as carbon source for the bioconversion of L-phenylamine to 2-phenylethanol by Yarrowia species. J. Chem. Technol. Biotechnol. 2021, 96, 2940–2949. [Google Scholar] [CrossRef]
- Xin, F.; Yan, W.; Quian, X.; Jiang, M.; Dong, W.; Ma, J.; Zhang, W.; Fang, Y. High-Stress-Resistance Yarrowia lipolytica and Application Thereof. Chinese Patent CN108441431 (A), 24 August 2018. [Google Scholar]
- Jo, Y.-S.; An, J.-U.; Oh, D.-K. γ-Dodecelactone production from safflower oil via 10-hydroxy-12(Z)-octadecenoic acid intermediate by whole cells of Candida boidinii and Stenotrophomonas nitritireducens. J. Agric. Food Chem. 2014, 62, 6736–6745. [Google Scholar] [CrossRef]
- Breuer, U.; Harms, H. Debaryomyces hansenii—An extremophilic yeast with biotechnological potential. Yeast 2006, 23, 415–437. [Google Scholar] [CrossRef]
- De Graeve, M.; De Maeseneire, S.L.; Roelants, S.L.K.W.; Soetaert, W. Starmerella bombicola, an industrially relevant, yet fundamentally underexplored yeast. FEMS Yeast Res. 2018, 18, foy072. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, X.; Too, H.-P. Microbial astaxanthin biosynthesis: Recent achievements, challenges, and commercialization outlook. Appl. Microbiol. Biotechnol. 2020, 104, 5725–5737. [Google Scholar] [CrossRef]
- Fernandes, T.; Silva-Sousa, F.; Pereira, F.; Rito, T.; Soares, P.; Franco-Duarte, R.; Sousa, M.J. Biotechnological importance of Torulaspora delbrueckii: From the obscurity to the spotlight. J. Fungi 2021, 7, 712. [Google Scholar] [CrossRef]
- Lane, M.M.; Morrissey, J.P. Kluyveromyces marxianus: A yeast emerging from its sister’s shadow. Fungal Biol. Rev. 2010, 24, 17–26. [Google Scholar] [CrossRef]
- Cerniglia, C.E.; Crow, S.A. Metabolism of aromatic hydrocarbons by yeasts. Arch. Microbiol. 1981, 129, 9–13. [Google Scholar] [CrossRef]
- Van der Walt, J.P.; von Arx, J.A. The yeast genus Yarrowia gen. nov. Antonie Leeuwenhoek 1980, 46, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Michely, S.; Gaillardin, C.; Nicaud, J.M.; Neuveglise, C. Comparative physiology of oleaginous species from the Yarrowia clade. PLoS ONE 2013, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Limtong, S.; Youngmanitchai, W.; Kawasaki, H.; Seki, T. Candida phangngensis sp. nov., an anamorphic yeast species in the Yarrowia clade, isolated from water in mangrove forests in Phang-Nga province, Thailand. Int. J. Syst. Evol. Microbiol. 2008, 58, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Groenewald, M.; Smith, M.T. The teleomorph state of Candida deformans Langeron & Guerra and description of Yarrowia yakushimensis comb. nov. Antonie Leeuwenhoek 2013, 103, 1023–1028. [Google Scholar] [CrossRef]
- Nagy, E.; Dlauchy, D.; Medeiros, A.O.; Péter, G.; Rosa, C.A. Yarrowia porcina sp. nov. and Yarrowia bubula f.a. sp. nov., two yeast species from meat and river sediment. Antonie Leeuwenhoek 2014, 105, 697–707. [Google Scholar] [CrossRef]
- Liu, K.-F.; Li, X.-H.; Hui, F.-L. Yarrowia brassicae f.a., sp. nov., a new yeast species from traditional chinese sauerkraut. Int. J. Syst. Evol. Microbiol. 2018, 68, 2024–2027. [Google Scholar] [CrossRef]
- Zhao, M.; Tao, Y.; Wu, X.; Xiao, Y. One-pot efficient biosynthesis of 4-hydroxyphenylacetic acid and its analogues from lignin-related p-coumaric and ferulic acids. ACS Sustain. Chem. Eng. 2021, 9, 6400–6409. [Google Scholar] [CrossRef]


| Entry 1 | Yeast | PE/PAA Ratio | PAA (g/L) 6 | PE + PAA Yield (%) 7 |
|---|---|---|---|---|
| 1 | Saccharomyces cerevisiae2 | 98/2 | 0.011 | 15.3 |
| 2 | Saccharomyces boulardi2,3 | 97/3 | 0.013 | 13.1 |
| 3 | Pichia pastoris2 | 97/3 | 0.013 | 14.8 |
| 4 | Yarrowia lipolytica (DSM 8218) 2 | 21/79 | 0.470 | 16.1 |
| 5 | Yarrowia lipolytica (DSM 8218) 4,5 | <1/99 | 1.675 | 40.0 |
| Entry 1 | Yeast | PE/PAA Ratio | PAA (g/L) 4 | PE + PAA Yield (%) 5 |
|---|---|---|---|---|
| 1 | Saccharomyces cerevisiae | 97/3 | 0.02 | 16.8 |
| 2 | Saccharomyces boulardii 2 | 96/4 | <0.01 | 3.7 |
| 3 | Pichia pastoris | 52/48 | 0.41 | 24.4 |
| 4 | Yarrowia lipolytica (DSM 8218) | <1/99 | 1.97 | 53.0 |
| 5 | Candida boidinii | 35/65 | 0.87 | 37.9 |
| 6 | Starmerella bombicola | <1/99 | 0.05 | 1.3 |
| 7 | Debaryomyces hansenii | 88/12 | 0.01 | 3.0 |
| 8 | Torulaspora delbrueckii | 87/13 | 0.06 | 13.4 |
| 9 | Kluyveromyces marxianus | 61/39 | 0.61 | 45.1 |
| 10 | Cryptococcus curvatus | 3 | 3 | - |
| 11 | Sporidiobolus johnsonii | 3 | 3 | - |
| 12 | Phaffia rhodozyma | 39/61 | 0.02 | 0.9 |
| Entry 1 | (L)-Phenylalanine Starting Concentration (g/L) | PAA Yield (%) 2 |
|---|---|---|
| 1 | 4.5 | 51.0 |
| 2 | 8.5 | 55.4 |
| 3 | 12.5 | 67.1 |
| 4 | 18.0 | 52.6 |
| 5 | 25.0 | 24.2 |
| Entry 1 | Yarrowia Strain | Fermentation Conditions | PAA Yield (%) 4 |
|---|---|---|---|
| 1 | Yarrowia lipolytica (DSM 8218) | Flask 2 | 67 |
| 2 | Yarrowia lipolytica (DSM 70562) | Flask 2 | 69 |
| 3 | Yarrowia deformans (DSM 70561) | Flask 2 | 64 |
| 4 | Yarrowia yakushimensis (CBS 10252) | Flask 2 | 24 |
| 5 | Yarrowia bubula (CBS 12934) | Flask 2 | 66 |
| 6 | Yarrowia phangngaensis (CBS 10407) | Flask 2 | 27 |
| 7 | Yarrowia brassicae (CBS 15225) | Flask 2 | 32 |
| 8 | Yarrowia lipolytica (DSM 8218) | Bioreactor 3 | 71 |
| 9 | Yarrowia lipolytica (DSM 70562) | Bioreactor 3 | 75 |
| 10 | Yarrowia deformans (DSM 70561) | Bioreactor 3 | 72 |
| 11 | Yarrowia bubula (CBS 12934) | Bioreactor 3 | 68 |
| Entry 1 | Phenylalanine Enantiomer | Fermentation Medium | PAA Yield (%) 2 |
|---|---|---|---|
| 1 | (L) | YM | 53.4 |
| 2 | (DL) | YM | 25.5 |
| 3 | (D) | YM | 12.1 |
| 4 | (D) | PF-YM | 5.5 |
| Entry 1 | Amino Acid | Compounds 2–6 2 | Other 2 |
|---|---|---|---|
| 1 | (L)-Phenyl alanine | 6a (58.3%) | - |
| 2 | (L)-Tyrosine | 6b (47.7%) | - |
| 3 | (L)-Tryptophan | 3c (16.0%); 6c (1.3%) | Anthranilic acid (33.6%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serra, S.; Castagna, A.; Marzorati, S.; Valentino, M. Biotransformation of the Proteogenic Amino Acids Phenylalanine, Tyrosine and Tryptophan by Yarrowia Species: An Application to the Preparative Synthesis of Natural Phenylacetic Acid. Catalysts 2022, 12, 1638. https://doi.org/10.3390/catal12121638
Serra S, Castagna A, Marzorati S, Valentino M. Biotransformation of the Proteogenic Amino Acids Phenylalanine, Tyrosine and Tryptophan by Yarrowia Species: An Application to the Preparative Synthesis of Natural Phenylacetic Acid. Catalysts. 2022; 12(12):1638. https://doi.org/10.3390/catal12121638
Chicago/Turabian StyleSerra, Stefano, Antonio Castagna, Stefano Marzorati, and Mattia Valentino. 2022. "Biotransformation of the Proteogenic Amino Acids Phenylalanine, Tyrosine and Tryptophan by Yarrowia Species: An Application to the Preparative Synthesis of Natural Phenylacetic Acid" Catalysts 12, no. 12: 1638. https://doi.org/10.3390/catal12121638